Abstract
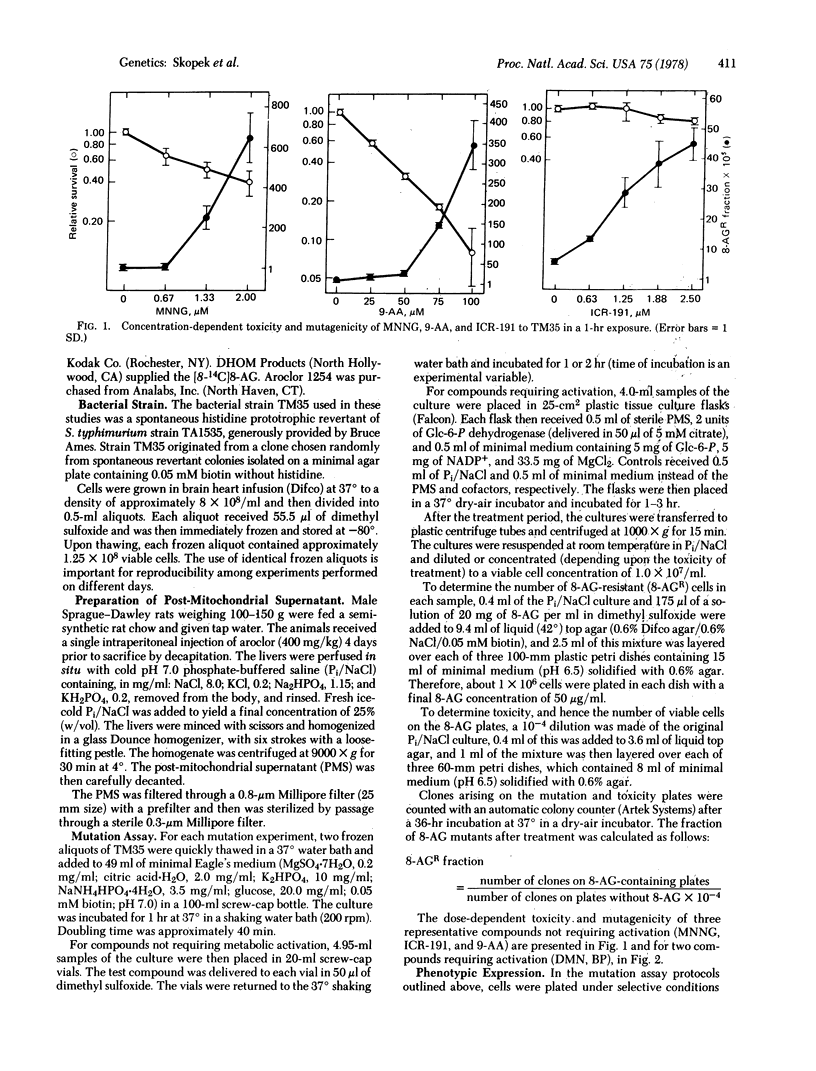
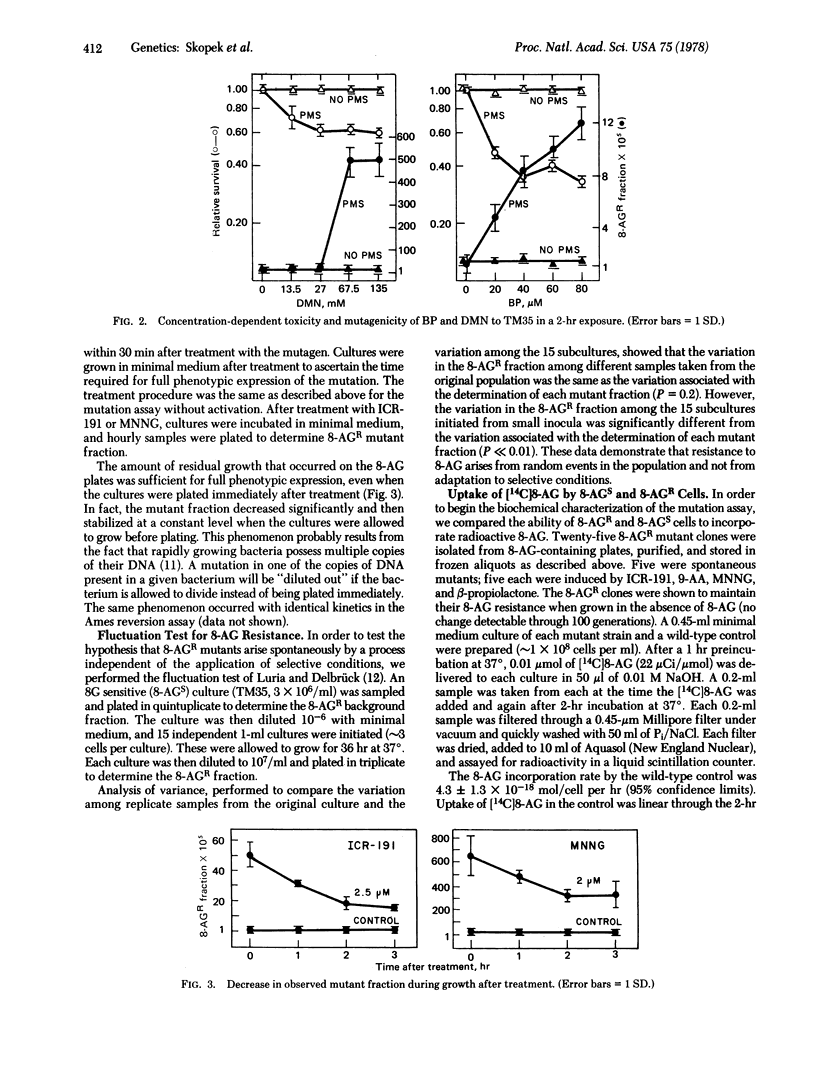
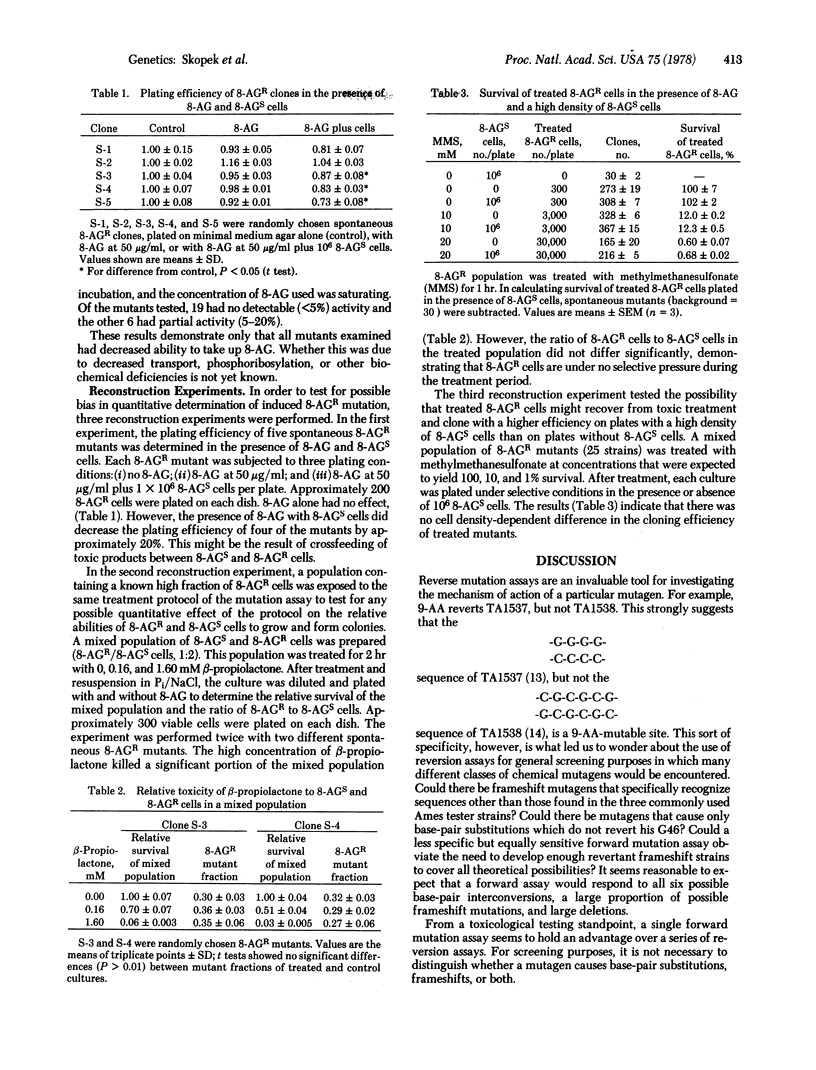
We have developed a quantitative forward mutation assay using Salmonella typhimurium, in which resistance to the purine analog 8-azaguanine is used as a genetic marker. We present the assay protocol, the concentration-dependent toxicity and mutagenicity of five known mutagens (N-methyl-N'-nitro-N-nitrosoguanidine, ICR-191, 9-aminoacridine, dimethylnitrosamine, and benzo[a]pyrene), and reconstruction experiments testing the assay for possible bias. The relative merits of forward versus reverse mutation assays are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Purine phosphoribosyltransferases of Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):1010–1013. doi: 10.1128/jb.112.2.1010-1013.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Deluca J. G., Krolewski J., Skopek T. R., Kaden D. A., Thilly W. G. 9-Aminoacridine-A frameshift mutagen for Salmonella typhimurium TA 1537 inactive at the hgprt locus in human lymphoblasts. Mutat Res. 1977 Feb;42(2):327–330. doi: 10.1016/s0027-5107(77)80034-1. [DOI] [PubMed] [Google Scholar]
- Ellenberger J., Mohn G. Mutagenic activity of cyclophosphamide, ifosfamide, and trofosfamide in different genes of escherichia coli and salmonella typhimurium after biotransformation through extracts of rodent liver. Arch Toxicol. 1975;33(3):225–240. doi: 10.1007/BF00311275. [DOI] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Shumas S. R. Genetic separation of hypoxanthine and guanine-xanthine phosphoribosyltransferase activities by deletion mutations in Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):910–916. doi: 10.1128/jb.112.2.910-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann G. R., Malling H. V. Azaguanine-resistant mutants induced by several mutagens in a neurospora heterokaryon. Mutat Res. 1975 Mar;27(3):307–318. doi: 10.1016/0027-5107(75)90287-0. [DOI] [PubMed] [Google Scholar]
- Isono K., Yourno J. Chemical carcinogens as frameshift mutagens: Salmonella DNA sequence sensitive to mutagenesis by polycyclic carcinogens. Proc Natl Acad Sci U S A. 1974 May;71(5):1612–1617. doi: 10.1073/pnas.71.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman L. E., Hochstadt J. Regulation of purine utilization in bacteria. VI. Characterization of hypoxanthine and guanine uptake into isolated membrane vesicles from Salmonella typhimurium. J Bacteriol. 1976 Apr;126(1):312–326. doi: 10.1128/jb.126.1.312-326.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakar J. H., Kalle G. P. Defective guanine uptake in an 8-azaguanine-resistant mutant of Salmonella typhimurium. J Bacteriol. 1968 Feb;95(2):458–464. doi: 10.1128/jb.95.2.458-464.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilly W. G., DeLuca J. G., Hoppe H., 4th, Penman B. W. Mutation of human lymphoblasts by methylnitrosourea. Chem Biol Interact. 1976 Sep;15(1):33–50. doi: 10.1016/0009-2797(76)90126-5. [DOI] [PubMed] [Google Scholar]


