Abstract
Tobramycin inhalation solution is used to treat chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients. We evaluated the efficacy and safety of a novel, light-porous-particle, dry-powder formulation of tobramycin, which was developed to improve delivery efficiency to the airways and substantially reduce the delivery time. In this randomized, double-blind study, patients with cystic fibrosis (age 6–21 years) received tobramycin inhalation powder (112 mg tobramycin) twice daily (n = 46) or placebo (n = 49) via the T-326 Inhaler for one cycle, followed by two open-label cycles (all patients). Cycles were 28 days on, 28 days off treatment. The primary endpoint was change in FEV1 % predicted from baseline to Day 28 of Cycle 1. The study was terminated early based on positive results in the interim analysis. Tobramycin inhalation powder significantly improved FEV1 % predicted versus placebo at Day 28 (difference 13.3, 95% CI 5.31, 21.28; P = 0.0016). Similar changes in FEV1 were seen in patients switching from placebo to tobramycin inhalation powder in Cycle 2; improvements were maintained over time. Tobramycin inhalation powder also reduced sputum Pseudomonas aeruginosa density, respiratory-related hospitalization and antipseudomonal antibiotic use versus placebo. The most common adverse event was cough; the frequency of cough was higher in patients receiving placebo (26.5%) versus tobramycin inhalation powder (13.0%) in Cycle 1. Tobramycin inhalation powder was not associated with ototoxicity or nephrotoxicity. Administration time was between 4 and 6 minutes. In conclusion, tobramycin inhalation powder was effective and well tolerated in cystic fibrosis patients, and may offer an important treatment option to decrease the treatment burden of cystic fibrosis pseudomonas lung infections.
Keywords: cystic fibrosis, tobramycin inhalation powder, pseudomonas aeruginosa
INTRODUCTION
The majority of individuals with cystic fibrosis (CF) develop chronic pulmonary infections that are difficult to treat.1 Pseudomonas aeruginosa is the predominant pathogen associated with progressive loss of lung function, as well as morbidity and mortality in patients with CF.2–5 Antibiotics by inhalation, including the aminoglycoside tobramycin, are commonly used to treat chronic P. aeruginosa lung infection in these patients.1,6–8 Tobramycin inhalation solution (TIS [TOBI®]) has been shown to significantly improve lung function, as well as decrease the rate of hospitalization and improve quality of life in patients with CF.9–12
Tobramycin inhalation solution 300 mg/5 mL twice daily (b.i.d.) delivered via the PARI-LC® PLUS nebulizer with a suitable compressor, is currently approved for the treatment of chronic P. aeruginosa pulmonary infections in patients with CF aged 6 years and older with forced expiratory volume in one second (FEV1) ≥25 to ≤75% predicted.13 Both US and European CF treatment guidelines recommend the use of TIS for treatment of P. aeruginosa pulmonary infection in CF patients.14,15 Administration time for TIS is approximately 15 minutes per dose, excluding the time required to clean and disinfect the nebulizer.16 To reduce the treatment burden for patients, a new drug-device combination using an inhalation powder formulation of tobramycin is being developed. Tobramycin inhalation powder (TIP™) is manufactured using Novartis PulmoSphere® technology, an emulsion-based spray drying process that yields spherical hollow porous particles (Fig. 1a). The new inhalation powder formulation of tobramycin is delivered via the T-326 Inhaler (Novartis Pharmaceuticals, San Carlos, CA, USA) (Fig. 1b).16 The device, which does not require a power source or electronics, was designed to enhance the delivery of the drug to the lung and shorten administration time. This may translate into increased patient adherence and therefore potentially improve clinical outcomes. The dose of tobramycin inhalation powder of 112 mg b.i.d. was selected from a Phase 1 dose-finding study.16 The present Phase 3 study (EVOLVE) was designed to assess the efficacy and safety of this new tobramycin inhalation powder formulation for treating CF patients with P. aeruginosa infection.
Fig. 1.
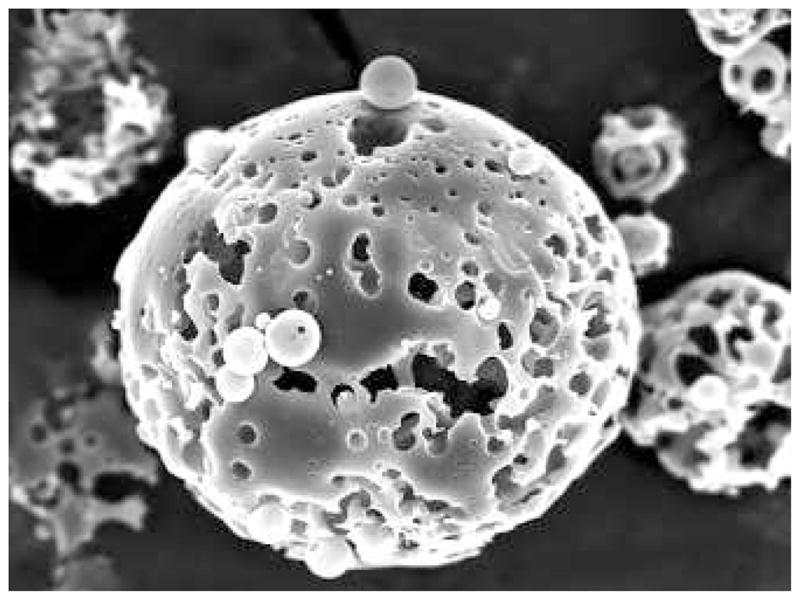
Tobramycin inhalation powder: a novel, light-porous-particle formulation of tobramycin delivered via a simple passive inhaler. a) Hollow, porous particles of tobramycin inhalation powder; b) the T-326 Inhaler
METHODS
This study was conducted between September 2005 and February 2007 in 38 centers in Europe (Bulgaria, Lithuania, Serbia), Latin America (Argentina, Brazil, Chile, Mexico) and the United States. The study was approved by an Institutional Review Board or Independent Ethics Committee for each center and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient or their legal representative prior to randomization.
Patients
CF patients aged 6–21 years with FEV1 ≥25 to ≤80% predicted based on Knudson criteria17, were eligible for enrollment. Diagnosis was confirmed by at least one clinical feature of CF plus sweat chloride test ≥60 mEq/l obtained by quantitative pilocarpine iontophoresis, known mutations in each cystic fibrosis transmembrane conductance regulator [CFTR] gene, or abnormal nasal transepithelial potential difference (characteristic of CF). Eligible patients were also required to have a positive sputum or throat culture for P. aeruginosa within 6 months of screening, and a positive sputum culture for P. aeruginosa at the screening visit.
Patients were excluded if they had positive cultures for Burkholderia cepacia within 2 years prior to screening or at screening; hemoptysis >60 cc at any time within 30 days of study drug administration; aminoglycoside hypersensitivity or adverse reaction to inhaled antibiotics; serum creatinine ≥2 mg/dl, blood urea nitrogen ≥40 mg/dl or abnormal urinalysis (≥2+ proteinuria). During the study, patients were allowed to use bronchodilators, macrolide antibiotics, dornase alfa, and inhaled steroids provided these were started at least 28 days prior to study drug administration. However, patients were excluded if they had received inhaled antipseudomonal antibiotics within 4 months prior to screening; systemic antipseudomonal antibiotics within 28 days prior to study drug administration and loop diuretics within 7 days of study drug administration. If patients required treatment with antipseudomonal antibiotics other than study drug for signs and/or symptoms of a pulmonary exacerbation, they were required to withdraw from the study.
Study design
This randomized two-arm trial comprised three cycles; each cycle consisted of 28 days on treatment followed by 28 days off treatment. Cycle 1 was double-blind and placebo-controlled with patients randomized 1:1 to tobramycin inhalation powder (112 mg) or placebo, both administered twice daily via the T-326 Inhaler. During Cycle 1 patients received tobramycin inhalation powder (four capsules each containing tobramycin 28 mg to be inhaled twice daily) or matching placebo capsules. The placebo was made from the excipients used in the spray-drying process to manufacture the study drug; distearylphosphatidylcholine (DSPC) and calcium chloride. After completing Cycle 1, all patients received open-label tobramycin inhalation powder for two additional cycles (Cycles 2 and 3). The total study duration was 24 weeks.
Efficacy measures
The primary efficacy measure was relative change in FEV1 % predicted from baseline (Day 1 pre-dose measurement) to end of Cycle 1 dosing (Day 28 pre-dose measurement) versus placebo. Other efficacy measures included change in sputum P. aeruginosa density (colony forming units [CFU] per gram of sputum), P. aeruginosa susceptibility to tobramycin (minimum inhibitory concentrations [MIC]), antipseudomonal antibiotic use, and respiratory-related hospitalizations.
Safety measures
Safety assessments included the incidence and severity of all adverse events (AEs) throughout the study, changes in vital signs, hematology, blood chemistry, urine protein, audiology (at selected sites) and the presence of airway reactivity secondary to the study drug (relative change in FEV1 % predicted from pre-dose to 30 minutes post-dose). In addition, serum tobramycin concentrations were measured in Cycles 1 and 2, or earlier in the case of early termination from the study.
Spirometry
Lung function was measured by spirometry in accordance with established guidelines.18 The same spirometer was used at each study visit. Forced expiratory volume in one second was measured and expressed as a percentage of predicted normal values; regression equations were used to calculate predicted values according to Knudson criteria.17
Pseudomonas aeruginosa susceptibility testing
Expectorated sputum or throat swabs were sent by courier to a central laboratory (ICON Central Laboratories) for analysis. Sputum samples were cultured quantitatively and throat swabs were cultured semi-quantitatively on selective agar, as previously described.19 Minimum inhibitory concentrations for tobramycin were established for all P. aeruginosa isolates via a Sensititre custom panel CMP4DCHS (CGI). Interpretation was based on current National Committee on Clinical Laboratory Standards (NCCLS) guidelines. Susceptibility testing was performed for each distinctive P. aeruginosa phenotype (mucoid, non-mucoid and small colony variant).
Statistical analyses
A sample size of 140 patients (70 per group) was estimated to provide 90% power at two-sided 0.05 significance level to detect a treatment difference of 11% in mean (assuming 20% standard deviation) relative change of FEV1 % predicted in Cycle 1 (baseline to Day 28).
The study included a planned interim analysis by an external independent Data Monitoring Committee (DMC) once 80 patients had completed dosing in Cycle 1. The objectives were to (a) estimate the common standard deviation for sample size re-estimation, (b) evaluate efficacy of tobramycin inhalation powder versus placebo for potential early termination of the study, and (c) assess safety in terms of AEs, airway reactivity, and bronchospasm. After reviewing the results, which showed a statistically significant benefit of tobramycin inhalation powder over placebo, the DMC recommended the trial be terminated early as pre-defined stopping criteria were fulfilled. Subsequently, concerns were raised regarding the quality of spirometry data from some Latin American centers. An expert panel of pulmonologists was therefore set up to review, in a blinded manner, the spirometry data from all Latin American Centers in the original interim analysis. The expert panel pre-defined a set of quality criteria to evaluate the adequacy of the FEV1 data: 1) Patients must have acceptable calibrations at screening day, Day 1, Day 8 and Day 28; 2) In addition to calibrations, patients must satisfy quality review at baseline (screening day or Day 1 pre-dose) and post-baseline (Day 8 or Day 28 pre-dose of Cycle 1). After reviewing the source spirometry data from all Latin American centers, the expert panel recommended that 18 patients (10 TIP-treated and 8 placebo-treated patients) from the original interim analysis should be excluded, due to unacceptable calibration of the spirometer or unacceptable FEV1 data quality. Subsequently, a sensitivity interim analysis (SIA) was performed by the DMC that excluded the 18 patients identified; this subpopulation (n = 61) is referred to as the modified intent-to-treat (mITT) population. Based on the results of the SIA, the DMC again recommended the trial be terminated early.
The primary efficacy analysis was based on the mITT population. The primary efficacy measure was assessed using an analysis of covariance (ANCOVA) model; factors of treatment, baseline FEV1 % predicted, age and region were included in the model. Due to interim analysis, the statistical significance level was set at 0.0044 for the SIA. All other efficacy measures used the all-treated patient population (includes all patients randomized who received at least one capsule of study medication, mITT population) and are reported descriptively with no formal statistical testing. All final analyses are based on observed data with no imputation performed for missing data. All reported AEs were recorded. Safety measures are summarized descriptively. The SAS® software version 8.2 (SAS Institute, Cary, NC, USA) was used.
RESULTS
Baseline demographic and clinical characteristics
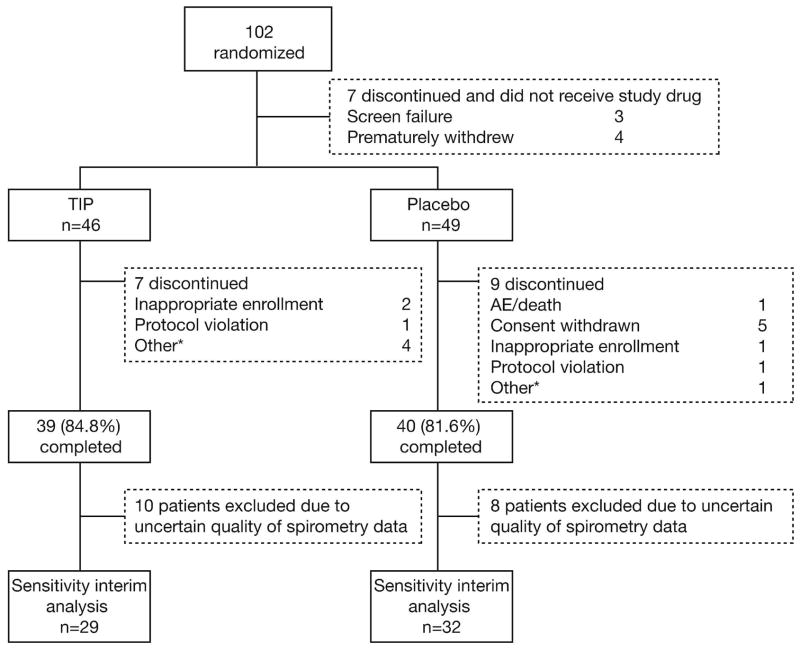
In total, 102 patients were randomized and 95 patients were treated (Fig. 2): 46 patients received tobramycin inhalation powder (39 patients [84.8%] completed) and 49 patients received placebo (40 patients [81.6%] completed) during Cycle 1 before all patients received open-label tobramycin inhalation powder for two additional cycles. Both treatment groups had generally high levels of adherence, which was assessed using dosing logs, number of used and unused capsules returned per patient and percentage of completed scheduled study visits. In Cycle 1, 91% adherence was observed for the patients in the tobramycin inhalation powder group and 89% in the placebo group. Over Cycles 2 and 3, both treatment groups showed greater than 95% adherence.
Fig. 2.
Study disposition (all randomized patients)
*Includes moved, intolerant of inhaler, non-compliance and self discontinuation; TIP = tobramycin inhalation powder
In the all-treated patient population, baseline demographics and clinical characteristics were similar between the two treatment groups (Table 1). Baseline demographics and clinical characteristics were also comparable between the all-treated and mITT patient population (data not shown).
TABLE 1.
Baseline demographic and clinical characteristics (all treated patients)
| Tobramycin inhalation powder (n = 46) | Placebo (n = 49) | |
|---|---|---|
| Age (years), mean (SD) | 13.4 (4.42) | 13.2 (3.91) |
| Age group, n (%) | ||
| ≥6 to <13 years | 21 (45.7) | 24 (49.0) |
| ≥13 to <22 years | 25 (54.3) | 25 (51.0) |
| Sex, n (%) | ||
| Females | 27 (58.7) | 26 (53.1) |
| Males | 19 (41.3) | 23 (46.9) |
| Race, n (%) | ||
| Black | 0 (0) | 1 (2.0) |
| Caucasian | 37 (80.4) | 43 (87.8) |
| Hispanic | 8 (17.4) | 4 (8.2) |
| Other | 1 (2.2) | 1 (2.0) |
| Body mass index (kg/m2), mean (SD) | 16.8 (3.6) | 17.1 (3.1) |
| FEV1 % predicted,* mean (SD) | 54.7 (18.89) | 58.5 (20.03) |
| FEV1 % predicted distribution*, n (%) | ||
| <25 | 1 (3.1) | 3 (8.1) |
| ≥25–<50 | 13 (40.6) | 11 (29.7) |
| ≥50–≤80 | 16 (50.0) | 18 (48.6) |
| >80 | 2 (6.3) | 5 (13.5) |
Excluding patients from Latin American sites with any potential spirometry quality concerns (TIP n = 32 and placebo n = 37); SD = standard deviation; FEV1 = forced expiratory volume in one second
Use of inhaled antipseudomonal antibiotics within the last 12 months prior to screening was low in both treatment groups: tobramycin inhalation powder group, 4.3%; placebo group, 4.1% (in accordance with the study inclusion criteria, none of the enrolled patients had used inhaled antipseudomonal antibiotics within the last 4 months prior to screening). During the study, concomitant therapy was used by 91.3% of patients in the tobramycin inhalation powder group and 98.0% in the placebo group. Dornase alfa was used by 58.7% and 73.5% of patients receiving tobramycin inhalation powder and placebo, respectively. Other commonly used concomitant medications were: pancreatic enzyme preparations, 73.9% in the tobramycin inhalation powder group and 85.7% in the placebo group (bioglan panazyme, pancrealipase), and selective β2-adrenoreceptor agonists, 45.7% in the tobramycin inhalation powder group and 61.2% in the placebo group (most frequently salbutamol).
Efficacy
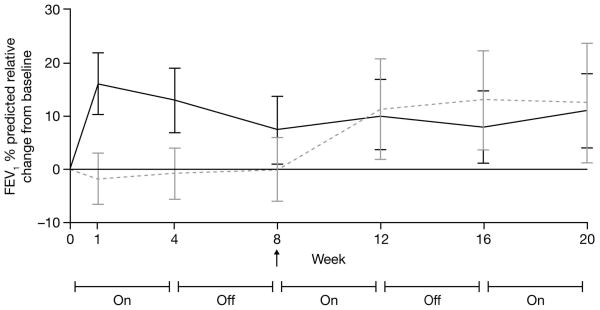
Tobramycin inhalation powder (112 mg tobramycin b.i.d.) significantly improved FEV1 % predicted from baseline to Day 28 of Cycle 1 compared with placebo in the mITT population (least squares mean difference 13.3, 95% confidence interval 5.31, 21.28; P = 0.0016). The results of ad hoc analyses performed using the original interim analysis ITT population (including patients from Latin American centers), are consistent with those of the mITT population (data not shown). The relative change from baseline in FEV1 % predicted over the three cycles is shown in Fig. 3. Improvements in FEV1 with tobramycin inhalation powder in Cycle 1 were maintained for all three cycles. When patients were switched from placebo to tobramycin inhalation powder in Cycle 2, FEV1 % predicted increased to the level observed in the tobramycin inhalation powder group and this was maintained throughout Cycles 2 and 3.
Fig. 3.
Relative change from baseline (95% confidence intervals) in forced expiratory volume in 1 second (FEV1) % predicted over three cycles in patients receiving tobramycin inhalation powder (112 mg tobramycin) (mITT population)
Grey dotted line = placebo [placebo for Cycle 1, TIP for Cycles 2 and 3]; black line = TIP [for all 3 cycles]; TIP = tobramycin inhalation powder
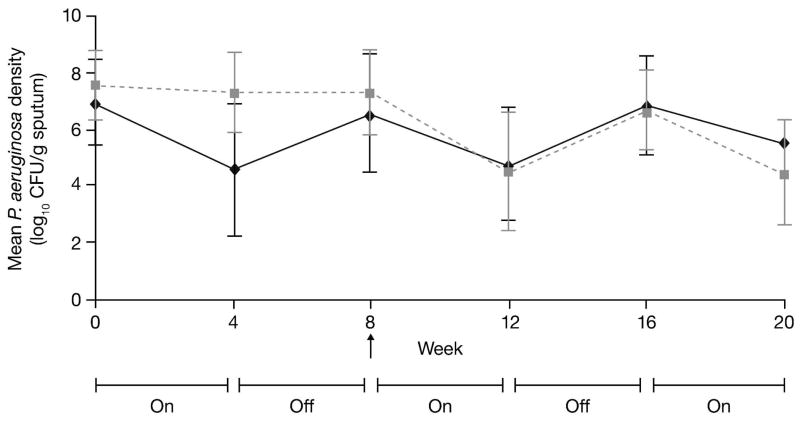
Treatment with tobramycin inhalation powder decreased the sputum density of both non-mucoid and mucoid phenotypes of P. aeruginosa compared with placebo at Day 28 of Cycle 1 (mean decrease [SD] 1.91 [2.54] vs 0.15 [0.68] log10 CFU/g for non-mucoid phenotype and 2.61 [2.53] vs 0.43 [1.05] log10 CFU/g for mucoid phenotype, respectively). After switching from placebo to tobramycin inhalation powder, the results were similar between the two groups in Cycles 2 and 3 with a trend showing P. aeruginosa density increasing after 28 days off treatment and then decreasing when on treatment (Fig. 4 presents combined mean of all phenotypes).
Fig. 4.
Mean Pseudomonas aeruginosa densities of all phenotypes (mucoid, non-mucoid and small colony variant) over three cycles of treatment in patients receiving tobramycin inhalation powder (112 mg tobramycin) (all treated patients)
Grey dotted line = placebo [placebo for Cycle1, TIP for Cycles 2 and 3]; black line = TIP [for all 3 cycles]; TIP = tobramycin inhalation powder; CFU = colony forming unit
The number of patients with MIC values >8 μg/mL for tobramycin for the mucoid and non-mucoid phenotypes of P. aeruginosa at baseline and at the end of Cycle 3 are shown in Table 2. There was a slightly higher number of patients with increased MIC values after 2–3 cycles of tobramycin inhalation powder treatment.
TABLE 2.
Prevalence of Pseudomonas aeruginosa phenotypes with a tobramycin minimum inhibitory concentration >8 μg/mL (all treated patients)
| Tobramycin inhalation powder (for all three cycles) | Placebo (placebo 1 cycle, tobramycin inhalation powder for two cycles) | |||
|---|---|---|---|---|
| No. of patients with MIC >8 μg/mL | No. patients with available MIC data | No. of patients with MIC >8 μg/mL | No. patients with available MIC data | |
| Mucoid phenotype | ||||
| Baseline | 3 | 33 | 1 | 40 |
| End of Cycle 3 | 5 | 27 | 2 | 27 |
| Non-mucoid phenotype | ||||
| Baseline | 2 | 30 | 2 | 34 |
| End of Cycle 3 | 4 | 14 | 3 | 10 |
MIC = minimum inhibitory concentration
The proportion of patients requiring any additional antipseudomonal antibiotic in Cycle 1 (including 28 days on and 28 days off treatment) was lower with tobramycin inhalation powder than placebo (13.0% vs 18.4%). In those patients requiring additional antipseudomonal antibiotics, the duration of use over the 56 days of the cycle was shorter in the tobramycin inhalation powder group (mean [SD] 13.3 [3.39] vs 19.3 [14.54] days). No patients receiving tobramycin inhalation powder were hospitalized during Cycle 1 for respiratory-related events, whereas 12.2% of patients on placebo had respiratory-related hospitalizations with an average duration of 12.3 days.
Administration time for tobramycin inhalation powder was approximately 4–6 minutes. Administration of the first dose was slightly longer (nearly 8 minutes) because of the need to train patients in the correct use of the T-326 Inhaler.
Safety
In Cycle 1, AEs were reported by 75.5% of placebo-treated versus 50.0% of tobramycin inhalation powder-treated patients (Table 3). In this cycle, the most common AEs in the tobramycin inhalation powder group were cough, lung disorders (generally reported as a pulmonary or cystic fibrosis exacerbation) and pharyngolaryngeal pain (sore throat), and in the placebo group were cough, lung disorders and productive cough. Most AEs were mild and transient. The most common AEs in patients receiving tobramycin inhalation powder over three cycles (including off treatment period) were cough, lung disorders, pharyngolaryngeal pain and pyrexia (Table 3). Cough was most commonly reported by patients receiving placebo in Cycle 1 and tobramycin inhalation powder in Cycles 2 and 3. The majority of cough events during the study were reported as an increase or worsening of cough above baseline. The frequency of lung disorders in any cycle appeared to be higher in the tobramycin inhalation group (21.7% over 3 cycles of treatment) than in the placebo group (12.2% over 2 cycles of treatment), whereas the incidence was comparable in Cycle 1 (10.9% vs 12.2%).
TABLE 3.
Most common adverse events (≥5% in any group) occurring in Cycle 1 and in any cycle* (all treated patients)
| n (%) | Cycle 1 | Any cycle | ||
|---|---|---|---|---|
| Tobramycin inhalation powder (n = 46) | Placebo (n = 49) | Tobramycin inhalation powder group [Tobramycin inhalation powder for 3 cycles (n = 46)] | Placebo group [Tobramycin inhalation powder inhalation powder for 2 cycles# (n = 41)] | |
| Any adverse event | 23 (50.0) | 37 (75.5) | 34 (73.9) | 30 (73.2) |
| Cough | 6 (13.0) | 13 (26.5) | 12 (26.1) | 10 (24.4) |
| Lung disorder† | 5 (10.9) | 6 (12.2) | 10 (21.7) | 5 (12.2) |
| Pharyngolaryngeal pain | 5 (10.9) | 0 (0) | 8 (17.4) | 0 (0) |
| Pyrexia | 3 (6.5) | 2 (4.1) | 6 (13.0) | 5 (12.2) |
| Dysgeusia | 3 (6.5) | 1 (2.0) | 4 (8.7) | 4 (9.8) |
| Upper respiratory tract infection | 2 (4.3) | 2 (4.1) | 4 (8.7) | 4 (9.8) |
| Dysphonia | 2 (4.3) | 0 (0) | 3 (6.5) | 4 (9.8) |
| Rhinitis | 2 (4.3) | 2 (4.1) | 3 (6.5) | 1 (2.4) |
| Productive cough | 1 (2.2) | 4 (8.2) | 2 (4.3) | 5 (12.2) |
| Nasopharyngitis | 1 (2.2) | 3 (6.1) | 2 (4.3) | 2 (4.9) |
| Nasal congestion | 1 (2.2) | 1 (2.0) | 4 (8.7) | 2 (4.9) |
| Abdominal pain | 1 (2.2) | 1 (2.0) | 3 (6.5) | 3 (7.3) |
| Hemoptysis | 1 (2.2) | 1 (2.0) | 3 (6.5) | 1 (2.4) |
| Aspartate aminotransferase increased | 1 (2.2) | 0 (0) | 3 (6.5) | 0 (0) |
| Headache | 0 (0) | 3 (6.1) | 2 (4.3) | 2 (4.9) |
Each cycle included 28 days on and 28 days off treatment;
Adverse events occurring in Cycles 2 and 3 in the placebo group;
Lung disorders were generally reported by the investigator as a pulmonary or cystic fibrosis exacerbation
The proportion of patients experiencing serious adverse events (SAEs) in Cycle 1 was lower in the tobramycin inhalation powder group than the placebo group (6.5% versus 14.3%). The most common SAEs in both treatment groups were lung disorders (6.5% and 8.2% in the tobramycin inhalation powder and placebo groups, respectively). During three cycles of tobramycin inhalation powder treatment (including the off treatment periods), SAEs were reported by 5 patients (10.9%), and during two cycles of tobramycin inhalation powder treatment in the placebo group, SAEs were reported by 6 patients (14.6%). The most common SAE in both groups was lung disorders (MedDRA coding for reported term lung exacerbation), occurring in 6.5% of patients in the tobramycin inhalation powder group and 9.8% of patients during the last two cycles of tobramycin inhalation powder treatment in the placebo group.
There was one death reported in the study. A placebo-treated patient took her last treatment on Day 8 during Cycle 1 and discontinued from the study because of an AE (pulmonary exacerbation) the next day. Her death due to decompensated chronic cor pulmonale occurred 39 days after the last study visit. This patient did not receive any tobramycin inhalation powder.
Serum tobramycin concentrations were determined before (trough level) and 60-minutes after (peak level) the administration of tobramycin inhalation powder. At the end of Cycle 1, mean ± SD trough and peak serum concentrations were 0.29 ± 0.27 μg/mL and 1.99 ± 0.59 μg/mL respectively. At the end of Cycle 2, the levels were 0.38 ± 0.44 μg/mL and 1.64 ± 0.96 μg/mL, respectively. One patient had a trough tobramycin serum concentration above 2 μg/mL (2.66 μg/mL) at the end of Cycle 2; however, there was no clinical evidence of nephrotoxicity or ototoxicity.
No major changes from baseline to pre-specified time points or between-treatment differences for biochemical or hematological measures were observed. No significant changes in renal function were observed as measured by increases in blood urea nitrogen, serum creatinine, or proteinuria. In addition, no major changes from baseline to pre-specified time points in vital signs, including heart rate and systolic and diastolic blood pressure were observed.
Six patients experienced a ≥20% decrease in FEV1 % predicted from pre-dose to 30 minutes post-dose (indicative of bronchospasm): one patient receiving tobramycin inhalation powder (Cycle 1, Day 1), one patient receiving placebo then tobramycin inhalation powder (Cycle 2, Day 28), and four patients receiving placebo in Cycle 1 (two on Day 1 and two on Day 28). Four of these patients received a bronchodilator prior to receiving study drug.
Audiology was performed at selected sites in 22 patients (13 in tobramycin inhalation powder group and 9 in placebo group). None of the patients reported any hearing complaints such as tinnitus, ear pressure or sensoneural hearing loss at any time during the three cycles. Five patients had a decrease in hearing as measured by a conductive audiology test. However, this was generally transient and not suspected to be drug related by the study investigators.
DISCUSSION
Tobramycin inhalation solution 300 mg/5 mL b.i.d. administered by nebulization is approved and widely used for the treatment of P. aeruginosa infection in CF patients. This study was designed to assess the efficacy and safety of a new drug-device combination of tobramycin inhalation powder (112 mg tobramycin) delivered by the T-326 Inhaler. This new formulation in development is intended to offer the same benefits as TIS (i.e. significantly improved lung function, reduced hospitalizations, and improved quality of life) with the advantage of greater convenience.
Tobramycin inhalation powder significantly (P = 0.0016) improved FEV1 % predicted compared with placebo following 28 days of treatment in Cycle 1 (primary efficacy measure) and improvements were maintained throughout the study. Tobramycin inhalation powder also reduced sputum P. aeruginosa density, and reduced the need for other antipseudomonal antibiotics as well as the incidence of respiratory-related hospitalizations compared with placebo. It should be noted that efficacy boundaries were reached at the interim analysis and the study was stopped early based on the recommendation of an independent Data Monitoring Committee; this new formulation has demonstrated benefits on key endpoints relevant to its pharmacological activity.
A single-dose, dose-escalation study showed that the serum and sputum pharmacokinetic profiles of tobramycin inhalation powder (112 mg tobramycin) were similar to that of TIS (300 mg tobramycin/5 mL preservative-free solution).16 These data suggest that the efficacy and safety profile of tobramycin inhalation powder is expected to be comparable to that of the TIS formulation; this was further explored in a recently completed study that directly compared tobramycin inhalation powder with TIS, the EAGER trial (ClinicalTrials.gov identifier NCT00388505).
In our study, tobramycin inhalation powder improved FEV1 % predicted from the first week of treatment and lung function remained above baseline values throughout the study, including the 28-day periods when tobramycin was not being administered. This is consistent with data presented by Ramsey et al (1999) who showed that TIS 300 mg/5 mL b.i.d. (28 days on and 28 days off for a total of 24 weeks) improved lung function during the first two weeks of treatment, and improvements were maintained above pre-treatment values for the duration of the trial.
Our results show that sputum P. aeruginosa density decreased by approximately 2 log10 CFU/g at the end of each of the three 28-day tobramycin inhalation powder treatment periods and values returned towards those at baseline during periods when the drug was withheld. Again, these results are consistent with the bactericidal effects of TIS 300 mg/5 mL described by Ramsey et al (1999). In that study, treatment effect was greatest during the first two cycles of treatment (average reductions of between 1.8–2.2 log10 CFU/g); however, the magnitude of bacterial reduction was smaller with the third cycle of therapy (average reductions of 0.8 log10 CFU/g). This was not observed in our study, where reductions in sputum density of P. aeruginosa were consistent across all three treatment cycles.
Exposure of bacteria to antibiotics may alter their susceptibility to these drugs.20 In our study, the number of tobramycin inhalation powder-treated patients with an MIC of >8 μg/mL increased slightly following 2–3 cycles of treatment. However, thresholds of susceptibility to tobramycin with parenteral therapy may not be relevant to inhaled therapy as the drug concentrations achieved at the site of infection with inhaled antibiotics can be significantly higher than systemic concentrations.16 Maximum tobramycin concentrations in sputum 30 minutes after single-dose administration of tobramycin inhalation powder (112 mg tobramycin) have been shown to be 1048 ± 1080 μg/g.16 This exceeds, by several times, the MIC of tobramycin measured in vitro in the vast majority of isolates from infected CF patients.
Inhaled antibiotics are an attractive option in the management of chronic lung infection. However, administration of aerosolized antibiotics such as TIS requires a compressor and nebulizer; TIS is approved for use with the PARI-LC® PLUS nebulizer only. Consequently, drug administration times with the approved TIS formulation are prolonged (15.8 ± 4.0 minutes, excluding cleaning and disinfection times).16 For patients on multiple therapies, time taken to administer, clean and disinfect the equipment can be burdensome and may contribute to poor adherence to treatment.21,22 This can result in poor clinical outcomes. In our study, total administration times for tobramycin inhalation powder were 4–6 minutes (as assessed by the study investigators), which is consistent with administration times previously reported for this formulation (4.9 ± 1.8 minutes).16
Treatment with tobramycin inhalation powder was well tolerated in this study. AEs were as expected for this population and this class of drug. The overall incidence rates of AEs and SAEs were generally higher in the placebo group relative to the tobramycin inhalation powder group in the first cycle of treatment. The frequency pattern of reported AEs in any cycle was generally similar to Cycle 1. The incidence of lung disorders was slightly lower in the tobramycin inhalation powder arm than in the placebo arm during Cycle 1. However, the incidence appears to be higher when comparing tobramycin inhalation powder over 3 cycles versus placebo for the last 2 cycles, when all patients have received tobramycin inhalation powder. This may be due to the differing duration of exposure to tobramycin inhalation powder in the two study arms. Serum tobramycin concentrations after inhalation of tobramycin inhalation powder (112 mg tobramycin) were assessed during Cycle 1 and 2, and in patients who were terminated from the study early. Serum concentrations were at least 6-fold lower than both the trough levels (>2 μg/mL) and maximum levels (>12 μg/mL) recommended for avoidance of toxicity associated with intravenous tobramycin therapy.23 There were no apparent major changes from baseline to specified time points or between treatment differences observed for any biochemical, hematology parameter and vital signs during the study. Clinically significant renal function changes as noted by rising blood urea nitrogen, serum creatinine and proteinuria were not reported. None of the patients reported hearing complaints (tinnitus, ear pressure or hearing loss) during the course of the study. In addition, transient hearing loss was not attributed to tobramycin by the study investigators. There were only two cases of airway reactivity to tobramycin inhalation powder treatment over the three treatment cycles (compared with four cases with placebo in Cycle 1).
In summary, alternate-month administration of tobramycin inhalation powder (112 mg tobramycin) b.i.d. was effective and well tolerated in the treatment of P. aeruginosa infection in cystic fibrosis patients. Tobramycin inhalation powder improved lung function, decreased sputum P. aeruginosa density and reduced respiratory-related hospitalizations and use of other antipseudomonal antibiotics. Results were consistent with those reported for the approved TIS formulation. The new drug-device combination for tobramycin inhalation powder could offer an important alternative for treating CF patients with P. aeruginosa infections. By reducing administration time, it may have beneficial effects on adherence and clinical outcomes.
Acknowledgments
Research funding support: This study was funded by Novartis Pharma AG, Basel, Switzerland
The authors would like to acknowledge the efforts of the participating cystic fibrosis centers, the Cystic Fibrosis Foundation Data Safety Monitoring Board and the Cystic Fibrosis Therapeutics Development Network during this study. Editorial assistance was provided by Melanie Stephens, ACUMED, UK. This assistance was funded by Novartis Pharma AG.
Footnotes
Clinical trial registration: NCT00125346
Dr Konstan has relationships with Aradigm Corp, Boehringer Ingelheim, CSL Behring, Genentech, GlaxoSmithKline, Gilead Sciences, Inc., NanoBio, Nektar, Novartis Pharmaceuticals, PTC Therapeutics, Roche, Transave, Inc., and Vertex Pharmaceuticals, Inc. Dr Geller has relationships with Bayer, CSL Behring, Discovery Labs, Genentech, Gilead Sciences, Inc., Inspire, MAP Pharmaceuticals, Mpex, NanoBio, Nektar, Novartis Pharmaceuticals and Philips Respironics. Dr Minić declares no conflicts of interest. Florian Brockhaus, Jie Zhang and Gerhild Angyalosi are employees of Novartis Pharmaceuticals.
References
- 1.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Repir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 2.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol. 1992;12:158–161. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 3.Ballmann M, Rabsch P, von der Hardt H. Long-term follow up of changes in FEV1 and treatment intensity during Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Thorax. 1998;53:732–737. doi: 10.1136/thx.53.9.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, Green CG, Collins J, Farrell PM. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32:277–287. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 5.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 6.Ryan G, Mukhopadhyay S, Singh M. Nebulised anti-pseudomonal antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2003:CD001021. doi: 10.1002/14651858.CD001021. [DOI] [PubMed] [Google Scholar]
- 7.Moskowitz SM, Silva SJ, Mayer-Hamblett N, Pasta DJ, Mink DR, Mabie JA, Konstan MW, Wagener JS Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis (ESCF) Shifting patterns of inhaled antibiotic use in cystic fibrosis. Pediatr Pulmonol. 2008;43:874–881. doi: 10.1002/ppul.20873. [DOI] [PubMed] [Google Scholar]
- 8.Ratjen F, Brockhaus F, Angyalosi G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: A review. J Cyst Fibros. 2009;8:361–369. doi: 10.1016/j.jcf.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev-K M, Borowitz D, Bowman CM, Marshall BC, Marshall S, Smith AL. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 10.Moss RB. Long-term benefits of inhaled tobramycin in adolescent patients with cystic fibrosis. Chest. 2002;121:55–63. doi: 10.1378/chest.121.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Quittner AL, Buu A. Effects of tobramycin solution for inhalation on global ratings of quality of life in patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr Pulmonol. 2002;33:269–276. doi: 10.1002/ppul.10074. [DOI] [PubMed] [Google Scholar]
- 12.Murphy TD, Anbar RD, Lester LA, Nasr SZ, Mickerson B, VanDevanter DR, Colin AA. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatr Pulmonol. 2004;38:314–320. doi: 10.1002/ppul.20097. [DOI] [PubMed] [Google Scholar]
- 13.TOBI® (Tobramycin) [Accessed September 9, 2009]; Prescribing information. Available from: http://www.tobitime.com/info/tools/prescribing.jsp.
- 14.Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, Rosenblatt R, Vender RL, Hazle L, Sabadosa K, Marshall B. Cystic Fibrosis Pulmonary Guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 15.Doring G, Hoiby N. Early intervention and prevention of lung disease in cystic fibrosis: a European consensus. J Cyst Fibros. 2004;3:67–91. doi: 10.1016/j.jcf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Geller DE, Konstan MW, Smith J, Noonberg SB, Conrad C. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulmonol. 2007;42:307–313. doi: 10.1002/ppul.20594. [DOI] [PubMed] [Google Scholar]
- 17.Knudson RJ, Lebowtiz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 18.Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.Burns JL, Van Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, Montgomery BA, Albers GM, Ramsey BW, Smith AL. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis. 1999;179:1190–1196. doi: 10.1086/314727. [DOI] [PubMed] [Google Scholar]
- 20.Davies J, Bilton D. Bugs, biofilms, and resistance in cystic fibrosis. Respir Care. 2009;54(5):628–638. doi: 10.4187/aarc0492. [DOI] [PubMed] [Google Scholar]
- 21.Abbott J, Havermans T, Hart A. Adherence to the medical regimen: clinical implications of new findings. Curr Opin Pulm Med. 2009 doi: 10.1097/MCP.0b013e3283310859. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Zemanick ET, Harris JK, Conway S, Konstan MW, Marshall B, Quittner AL, Retsch-Bogart G, Saiman L, Accurso FJ. Measuring and improving respiratory outcomes in cystic fibrosis lung disease: Opportunities and challenges to therapy. J Cyst Fibros. 2010;9:1–16. doi: 10.1016/j.jcf.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweetman SC, editor. Martindale: The Complete Drug Reference. London: Pharmaceutical Press; 2009. [Accessed 29 Jun 2009]. online. Available from: http://www.medicinescomplete.com. [Google Scholar]