Abstract
Introduction
This study evaluated whether Schwann cells (SCs) from different nerve sources transplanted into cold-preserved acellular nerve grafts (CP-ANGs) would improve functional regeneration compared to nerve isografts.
Methods
SCs isolated and expanded from motor and sensory branches of rat femoral and sciatic nerves were seeded into 14mm CP-ANGs. Growth factor expression, axonal regeneration, and functional recovery were evaluated in a14 mm rat sciatic injury model and compared to isografts.
Results
At 14 days, motor or sensory-derived SCs increased expression of growth factors in CP-ANGs versus isografts. After 42 days, histomorphometric analysis found CP-ANGs with SCs and isografts had similar numbers of regenerating nerve fibers. At 84 days, muscle force generation was similar for CP-ANGs with SCs and isografts. SC source did not affect nerve fiber counts or muscle force generation.
Discussion
SCs transplanted into CP-ANGs increase functional regeneration to isograft levels; however SC nerve source did not have an effect.
Keywords: cell transplantation, reinnervation, peripheral nerve injury, nerve regeneration, growth factor
INTRODUCTION
Direct end-to-end anastomosis of a severed peripheral nerve provides the optimal clinical outcome following injury. In most clinical cases, primary nerve repair is not possible, and a bridging component that allows for tension-free reconstruction must be used to achieve functional recovery1. Nerve autografts, the standard for peripheral nerve reconstruction, are limited by lack of sufficient donor tissue and size mismatches with the injury site2,3. Fresh cadaveric allografts can function as well as nerve autografts4–8, but they require host immuno-suppression and attendant morbidity. To circumvent these problems, investigators have sought alternatives in synthetic conduits and acellular nerve allografts, both of which are currently commercially available for clinical use in the United States.
Acellular nerve grafts (ANGs) can be prepared using freeze thaw cycles9,10, cold-preservation9, or detergent11,12 treatment. These processing methods remove antigenic cellular components, thus reducing the immunological response to ANGs. Compared with nerve conduits, ANGs support superior nerve regeneration, likely due to the intact endoneurial microstructure of extracellular matrix (ECM) proteins that can guide regenerating axons13–15. Surgical reconstruction of nerves using conduits has generally been limited to short gaps and sensory nerve defects due to their inferior regeneration potential compared to autografts16–18. Preclinical and early clinical studies on commercially-available ANGs have shown regeneration across gap lengths up to 28 mm15,19. However, the lack of SCs, which are critical to peripheral nerve regeneration, limits regeneration in ANGs and makes them inferior to autografts14,15,20–26.
In uninjured nerves, SCs myelinate axons and secrete both ECM molecules and growth factors to promote neuronal survival20–23. After nerve injury, SCs present at the injury site are essential to regeneration. SCs proliferate and align themselves in the remaining basal lamina to guide regenerating axons to their distal targets2,24. ECM molecules and soluble growth factors, such as nerve growth factor (NGF), glial derived neurotrophic factor (GDNF), and brain derived neurotrophic factor (BDNF), are secreted by SCs to stimulate and guide axons from the proximal stump toward their target end-organ25–27. It has been shown that SCs seeded within semi-permeable nerve guidance conduits promote nerve regeneration equivalent to isografts by histological measures28–30. In another study, SCs derived from the proximal stump were transplanted within acellular nerve grafts. Although nerve regeneration was similar to isografts by histology, functional recovery was poor31. In all these prior studies, SC treatments were sufficient to promote bridging of the graft or conduit, but they failed to promote functional recovery as a result of the transplanted SCs. In this study, we transplanted SCs expanded in culture from healthy nerve into isogenic nerve allografts derived from rat sciatic nerve and assessed the regeneration capacity of this treatment by both histological and functional recovery measures.
Previous research suggests that SCs exhibit a specific phenotype (i.e. motor or sensory) based on their source that may influence the regeneration of axons toward their correct target end-organ (muscle or sensory)32–38. Based on these studies, we hypothesized that the source of SCs (i.e. motor or sensory nerve) could influence sensory or motor-specific functional regeneration. We quantified previously phenotype-specific gene expression to confirm that the motor and sensory branches of the femoral nerve are a source of phenotype-specific SCs39. The current study was designed to determine if addition of SCs would increase functional regeneration through ANGs in a 14 mm rat sciatic nerve injury model, and if the source of SCs had an effect on regeneration.
METHODS
Animals
Adult (225–250 g) male Lewis rats (Harlan Sprague-Dawley, Indianapolis, IN) were maintained in a central housing facility. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Washington University and were done in strict accordance with the guidelines set forth by NIH.
Experimental Design
Animals were randomized into 5 groups (n = 18 per group) corresponding to the type of graft used to repair a 14 mm sciatic nerve gap. Additional animals were used as donors for nerve grafts or SCs. The first group (isograft) served as the positive control and received a 14 mm reversed isograft repair obtained from another Lewis donor. Three more groups received 14 mm CP-ANGs injected with 106 SCs derived and expanded from the sciatic nerve, femoral motor, and femoral sensory nerve branches, respectively. The last group served as the negative control, receiving a 14 mm CP-ANG with no SCs. Of the 18 animals in each group, 4 were used for quantitative real time polymerase chain reaction (qRT-PCR) analysis at 2 weeks post-transplantation, 8 were evaluated for histomorphometry 6 weeks post transplantation, and 6 underwent functional muscle force testing 12 weeks post transplantation.
Processing of Donor Nerve Grafts
Sciatic nerve segments from donor Lewis rats were immediately transferred into sterile six-well plates with 10 mL of a solution containing University of Wisconsin solution40 (15 ml; NPBI International BV, Emmer Compascuum, The Netherlands), penicillin G (200,000 U/L), regular insulin (40 U/L), and dexamethasone (16 mg/L). The solution was changed weekly in a sterile hood for 7 weeks and stored at 4°C as described previously41.
Isolation and expansion of SCs
SCs were isolated from the motor and sensory branches of the rat femoral nerve. Motor-derived SCs were harvested from the motor branch of the femoral nerve, which we have previously shown to exhibit similar expression patterns of phenotypic markers as SCs derived from the ventral root38,39. To determine whether a population of mixed (motor and sensory) SCs would promote similar regeneration to motor and sensory SCs, SCs derived from the rat sciatic nerve were also used in this study. SC cultures were prepared as previously described42–44.
Preparation of SCs for Injection
SCs were prepared for injection under the epineurium as previously described45. Briefly, SCs were washed twice with Hanks balanced saline solution (Invitrogen) and incubated with 0.25% trypsin for 3 min at 37°C. After centrifugation for 5 min at 130 × g, the supernatant was removed, and the cells were resuspended at 106 cells/5μL in culture media containing DMEM supplemented with 10% FBS and 1% ABAM. A 10 nM Qtracker® (Invitrogen) solution was prepared for labeling, as recommended by the manufacturer. After mixing 200 μL of fresh media in 1.5 mL of prepared Qtracker® solution, 1×106 SCs were added and incubated at 37°C for 60 min to label the cells prior to transplantation. The resulting labeled cells were washed with media twice and concentrated as needed in culture media.
Donor Graft Harvest
Animals were anesthetized with a subcutaneous injection of ketamine (75 mg/kg, Ketaset®, Fort Dodge Animal Health, Fort Dodge, IA) and medetomidine (0.5 mg/kg, Dormitor®, Orion Corporation, Espoo, Finland). Under aseptic conditions, both hind limbs were prepared for incision. A 3 cm skin incision was made from the top of the femur toward the kneecap, and the gluteal muscles were separated to expose the sciatic nerve. A 30–35 mm sciatic nerve segment was excised bilaterally and used for immediate isograft repair or cold-preservation treatment. The animals were subsequently euthanized with intracardiac injection of Euthasol® (150 mg/kg, Delmarva Laboratories, Des Moines, IA).
Graft Implantation
After achieving adequate anesthesia, the right sciatic nerve was exposed, neurolysed, and sharply transected with micro-scissors 5 mm proximal to the trifurcation. For groups that received the isograft treatment, a 14 mm sciatic nerve segment was removed, reversed, and sutured to the proximal and distal stumps with 1 10-0 nylon suture at each end and secured with fibrin sealant (Tisseel™, Baxter International Inc., Deerfield, IL). For groups that received SC treatments, injections were done after removal of a 14 mm sciatic nerve segment. With the CP-ANG fastened to the proximal stump, a 27-gauge Hamilton™ syringe (Hamilton Company, Reno, NV) was inserted longitudinally just under the epineurium, and a solution with 1 × 106 SCs/5 μL was injected as described previously45. To confirm adequate injection of the SCs, the labeled SCs were visualized in the CP-ANG with a fluorescence Olympus MVX10 dissecting microscope (Olympus Corporation, Japan) fitted with a cooled CCD digital camera (Hamamatsu ORCA-ER; Hamamatsu City, Japan) and analyzed with MetaMorph version 7.0 (Universal Imaging Corporation, PA). After wound irrigation, the muscles and skin were reapproximated with interrupted 6-0 Vicryl (Ethicon, Somerville, NJ) and 4-0 nylon sutures, respectively. Animals were recovered with a subcutaneous injection of atipamezole HCl (1mg/kg, Antisedan®, Orion Corporation) and placed on a warming pad post-operatively. Following surgery and post-operative care, animals were returned to a central housing facility.
Graft Harvest
Animals used for qRT-PCR analysis were re-anesthetized 2 weeks after surgery. After the graft was identified and neurolysed, it was dissected at both suture sites to prevent any host nerve contamination46,47, and the grafts were stored in RNAlater™ (Ambion®, Austin, TX) for PCR analysis. Animals were euthanized with intracardiac injection of Euthasol® (150 mg/kg, Delmarva Laboratories, Des Moines, IA) immediately following harvest.
Animals for histomorphometry were re-anesthetized 6 weeks post-operatively. The right sciatic nerve and graft were explanted together with 5 mm portions of the proximal and distal nerve stumps and placed in 3.5% glutaraldehyde at 4°C for histomorphometric analysis48. Animals were euthanized as described above.
Functional Recovery Testing
Animals for muscle force testing were re-anesthetized at 12 weeks post-operatively. Sciatic nerve function was assessed by measuring the evoked compound muscle action potential in reinnervated extensor digitorum longus (EDL) muscle upon electrical stimulation of the repaired sciatic nerve as described previously49. Animals were immobilized in an automated functional assessment station (FASt System, Red Rock Laboratories, St. Louis, MO) with the distal portion of the EDL muscle fixed to a 5 N load cell. Cathodic, monophasic electrical impulses (duration=200 ms, frequency = single–200 Hz, burst width = 300 ms, amplitude = 0–1000 μA) were applied to the sciatic nerve proximal to the interposed nerve graft via silver wire electrodes, and the resulting force production in the EDL was recorded using custom data acquisition software (RRL V.1.0, Red Rock Laboratories).
Twitch contractions were utilized to determine the optimal stimulus amplitude (Vo) and optimal muscle length (Lo) for isometric force production in the EDL muscle. All subsequent isometric force measurements were made at Vo and Lo. Single twitch contractions were recorded, and maximum twitch force (Ft) was calculated. Tetanic contractions were recorded at increasing frequencies of stimulation (5–200 Hz), allowing 2 min intervals between stimuli to prevent muscle fatigue. Maximum isometric tetanic force (Fo) was automatically calculated from the resulting sets of recorded force traces.
Physiological cross-sectional area (PCSA) of the EDL muscle was calculated using the following equation:
where the PCSA is the physiological muscle cross-sectional area (cm2), M is the EDL muscle mass (g), cos θ is the angle of pennation of the EDL muscle (~0°), ρ is the density of mammalian skeletal muscle (1.06 g/cm3), Lo is the optimal muscle length (cm), and 0.44 is the ratio of fiber length to muscle length (Lf/Lm) in rat EDL muscle. Maximum specific isometric force was calculated as the maximum isometric force normalized to muscle PCSA. Values were reported relative to measurements taken from healthy, unoperated animals. Following assessment, both denervated/reinnervated and healthy, unoperated EDL muscles were harvested and weighed. Animals were euthanized as described above.
RNA Isolation
Total RNA was extracted from the explanted CP-ANGs 2 weeks after nerve repair using an acid phenol extraction (TRIzol Reagent, Invitrogen). The aqueous layer was collected, and the samples were purified using an RNeasy Mini Kit (Qiagen). The presence of RNA was assessed by electrophoresis using 2% agarose gels after running reverse transcriptase PCR with a β-actin primer. To verify that the mRNA extracted from the nerves met the quality standards for further experiments, mRNA concentration was determined using an absorbance ratio of A260/A280. The ratio threshold was at 1.8, which denotes a high purity of RNA in the sample50. Since the majority of the cells in the nerve are SCs (~70%)51, the harvested RNA was assumed to be representative of SC RNA present in the explanted CP-ANGs after 2 weeks.
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
cDNA was synthesized from the isolated RNA using the Quantitect Reverse Transcription Kit (Qiagen). Using the Quantitect SYBR Green PCR Mastermix (Qiagen) in combination with gene specific QuantiTect primer assays, qRT-PCR was performed using an Applied Biosystems 7000 Real-Time PCR thermocycler for genes chosen from the literature and identified from previous experiments: vascular endothelial cell growth factor (VEGF), NGF, BDNF, pleiotrophin (PTN), GDNF, myelin basic protein (MBP), protein kinase C iota (PRKCi), neural cell adhesion molecule 1 (NCAM1), and neurofilament (NEFL)38,39. The qRT-PCR was conducted using the following conditions: (1) 50°C for 2 min to eliminate any PCR products containing dUTP from carryover contamination; (2) 95°C for 15 min, to activate the polymerase; (3) 40 cycles of 95°C for 15 seconds to anneal, 55°C for 30 seconds to extend, and 72°C for 30 seconds to amplify with the fluorescent signal detected at 72°C52. Target genes were normalized to an internal control (β-actin) to account for variation in cDNA concentration between samples. No template was used as a negative control. The Quantitect primer assays are validated to have a PCR efficiency of 100%. The differences in gene expression levels between 2 different samples were calculated using the comparative delta crossover threshold (Ct) method53.
Histomophometry
All harvested nerves were post-fixed with 1% osmium tetroxide, serially dehydrated in ethanol and embedded in Araldite 502 (Polyscience Inc., Warrington, PA). Tissues were cut into 1 μm cross-sections using an ultramicrotome and stained with 1% toluidine blue in preparation for light microscopy imaging and qualitative analysis. Using a semiautomated program described previously48, an observer blinded to experimental groups measured total nerve fiber number, fiber width (μm), and percent neural tissue (100 x neural area/intrafascicular area) at the midgraft and at areas 3–5 mm distal to the graft. The percent neural tissue metric helps determine how compact the area of regeneration is and what percent of the regenerated tissue is occupied by myelinated axons.
Statistical Analysis
Statistical analyses were run using SigmaStat 3.0 (Systat Software, San Jose, CA). Multiple groups were compared with a one-way analysis of variance (ANOVA) if conditions of normality (assessed with the Kolmogorov-Smirnoff normality test) and equal variance (assessed with the Levene Median test) were met. If ANOVA returned a statistically significant P value, a post-hoc Student-Newman-Keuls test was used to isolate significant differences between groups with correction for multiple comparisons. Significance was set at P<0.05, and all results are reported as mean ± standard deviation.
RESULTS
Gene Expression Analysis
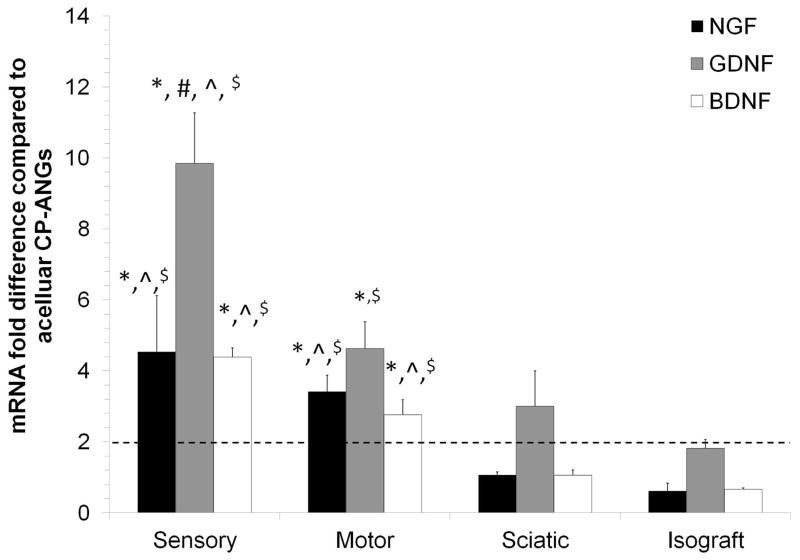
qRT-PCR was used to assess the effect of SC transplantation on growth factor expression in the acute phase after injury54,55 compared to nerve isografts and acellular CP-ANGs controls. NGF, GDNF and BDNF were chosen as representative growth factors because they have been shown to promote neuronal survival and axon regeneration after peripheral nerve injury54,56,55,57–59. Two weeks after transplantation, all growth factor expression levels (NGF, GDNF, and BDNF) were upregulated in the CP-ANGs injected with sensory and motor-derived SCs compared to the acellular CP-ANGs (Figure 1). When compared to isografts, CP-ANGs injected with sensory and motor-derived SCs also showed increased expression of NGF (sensory ~4-fold increase, motor ~3-fold increase), GDNF (sensory ~10-fold, motor ~4-fold), and BDNF (sensory ~4-fold, motor ~ 3-fold), (Figure 1). The growth factor expression levels of CP-ANGs injected with sciatic-derived SCs were similar to that of isografts, as expected (Figure 1). The increased level of growth factor expression in the CP-ANGs injected with sensory and motor-derived SCs compared to acellular CP-ANGs and isografts demonstrates that transplantation of sensory and motor nerve-derived SCs into CP-ANGs increases growth factor expression 2 weeks after injury.
Figure 1. Sensory and motor-derived Schwann cells increase growth factor expression at 2 weeks.
qRT-PCR was used to determine the gene expression level of each marker with the values normalized to β-actin. The groups injected with sensory and motor-derived SCs showed greater expression of all growth factors examined compared to the isograft and acellular CP-ANG groups. The mRNA fold difference was calculated versus the acellular CP-ANG. ** the dotted line at 2 is the threshold value for upregulation versus the CP group. Error bars represent the standard deviation (n = 3). * denotes P< 0.05 when compared to isograft, ^ denotes P< 0.05 when compared to sciatic, # denotes P< 0.05 when compared to motor, $ denotes P< 0.05 when compared to acellular CP-ANG.
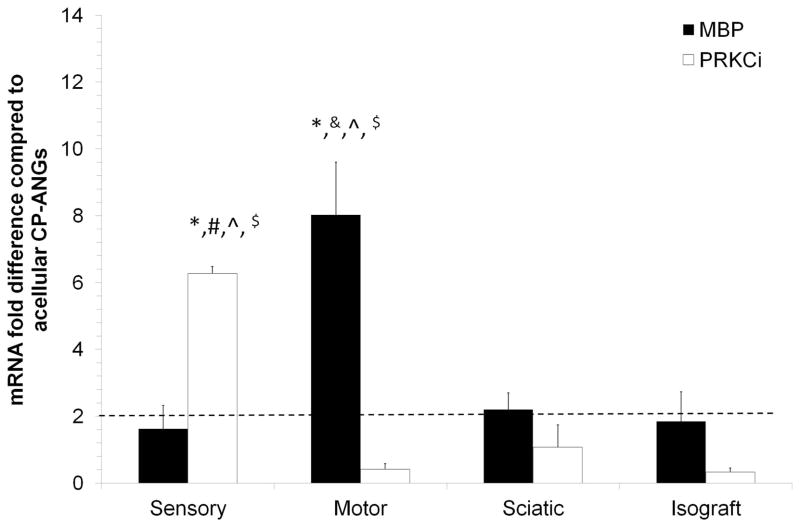
Prior to transplantation, SCs were harvested and expanded in culture, which has been shown to alter their gene expression patterns39. To determine whether transplantation of SCs into CP-ANGs would promote re-differentiation of SCs back into their native phenotypes, the expression levels of phenotypic markers for sensory or motor-derived SCs (Table 1)39 were quantified. Of the phenotypic markers evaluated, only MBP and PRKCi showed significant changes in expression patterns compared to other experimental groups. MBP was previously identified as a marker of sensory-derived SCs39. However, in this study, MBP was upregulated 4-fold in CP-ANGs injected with motor-derived SCs versus CP-ANGs injected with sensory-derived SCs (Figure 2). Previously, PRKCi was reported to be a marker of motor-derived SCs39. Expression of PRKCi was 6-fold higher in CP-ANGs injected with sensory-derived SCs compared to CP-ANGs injected with motor-derived SCs (Figure 2). In a previous study, our lab demonstrated that the expression of MBP and PRKCi was dysregulated in sensory and motor-derived SCs after 30 days of expansion culture in vitro, compared to fresh nerve tissue39. These results suggest that the phenotypic marker expression patterns of MBP and PRKCi remain dysregulated in the CP-ANGs 2 weeks post-transplantation in this sciatic nerve injury model.
Table 1.
List of Genes used for qRT-PCR analysis 2 weeks post-transplantation
| Gene | Gene Common Name | Upregulated in Motor, Sensory, or Similar |
|---|---|---|
| Vascular Endothelial Growth Factor | VEGF | Motor |
| Pleiotrophin | PTN | Motor |
| Protein Kinase C iota | PRKCi | Motor |
| Neurofilament | NEFL | Motor |
| Brain Derived Neurotrophic Factor | BDNF | Sensory |
| Glial Derived Neurotrophic Factor | GDNF | Sensory |
| Myelin Basic Protein | MBP | Sensory |
| Neural Cell Adhesion Molecule | NCAM1 | Sensory |
| Nerve growth Factor | NGF | Similar expression in both |
Figure 2. SC gene expression patterns remain dysregulated 2 weeks after transplantation in CP-ANGs.
qRT-PCR was used to determine the gene expression of each marker with the values normalized to β-actin. MBP, a sensory marker, showed increased expression in the motor group when compared to all other groups. PRKCi, a motor marker, showed increased expression in the sensory group. The mRNA fold difference was calculated versus the acellular CP-ANG. ** the dotted line at 2 is the threshold value for upregulation versus the CP group. Error bars represent the standard deviation (n = 3). * denotes P< 0.05 when compared to isograft, & denotes P< 0 05 when compared to sensory, ^ denotes P< 0.05 when compared to sciatic, # denotes P< 0.05 when compared to motor, $ denotes P< 0.05 when compared to acellular CP-ANG.
Histomorphometric Analysis
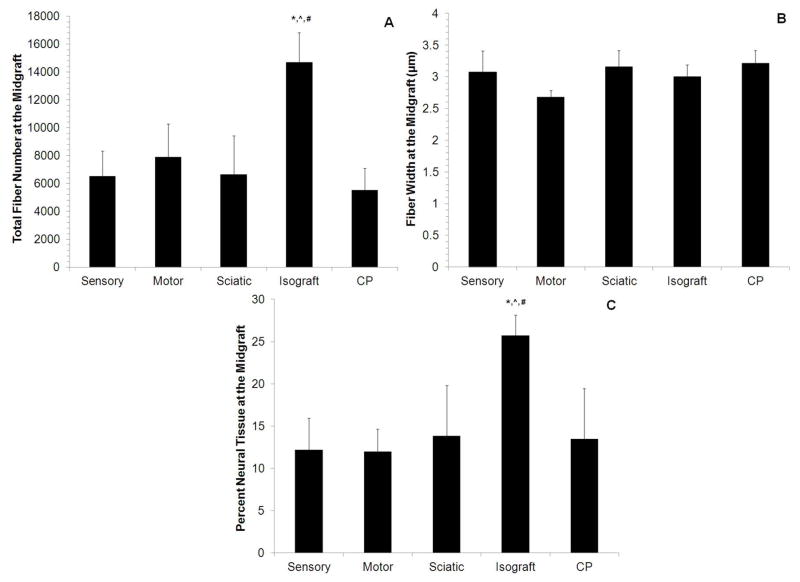
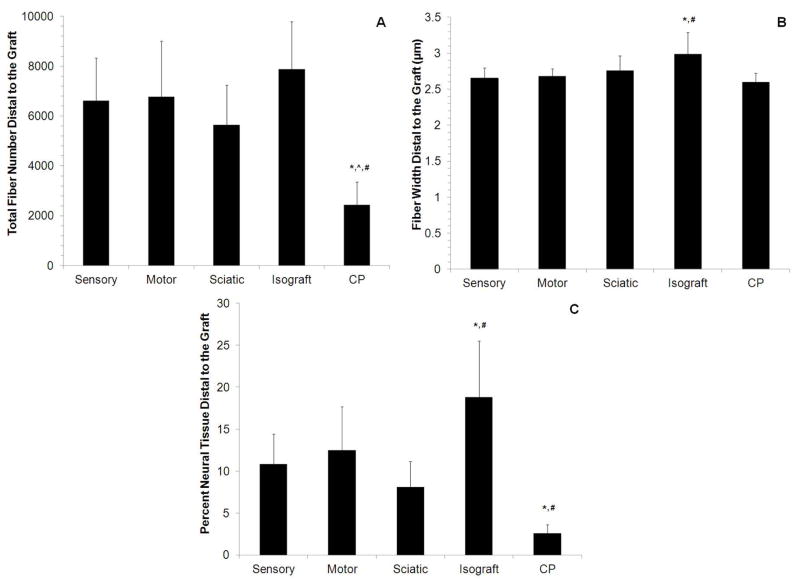
To determine whether CP-ANGs injected with SCs had similar regenerative capacity as isografts and whether SC source had an effect on nerve regeneration, histomorphometric analysis was conducted 6 weeks after injury to evaluate the total number of myelinated nerve fibers, fiber width, and percent of neural tissue present at midgraft and distal to the nerve grafts. The total number of regenerating fibers in the midgraft for all CP-ANG groups was lower than that in the isografts (Figure 3A). However for all SC-injected CP-ANGs, the total myelinated fiber counts in the nerve distal to the graft were similar to isografts (Figure 4A). In contrast, acellular CP-ANGs had a reduced number of nerve fibers (less than 2500) distal to the graft compared to isografts (7890 ± 1896). The similar fiber counts for the isograft and SC-injected CP-ANGs distal to the graft demonstrated that the transplantation of SCs, regardless of source, into ANGs promotes regeneration of myelinated nerve fibers at 6 weeks after injury, and this regeneration was improved compared to acellular ANGs.
Figure 3. Histomorphometry on the midgraft of the regenerating nerve grafts 6 weeks post-transplantation.
(A) The isograft group had a higher number of total myelinated nerve fibers compared to all other groups. (B) All groups had regenerated fibers of similar width. (C) A higher percentage of neural tissue was observed in the isograft group compared to all other groups. Error bars represent the standard deviation (n = 8). * denotes P<0.05 versus sensory and motor. ^ denotes P<0.05 versus sciatic, # denotes P < 0.05 versus CP.
Figure 4. Histomorphometry in the distal segment of nerve grafts 6 weeks post-transplantation.
(A) The groups injected with sensory, motor, and sciatic nerve-derived SCs, showed nerve regeneration similar to the positive control (isograft group). These 4 groups had more total myelinated nerve fibers than the acellular CP-ANG (CP). (B) The isograft group regenerated thicker fibers (an indicator of fiber maturity) than the motor, sensory, and CP groups. (C) A higher percentage of neural tissue was observed in the isograft group compared to all other groups, but the groups with injected SCs (sensory, motor, and sciatic nerve-derived) all had more neural tissue than the CP group. Error bars represent the standard deviation (n = 8). * denotes P<0.05 versus sensory and motor. ^ denotes P<0.05 versus sciatic, # denotes P < 0.05 versus CP.
To determine fiber maturity, the myelinated nerve fiber width was measured at midgraft and distal to the graft. At midgraft, the fiber widths of all groups were similar (Figure 3B). However, the fiber width distal to the graft for CP-ANGs injected with sensory-derived SCs, CP-ANGs injected with motor-derived SCs, and acellular CP-ANG groups were significantly thinner (~2.60 ± 0.15 – 2.68 ± 0.10 μm) than isografts (~3.00 ± 0.30 μm) (Figure 4B). The nerve fibers in the CP-ANGs injected with sciatic-derived SCs had similar widths (2.75 ± 0.21 μm) to those found in isografts at both the midgraft and the distal to the graft. These results demonstrate that the isograft promoted wider nerve fibers, which is suggests increased fiber maturity. However, the fibers distal to the graft in the isograft group were thinner than found in normal rat sciatic nerve (6.5 μm60).
The percent neural tissue was calculated for each group at midgraft and distal to the graft. At midgraft, the isografts had a higher percentage of neural tissue than all other groups (Figure 3C). Distal to the graft, however, all CP-ANGs injected with SCs showed a higher percentage of neural tissue (8–12%) than acellular CP-ANGs (3%). The isografts outperformed all other groups with 20% neural tissue present distally at 6 weeks post-repair (Figure 4C), implying that repairing nerve defects with isografts increased the quality of regeneration. However transplantation of SCs, regardless of source, into CP-ANGs promoted nerve regeneration of higher quality than acellular CP-ANGs.
To visually assess myelinated nerve fibers, light microscopy was performed on distal sections for each group. CP-ANGs injected with SCs had a similar number of myelinated axons compared to the isografts, while the acellular CP-ANGs had fewer myelinated axons distal to the graft (Figure 5). The histomorphomery results demonstrate that the injection of SCs into CP-ANGs promoted regeneration similar to the isograft by some measures and better than acellular CP-ANGs, regardless of SC source.
Figure 5. Light micrographs of nerve distal to the grafts 6 weeks post-transplantation.
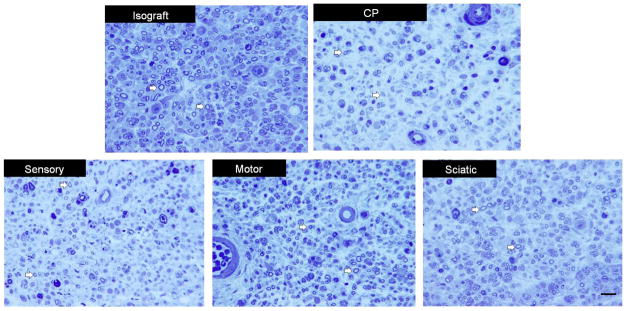
The isograft group shows more myelinated nerve fibers (white arrows) than the acellular CP-ANG (CP) group. By visual inspection, the groups injected with sciatic, motor, and sensory-derived SCs closely approximate the isograft, in contrast to the CP group. Scale bar = 30 μm.
Muscle Force Assessment
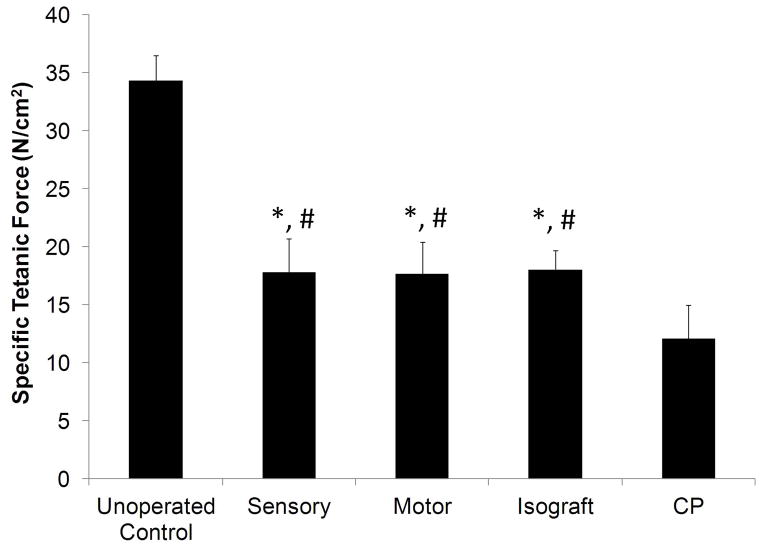
To assess the degree of regenerating motor reinnervation of distal motor targets, evoked force production in the EDL muscle was measured upon electrical stimulation of repaired sciatic nerve 12 weeks after injury. Specific tetanic force measurements were calculated by normalizing the maximum tetanic force to the cross-sectional area of the EDL muscle. This metric helped to measure deficits in functional recovery independent of muscle atrophy49. Although none of the experimental groups matched the specific tetanic force measurements (12–18 N/cm2) of the unoperated nerve (34.4 ± 2.2 N/cm2), the CP-ANGs groups injected with SCs generated forces similar to the isografts and greater than the acellular CP-ANGs (Figure 6). These results demonstrate that injection of SCs into CP-ANGs improved functional recovery compared to acellular ANGs, regardless of SC source.
Figure 6. Evoked muscle force measurements in EDL reveal similar functional recovery in distal musculature 12 weeks after implantation of CP-ANGs seeded with SCs.
Specific force measurements demonstrate the positive effect of SC supplementation on the neuroregenerative capacity of CP-ANGs seeded with SCs. Specific tetanic force measurements normalized to EDL muscle mass demonstrate that isografts and CP-ANGs supplemented with either sensory or motor-derived SCs support increased reinnervation of distal musculature compared to acellular CP-ANGs (CP). Observation of normal tetanic responses in all EDL muscles innervated by repaired sciatic nerve confirms normal function of regenerated motor axons and corresponding motor units. Error bars represent the standard deviation (n = 6). * - denotes P < 0.05 versus isograft, # - denotes P < 0.05 versus CP.
DISCUSSION
Typically, SCs dedifferentiate into an immature state after injury, which promotes the upregulation of NGF, GDNF, and BDNF to guide the regenerating axons to their distal targets25,26,61,62. Similarly, when SCs are harvested from fresh nerve tissue and expanded in vitro, they may revert to an immature phenotype and thus upregulate growth factor expression levels. Therefore, we hypothesized that injection of SCs into CP-ANGs would increase growth factor expression levels compared to acellular CP-ANGs, and this was confirmed for sensory and motor-derived SCs by qRT-PCR 2 weeks post-transplantation. However, these differences may be due to the use of proliferative media for SC expansion in vitro prior to transplantation.
Furthermore, higher growth factor expression levels were observed in sensory and motor-derived SCs compared to the sciatic-derived isograft. This may be due to differential upregulation of growth factors in different populations of SCs38,39 and could potentially influence the number of regenerating axons through the CP-ANGs. This difference may be attributed to the fact that sciatic-derived SCs were transplanted into their native ECM environment and thus did not receive as many cues for gene upregulation. However, future studies need to be done to assess the differences in structure and composition of acellular grafts derived from different sources to evaluate whether this influences SC gene expression.
SCs derived from the sensory and motor branches of the rat femoral nerve have been shown to exhibit differential gene expression patterns39. However, as SCs are expanded in vitro, which is necessary to provide sufficient cells for transplantation, the expression patterns of phenotypic markers are dysregulated, possibly due to the lack of environmental cues in vitro39. Therefore, we hypothesized that transplantation of dedifferentiated SCs into CP-ANGs would promote expression of their native phenotypic markers. However, the expression of MBP and PRKCi by SCs in the CP-ANGs remained dysregulated at 2 weeks. These results suggest that the environment within a sciatic nerve-derived CP-ANG may not provide sufficient cues for SCs to express their native phenotype within the 2 weeks. If the CP-ANGs were derived from sensory or motor nerves, the graft might provide stronger cues to promote the expression of native phenotype-specific markers.
Despite the differences in growth factor expression observed between CP-ANGs injected with SCs (motor, sensory, or sciatic-derived), a similar number of regenerating nerve fibers was observed for all CP-ANGs injected with SCs and isografts. However, fiber width, an indication of maturity63–65, was lower for CP-ANGs injected with SCs compared to isografts, suggesting that there may be other cues (such as fibroblast-derived cues66 and smaller ECM molecules11,12) that were removed from CP-ANGs during processing that may facilitate the maturation of myelinated fibers. Together, these results demonstrate that SCs derived from any nerve source can be injected into CP-ANGs to promote improved regeneration compared to acellular CP-ANGs.
We also found that there were no differences between the CP-ANGs injected SCs and isografts when assessing functional recovery, while acellular ANGs showed less functional recovery. These results further reinforce the critical role of SCs in functional recovery after nerve grafting. The maximum recovery to 52% of unoperated specific tetanic force following isograft repair reveals the functional limitations of nerve grafting67,68. However, the evoked muscle force generation showed that injection of sensory or motor derived SCs into CP-ANGs promotes similar functional recovery in an isograft, which mimics the autografts currently used for clinical nerve repair.
Currently, sensory nerves are used as an autograft source to repair large peripheral nerve gaps18. However, studies have shown that use of a sensory autograft has poor functional outcomes for motor nerves69. Results from this study suggest that as an alternative, a sensory nerve could be used as a SC source in combination with cadaver acellular motor nerve graft to obtain a better size match for the grafted nerve combined with SCs to improve functional recovery. The use of SCs from a sensory nerve source to repair peripheral motor nerve injuries would prevent the need for an autologous motor nerve harvest and sacrifice of motor function to obtain SCs for transplantation to promote functional recovery. Furthermore, with the possibility of introducing additional cues (growth factors56,57 or fibroblasts66) within therapies that utilize CP-ANGs, the regeneration capacity of the autografts may be surpassed, which is a highly desirable outcome.
In summary, this study demonstrates that the transplantation of SCs (that were expanded in vitro) into CP-ANGs improves nerve regeneration and functional recovery to the level of the isograft, regardless of SC source. Currently in the clinic, SCs can be obtained from a transected nerve by removal of a piece of nerve from the injured nerve stump or sacrifice of a healthy autologous nerve. If the peripheral nerve source that the SCs are derived from does not have an influence on the regeneration of the nerve and the functional recovery of the patient, then SCs can be derived from the nerve source that provides the least donor site morbidity. The use of SCs from any autologous nerve source to repair peripheral motor nerve injuries will prevent the need for an autologous motor nerve harvest to obtain SCs for cell transplantation therapies to promote peripheral nerve regeneration and functional recovery.
Acknowledgments
The authors were funded and supported by the NIH RO1 grant R01NS033406. We would also like to acknowledge the Hope Center for Neurological Disorders at Washington University in St. Louis, MO, USA for funding and use of the qRT-PCR thermocycler
References
- 1.Siemionow M, Brzezicki G. Chapter 8: Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141–172. doi: 10.1016/S0074-7742(09)87008-6. [DOI] [PubMed] [Google Scholar]
- 2.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurgical focus. 2004;16(5):E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 4.Bain JR, Mackinnon SE, Hudson AR, Falk RE, Falk JA, Hunter DA, Makino A. Preliminary report of peripheral nerve allografting in primates immunosuppressed with cyclosporin A. Transplant Proc. 1989;21(1 Pt 3):3176–3177. [PubMed] [Google Scholar]
- 5.Midha R, Mackinnon SE, Evans PJ, Best TJ, Hare GM, Hunter DA, Falk-Wade JA. Comparison of regeneration across nerve allografts with temporary or continuous cyclosporin A immunosuppression. J Neurosurg. 1993;78(1):90–100. doi: 10.3171/jns.1993.78.1.0090. [DOI] [PubMed] [Google Scholar]
- 6.Nakao Y, Mackinnon SE, Mohanakumar T, Miyasaka M, Nakayama K, Nakafusa Y, Horiuchi Y, Yabe Y. Monoclonal antibodies against ICAM-1 and LFA-1 (CD11A) induce specific tolerance to peripheral nerve allograft in rats. Transplant Proc. 1995;27(1):373–377. [PubMed] [Google Scholar]
- 7.Strasberg SR, Hertl MC, Mackinnon SE, Lee CK, Watanabe O, Tarasidis G, Hunter DA, Wong PY. Peripheral nerve allograft preservation improves regeneration and decreases systemic cyclosporin A requirements. Experimental neurology. 1996;139(2):306–316. doi: 10.1006/exnr.1996.0104. [DOI] [PubMed] [Google Scholar]
- 8.Strasberg SR, Mackinnon SE, Genden EM, Bain JR, Purcell CM, Hunter DA, Hay JB. Long-segment nerve allograft regeneration in the sheep model: experimental study and review of the literature. J Reconstr Microsurg. 1996;12(8):529–537. doi: 10.1055/s-2007-1006625. [DOI] [PubMed] [Google Scholar]
- 9.Gulati AK, Cole GP. Immunogenicity and regenerative potential of acellular nerve allografts to repair peripheral nerve in rats and rabbits. Acta Neurochir (Wien) 1994;126(2–4):158–164. doi: 10.1007/BF01476427. [DOI] [PubMed] [Google Scholar]
- 10.Zalewski AA, Gulati AK. Evaluation of histocompatibility as a factor in the repair of nerve with a frozen nerve allograft. J Neurosurg. 1982;56(4):550–554. doi: 10.3171/jns.1982.56.4.0550. [DOI] [PubMed] [Google Scholar]
- 11.Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10(9–10):1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 12.Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10(11–12):1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 13.Johnson PJ, Newton P, Hunter DA, Mackinnon SE. Nerve endoneurial microstructure facilitates uniform distribution of regenerative fibers: a post hoc comparison of midgraft nerve fiber densities. J Reconstr Microsurg. 2011;27(2):83–90. doi: 10.1055/s-0030-1267834. [DOI] [PubMed] [Google Scholar]
- 14.Moore AM, MacEwan M, Santosa KB, Chenard KE, Ray WZ, Hunter DA, Mackinnon SE, Johnson PJ. Acellular nerve allografts in peripheral nerve regeneration: a comparative study. Muscle Nerve. 2011;44(2):221–234. doi: 10.1002/mus.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, Moore AM, Tong AY, Mackinnon SE, Borschel GH. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39(6):787–799. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
- 16.Mackinnon SE, Dellon AL. A study of nerve regeneration across synthetic (Maxon) and biologic (collagen) nerve conduits for nerve gaps up to 5 cm in the primate. J Reconstr Microsurg. 1990;6(2):117–121. doi: 10.1055/s-2007-1006810. [DOI] [PubMed] [Google Scholar]
- 17.Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990;85(3):419–424. doi: 10.1097/00006534-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Meek MF, Coert JH. Clinical use of nerve conduits in peripheral-nerve repair: review of the literature. J Reconstr Microsurg. 2002;18(2):97–109. doi: 10.1055/s-2002-19889. [DOI] [PubMed] [Google Scholar]
- 19.Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y) 2009;4(3):245–249. doi: 10.1007/s11552-009-9195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner MJ, Lowe JB, 3rd, Fox IK, Mackinnon SE, Hunter DA, Darcy MD, Duncan JR, Wood P, Mohanakumar T. Effects of Schwann cells and donor antigen on long-nerve allograft regeneration. Microsurgery. 2005;25(1):61–70. doi: 10.1002/micr.20083. [DOI] [PubMed] [Google Scholar]
- 21.Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242(1 Suppl 1):S19–21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- 22.Nagarajan R, Le N, Mahoney H, Araki T, Milbrandt J. Deciphering peripheral nerve myelination by using Schwann cell expression profiling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8998–9003. doi: 10.1073/pnas.132080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds ML, Woolf CJ. Reciprocal Schwann cell-axon interactions. Current opinion in neurobiology. 1993;3(5):683–693. doi: 10.1016/0959-4388(93)90139-p. [DOI] [PubMed] [Google Scholar]
- 24.Waller AV. Experiments on the glossopharyngeal and hypoglossal nerves of the frog and observations produced thereby in the structure of their primative fibres. Phil Trans R Soc Lond. 1850;140(423) [Google Scholar]
- 25.Chan JR, Cosgaya JM, Wu YJ, Shooter EM. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(25):14661–14668. doi: 10.1073/pnas.251543398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwase T, Jung CG, Bae H, Zhang M, Soliven B. Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. J Neurochem. 2005;94(6):1488–1499. doi: 10.1111/j.1471-4159.2005.03290.x. [DOI] [PubMed] [Google Scholar]
- 27.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18(7):397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12(9):3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DH, Connolly SE, Kline DG, Voorhies RM, Smith A, Powell M, Yoes T, Daniloff JK. Labeled Schwann cell transplants versus sural nerve grafts in nerve repair. J Neurosurg. 1994;80(2):254–260. doi: 10.3171/jns.1994.80.2.0254. [DOI] [PubMed] [Google Scholar]
- 30.Levi AD, Guenard V, Aebischer P, Bunge RP. The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J Neurosci. 1994;14(3 Pt 1):1309–1319. doi: 10.1523/JNEUROSCI.14-03-01309.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aszmann OC, Korak KJ, Luegmair M, Frey M. Bridging critical nerve defects through an acellular homograft seeded with autologous schwann cells obtained from a regeneration neuroma of the proximal stump. J Reconstr Microsurg. 2008;24(3):151–158. doi: 10.1055/s-2008-1076091. [DOI] [PubMed] [Google Scholar]
- 32.Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8(3):1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13(6):2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madison RD, Archibald SJ, Brushart TM. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J Neurosci. 1996;16(18):5698–5703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madison RD, Archibald SJ, Lacin R, Krarup C. Factors contributing to preferential motor reinnervation in the primate peripheral nervous system. J Neurosci. 1999;19(24):11007–11016. doi: 10.1523/JNEUROSCI.19-24-11007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madison RD, Robinson GA, Chadaram SR. The specificity of motor neurone regeneration (preferential reinnervation) Acta physiologica (Oxford, England) 2007;189(2):201–206. doi: 10.1111/j.1748-1716.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 37.Madison RD, Sofroniew MV, Robinson GA. Schwann cell influence on motor neuron regeneration accuracy. Neuroscience. 2009;163(1):213–221. doi: 10.1016/j.neuroscience.2009.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26(38):9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jesuraj NJ, Nguyen PK, Wood MD, Moore AM, Borschel GH, Mackinnon SE, Sakiyama-Elbert SE. Differential gene expression in motor and sensory Schwann cells in the rat femoral nerve. J Neurosci Res. 2012;90(1):96–104. doi: 10.1002/jnr.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–247. doi: 10.1146/annurev.med.46.1.235. [DOI] [PubMed] [Google Scholar]
- 41.Fox IK, Jaramillo A, Hunter DA, Rickman SR, Mohanakumar T, Mackinnon SE. Prolonged cold-preservation of nerve allografts. Muscle Nerve. 2005;31(1):59–69. doi: 10.1002/mus.20231. [DOI] [PubMed] [Google Scholar]
- 42.Brockes JP, Raff MC. Studies on cultured rat Schwann cells. II. Comparison with a rat Schwann cell line. In Vitro. 1979;15(10):772–778. doi: 10.1007/BF02618303. [DOI] [PubMed] [Google Scholar]
- 43.Pruss RM. The potential use of cultured cells to detect non-neuronal targets of neuropeptide action. Peptides. 1982;3(3):231–233. doi: 10.1016/0196-9781(82)90083-3. [DOI] [PubMed] [Google Scholar]
- 44.Raff MC, Abney E, Brockes JP, Hornby-Smith A. Schwann cell growth factors. Cell. 1978;15(3):813–822. doi: 10.1016/0092-8674(78)90266-0. [DOI] [PubMed] [Google Scholar]
- 45.Jesuraj NJ, Santosa KB, Newton P, Liu Z, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE, Johnson PJ. A systematic evaluation of Schwann cell injection into acellular cold-preserved nerve grafts. J Neurosci Methods. 2011;197(2):209–215. doi: 10.1016/j.jneumeth.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguayo AJ, Charron L, Bray GM. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J Neurocytol. 1976;5(8):565–573. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- 47.Aguayo AJ, Epps J, Charron L, Bray GM. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976;104(1):1–20. doi: 10.1016/0006-8993(76)90643-0. [DOI] [PubMed] [Google Scholar]
- 48.Hunter DA, Moradzadeh A, Whitlock EL, Brenner MJ, Myckatyn TM, Wei CH, Tung TH, Mackinnon SE. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166(1):116–124. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood MD, Macewan MR, French AR, Moore AM, Hunter DA, Mackinnon SE, Moran DW, Borschel GH, Sakiyama-Elbert SE. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol Bioeng. 2010;106(6):970–979. doi: 10.1002/bit.22766. [DOI] [PubMed] [Google Scholar]
- 50.Wilfinger WW, Mackey K, Chomczynski P. Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques. 1997;22(3):474–476. 478–481. doi: 10.2144/97223st01. [DOI] [PubMed] [Google Scholar]
- 51.Salonen V, Aho H, Roytta M, Peltonen J. Quantitation of Schwann cells and endoneurial fibroblast-like cells after experimental nerve trauma. Acta Neuropathol. 1988;75(4):331–336. doi: 10.1007/BF00687785. [DOI] [PubMed] [Google Scholar]
- 52.Gaumond G, Tyropolis A, Grodzicki S, Bushmich S. Comparison of direct fluorescent antibody staining and real-time polymerase chain reaction for the detection of Borrelia burgdorferi in Ixodes scapularis ticks. J Vet Diagn Invest. 2006;18(6):583–586. doi: 10.1177/104063870601800610. [DOI] [PubMed] [Google Scholar]
- 53.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 54.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, et al. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363(6426):266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Wu W, Lin LF, Lei M, Oppenheim RW, Houenou LJ. Rescue of adult mouse motoneurons from injury-induced cell death by glial cell line-derived neurotrophic factor. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(21):9771–9775. doi: 10.1073/pnas.92.21.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee AC, Yu VM, Lowe JB, 3rd, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184(1):295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 57.Wood MD, Moore AM, Hunter DA, Tuffaha S, Borschel GH, Mackinnon SE, Sakiyama-Elbert SE. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomater. 2009;5(4):959–968. doi: 10.1016/j.actbio.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X, Yu H, Gao S, Ma HQ, Leong KW, Wang S. Polyphosphoester microspheres for sustained release of biologically active nerve growth factor. Biomaterials. 2002;23(17):3765–3772. doi: 10.1016/s0142-9612(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 59.Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373(6512):341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 60.Mackinnon SE, Dellon AL, O’Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14(11):1116–1122. doi: 10.1002/mus.880141113. [DOI] [PubMed] [Google Scholar]
- 61.Mirsky R, Jessen KR. Schwann cell development, differentiation and myelination. Current opinion in neurobiology. 1996;6(1):89–96. doi: 10.1016/s0959-4388(96)80013-4. [DOI] [PubMed] [Google Scholar]
- 62.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 63.Aitken JT, Sharman M, Young JZ. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947;81(1):1–22. [PubMed] [Google Scholar]
- 64.Williams PL, Wendell-Smith CP. Some additional parametric variations between peripheral nerve fibre populations. J Anat. 1971;109(Pt 3):505–526. [PMC free article] [PubMed] [Google Scholar]
- 65.Fraher J, Dockery P. A strong myelin thickness-axon size correlation emerges in developing nerves despite independent growth of both parameters. J Anat. 1998;193 (Pt 2):195–201. doi: 10.1046/j.1469-7580.1998.19320195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parrinello S, Napoli I, Ribeiro S, Digby PW, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 143(1):145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urbanchek MS, Chung KC, Asato H, Washington LN, Kuzon WM., Jr Rat walking tracks do not reflect maximal muscle force capacity. J Reconstr Microsurg. 1999;15(2):143–149. doi: 10.1055/s-2007-1000085. [DOI] [PubMed] [Google Scholar]
- 68.Viguie CA, Lu DX, Huang SK, Rengen H, Carlson BM. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat Rec. 1997;248(3):346–354. doi: 10.1002/(SICI)1097-0185(199707)248:3<346::AID-AR7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 69.Dellon AL, Mackinnon SE. An alternative to the classical nerve graft for the management of the short nerve gap. Plast Reconstr Surg. 1988;82(5):849–856. doi: 10.1097/00006534-198811000-00020. [DOI] [PubMed] [Google Scholar]
- 70.Hirata H, Hibasami H, Yoshida T, Ogawa M, Matsumoto M, Morita A, Uchida A. Nerve growth factor signaling of p75 induces differentiation and ceramide-mediated apoptosis in Schwann cells cultured from degenerating nerves. Glia. 2001;36(3):245–258. doi: 10.1002/glia.1113. [DOI] [PubMed] [Google Scholar]
- 71.Galla TJ, Vedecnik SV, Halbgewachs J, Steinmann S, Friedrich C, Stark GB. Fibrin/Schwann cell matrix in poly-epsilon-caprolactone conduits enhances guided nerve regeneration. Int J Artif Organs. 2004;27(2):127–136. doi: 10.1177/039139880402700208. [DOI] [PubMed] [Google Scholar]
- 72.Suri S, Schmidt CE. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A. 2010;16(5):1703–1716. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]