Abstract
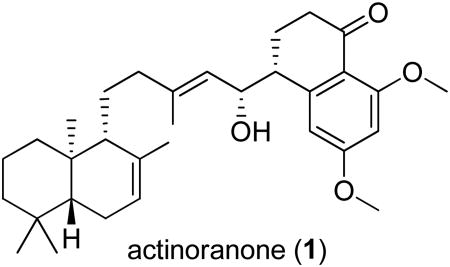
The isolation and structure elucidation of a new meroterpenoid, actinoranone (1), produced by a marine bacterium closely related to the genus Streptomyces is reported. Actinoranone is composed of an unprecedented dihydronaphthalenone polyketide linked to a bicyclic diterpenoid. The stereochemistry of 1 was defined by application of the advanced Mosher's method and by interpretation of spectroscopic data. Actinoranone (1) is significantly cytotoxic to HCT-116 human colon cancer cells with an LD50 = 2.0 μg/mL.
The variability of environmental chemical and physical conditions in the oceans has clearly played a major role in the evolution of a great diversity of microorganisms.1 Our evidence suggests that obligate marine microorganisms have, in part, adapted to life in the sea through the production of unique secondary metabolites that distinguish them from their terrestrial counterparts.2 Thus, investigations of the chemistry and biology of these unique molecules could lead to the discovery of a significant number of new drug leads.3
The chemically-prolific genus Streptomyces, which is abundant and well-known in terrestrial habitats, also appears to be represented in marine ecosystems. In recent years, expanding studies of marine-derived and obligate marine actinomycetes have yielded bioactive molecules with diverse chemical structures. 4 Phylogenetic classification, by analysis of 16S rRNA gene sequence data, has indicated that new taxa at both the genus and species levels are present.5
As part of our continuing program to explore the biomedical potential of marine actinomycetes, we have previously isolated and reported the structures of several highly modified peptides, actinoramides A-C, from our marine actinomycete strain CNQ-027 (GenBank accession number EU214912).6 This seawater-requiring strain shares only 97.6% 16S rRNA gene sequence identity with an obligate marine Streptomyces species, S. marinus, suggesting it may represent a new Streptomyces species adapted to live in the sea. 7
A more extensive study of the secondary metabolite composition of this strain, has now yielded a new meroterpenoid, actinoranone (1), which possesses a new carbon skeleton and significant cancer cell cytotoxicity. Details of the isolation, characterization, and biological activity of actinoranone are presented here.
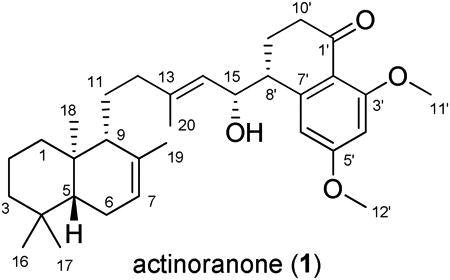
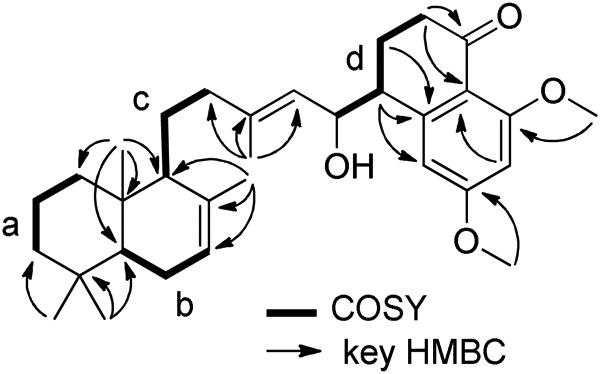
The molecular formula of actinoranone (1)8 was defined as C32H46O4, based on analysis of HRESIMS data (a pseudomolecular ion peak at m/z 495.3450 [M+H]+), and on interpretation of 13C NMR data. The 1H NMR spectrum of 1 displayed meta-coupled aromatic protons [δ 6.56 (d, J = 2.0 Hz), 6.50 (d, J = 2.0 Hz)], two olefinic protons [δ 5.38 (br s), 5.25 (d, J = 8.5 Hz)], and one downfield methine protons [δ 4.47 (dd, J = 8.5, 8.5 Hz)]. The 1H NMR spectrum also showed two methoxyl groups [δ 3.87, 3.83], and five methyl singlets [δ 1.71, 1.63, 0.90, 0.86, 0.78](Table 1). Analysis of 13C NMR and gHSQC spectral data revealed seven methyl, eight methylene, eight methine, and nine fully-substituted carbons. Analysis of the gCOSY spectroscopic data for 1 revealed the connectivity of four partial structures; substructure a (C-1 to C-3), substructure b (C-5 to C-7), substructure c (C-9 to C-11 and C-11 to C-12), and substructured (C-15 to C-8′, and C-8′ to C-10′), as shown in Figure 1. Substructures (a and b) and gHMBC correlations from gem-dimethyl singlets (H-16 and H-17) to carbons C-3, C-4, and C-5, and from a methyl singlet H-18 to carbons C-1, C-5, C-9, and C-10, and from a methyl singlet H-19 to carbons C-7, C-8, C-9 allowed the terpenoid bicyclic ring to be defined. The dihydronaphthalenone moiety was also constructed by interpretation of gCOSY and gHMBC spectroscopic data. The presence of meta-coupled aromatic protons [δ 6.56 (d, J = 2.0 Hz), 6.50 (d, J = 2.0 Hz)] indicated a 1, 2, 3, 5-tetrasubstituted benzene ring. The composition of substructure d and gHMBC correlations from H-9′ to carbons C-1′, C-7′, and from H-8′ to carbons C-2′, C-6′, and C-7′, and from H-11′ to C-3′, and from H-12′ to C-5′, and from H-4′ to C-2′, C-6′ confidently established the dihydronaphthalenone ring. Last, gHMBC correlations from the H-20 methyl singlet to carbons C-12, C-13, and C-14 permitted the connectivity of C-14 and C-15, thus completing the assignment of the planar structure of 1 (Figure 1).
Table 1. NMR Data for Actinoranone 1 (methanol-d4)a.
| no | δC, #H.b | δH (mult, J (Hz)) | HMBC |
|---|---|---|---|
| 1 | 39.3, CH2 | 1.85* 0.94 dt (13.5, 3.6) | 3, 18 |
| 2 | 25.4, CH2 | 1.55, m 1.29, m | 4, 10 |
| 3 | 42.3, CH2 | 1.42, m 1.16* | 5 |
| 4 | 32.7, C | ||
| 5 | 50.4, CH | 1.16, m | 7 |
| 6 | 23.7, CH2 | 1.97, m 1.86, m | 7, 8 |
| 7 | 122.2, CH | 5.38, br s | 6, 8, 19 |
| 8 | 135.0, C | ||
| 9 | 54.2, CH | 1.61, m | 11, 12 |
| 10 | 36.7, C | ||
| 11 | 25.4, CH2 | 1.55, m 0.78, m | 8, 13 |
| 12 | 42.1, CH2 | 2.24, m 2.01, m | 9, 13, 14 |
| 13 | 139.5, C | ||
| 14 | 126.8, CH | 5.25, d (8.5) | 15, 20 |
| 15 | 70.4, CH | 4.47, dd (8.5, 8.5) | 8′ |
| 16 | 32.5, CH3 | 0.86, s | 3, 4, 5, 17 |
| 17 | 21.0, CH3 | 0.90, s | 3, 4, 5, 16 |
| 18 | 12.8, CH3 | 0.78, s | 1, 5, 9, 10 |
| 19 | 21.5, CH3 | 1.71, s | 7, 8, 9 |
| 20 | 15.6, CH3 | 1.63, s | 12, 13, 14 |
| 1′ | 197.3, C | ||
| 2′ | 115.5, C | ||
| 3′ | 162.7, C | ||
| 4′ | 97.2, CH | 6.50, d (2.0) | 2′, 5′, 6′ |
| 5′ | 164.4, C | ||
| 6′ | 107.5, CH | 6.56, d (2.0) | 2′, 4′, 5′, 7′, 8′ |
| 7′ | 150.4, C | ||
| 8′ | 45.3, CH | 2.97, m | 15, 2′, 6′, 7′, 9′, 10′ |
| 9′ | 23.8, CH2 | 2.12, m | 1′, 7′ |
| 10′ | 36.3, CH2 | 2.44, ddd (18.0, 11.0, 8.0) 2.59, dt (18.0, 4.5) | 1′, 2′, 8′, 9′ |
| 11′ | 54.9, CH3 | 3.83, s | 3′ |
| 12′ | 55.0, CH3 | 3.87, s | 5′ |
500 MHz for 1H NMR and 75 MHz for 13C NMR.
The number of attached protons was determined by analysis of 2D spectroscopic data.
The coupling constant was not determined because of overlapping signals.
Figure 1. gCOSY and key gHMBC correlations assisting in the structure elucidation of actinoranone (1).
The relative configuration of the bicyclic terpenoid ring in 1 was assigned by interpretation of ROESY NMR correlations. Correlations between the H-18 methyl singlet and the H-11 methylene protons, and between H-5 and H-9, allowed the relative configurations to be assigned as 5R*, 9R*, and 10R*. The 7Z and 13E doublebond geometries were also assigned by the observation of ROESY NMR correlations between the H-7 olefinic proton and the H-19 methyl singlet, and between the H-12 methylene protons and the H-14 olefinic proton, respectively.
Next, application of the advanced Mosher's method allowed the absolute configuration at C-15 to be defined.9 Treatment of 1 with (R)- and (S)-MTPA-Cl [α-methoxy-α-(trifluoromethyl) phenylacetyl chloride] yielded the (S)- and (R)-MTPA ester derivatives, respectively. Calculation of the 1H NMR ΔδS-R values for the mono-MTPA esters of 1 established the absolute configuration at C-15 as R (Figure 2a). HETLOC 10 and HSQMBC 11 NMR experiments were undertaken to determine the configuration of C-8′, but we were unable to measure the coupling constants due to the complexity of signals (data not shown). As an alternative approach, we carefully analyzed the ROESY NMR data hoping to assign the absolute configuration of C-8′. There were only two possible stereoisomers, (15R, 8′ R) or (15R, 8′S), for 1. The median value of the coupling constant (3JH15-H8′ = 8.0 Hz) and ROESY correlations from H-8′ and H-6′ to H-15′, and from H-8′ and H-9′ to H-14 supported our assignment of an 8′R configuration (Figure 2b).
Figure 2.
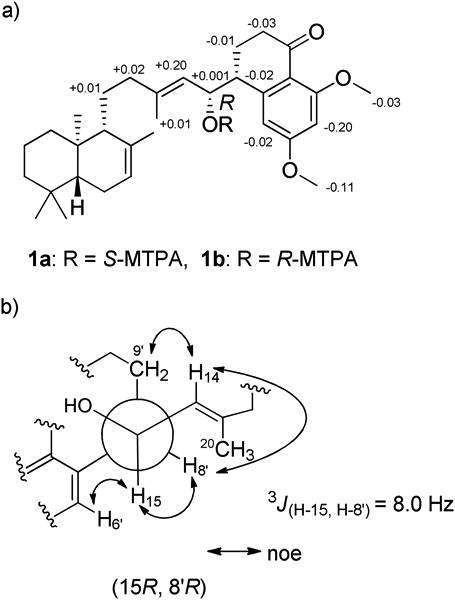
a) Mosher's ester analysis of actinoranone (1), ΔδS-R of 1H for S- and R-mono-MTPA esters of 1, and b) A Newman projection of the C-8′ — C-15 bond illustrating the assigned C-8′ relative configuration.
In an attempt to determine the absolute configurations for C-5, C-9, and C-10, we performed a series of synthetic modifications. We protected the hydroxyl group at C-15 of 1 by acetylation and then we treated the 15-acetoxyl derivative 2 with OsO4 to afford a mixture of 7, 8, 13, 14-tetra-ol products, which were then subjected to HPLC purification to obtain a major stereoisomer 3. 12 The relative configuration of the bicyclic ring system of this stereoisomer was suggested as 5R*, 7S*, 8R*, 9S*, and 10R* based upon ROESY correlations between H-7 and H-19, between H-19 and H-11, and between H-11 and H-18 (see supporting information). Treatment of this stereoisomer with (R)- and (S)-MTPA-Cl produced crude bis-(S)- and bis-(R)-MTPA ester derivatives, respectively. However, on HPLC purification these ester derivatives spontaneously decomposed under all conditions explored. Next, we attempted chemical reactions to eliminate the stereocenters at C-15 and C-8′ in an effort to compare the optical rotations of derivatives of 1 with the known (+) isozonarol13a and (-) isozonarol.13b Actinoranone A was treated with p-toluenesulfonic acid (TSA) hoping to obtain the Δ13,14,15,8′-diene.14a However, we obtained the undesired dehydration product possessing the Δ11,12,13,14-diene. We also attempted a base dehydration of actinoranone A using p-toluenesulfonyl chloride (TsCl),14b but this yielded the same result. In addition, attempted aromatization of these unexpected products with Pd/C14c did not produce any isolable products. Then, actinoranone A was treated with Dess-Martin periodinane (DMP) 14d to oxidize the secondary alcohol to ketone 5, and then further oxidized by using 2-iodoxybenzoic acid (IBX)14e hoping to obtain the C-8′-C-9′ α,β-unsaturated ketone. The reaction did not yield identifiable products.
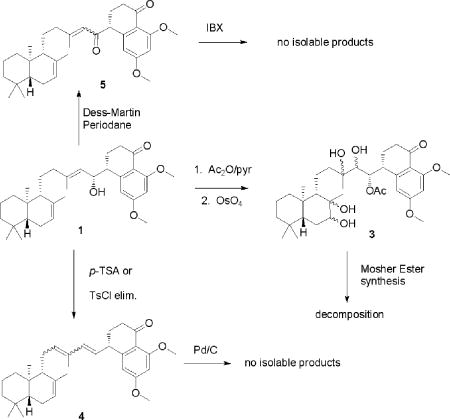
All attempts defined above to eliminate the stereocenters at C-15 and C-8′ were unsuccessful (see supporting information for synthetic details). Thus, only the relative stereochemistry of 1 could be defined as 5R*, 9R*, 10R*, along with the absolute configurations 15R and 8′R.
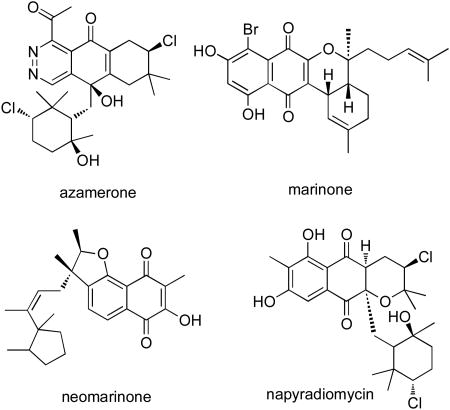
Traditionally, actinomycetes are not considered producers of terpenoids. However, strains of a largely marine lineage we have classified as MAR4 consistently produce diverse meroterpenoids. 15 Three classes of meroterpenoids, exempified by azamerone, 16 marinone and neomarinone, 17 and the many members of the napyradiomycin family,18 have been isolated from these organisms. They commonly share a 2,3-dihydronaphthalene-1,4-dione or naphthalene-1,4-dione derived from the polyketide precursor 1,3,6,8-tetrahydroxynaphthalene (THN) and terpenoid substituents in the molecules. Given these comparisons, it is surprising that strain CNQ-027 does not belong to the MAR4 group of marine actinomycetes.
To the best of our knowledge, this is the first observation of the common diterpenoid “labdane” bicyclic ring system in microbial natural products. This is also the first report of a meroterpenoid isolated from a marine-derived actinomycete possessing the dihydronaphthalenone moiety. Actinoranone (1) showed in vitro cytotoxicity against HCT-116 human colon carcinoma with an LD50 = 2.0 μg/mL. Testing in a broader number of diverse cancer cell lines is in progress.
Supplementary Material
Acknowledgments
Financial support was provided by the National Cancer Institute, NIH (under grant R37 CA044848). We thank Chambers C. Hughes, Scripps Institution of Oceanography, UCSD, for his generous advice in the determination of the stereochemistry of actinoranone, and Katherine N. Maloney, Scripps Institution of Oceanography, UCSD for providing preliminary information about the strain CNQ-027.
Footnotes
Supporting Information Available: Details of the isolation procedures, reactions and 1H, 13C, and 2D NMR spectra of actinoranone, 1H NMR spectra of mono-MTPA esters (1a, 1b), and other derivatives, are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Colin M. Marine Microbiology: Ecology and Applications. Garland Science; New York: 2012. Chapter 1. [Google Scholar]
- 2.Murphy BT, Maloney KN, Fenical W. In: Handbook of Marine Natural Products. Fattorusso E, Gerwick WH, Taglialatela-Scafati O, editors. Vol. 1. Springer; New York: p. 153. [Google Scholar]
- 3.a) Harvey A. Drug Discov Today. 2000;5:294. doi: 10.1016/s1359-6446(00)01511-7. [DOI] [PubMed] [Google Scholar]; b) Zotchev SB. J Biotechnol. 2012;158:168. doi: 10.1016/j.jbiotec.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Fenical W, Jensen PR. Nat Chem Biol. 2006;2 doi: 10.1038/nchembio841. 666 and therein. [DOI] [PubMed] [Google Scholar]
- 5.a) Jensen PR, Gontang EA, Mafnas C, Mincer TJ, Fenical W. Environ Microbiol. 2005;7:1039. doi: 10.1111/j.1462-2920.2005.00785.x. [DOI] [PubMed] [Google Scholar]; b) Gontang EA, Fenical W, Jensen PR. Appl Environ Microbiol. 2007;73:3272. doi: 10.1128/AEM.02811-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nam SJ, Kauffman CA, Jensen PR, Fenical W. Tetrahedron. 2011;67:6707. doi: 10.1016/j.tet.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prieto-Davó A, Fenical W, Jensen PR. Aquat Microbial Ecol. 2008;52:1. [Google Scholar]
- 8.Actinoranone: [α]D21 +3 (c 0.7, MeOH); IR (KBr) ν max 3429, 2921, 1720, 1664, 1602, 1464, 1258, 1148 cm-1; UV (MeOH) λmax (log ε) 230 (3.2), 289 (2.8), 308 (2.0) nm; 1H and 13C NMR data, See Table 1; ESI-TOF m/z 495 [M+H]+; HR ESI-TOF m/z 495.3450 (calcd. for C32H47O4, 495.3469). Details of the isolation of actinoranone are provided in the supporting information.
- 9.Uhrin D, Barlow PN. J Magn Reson. 1997;126:248. [Google Scholar]
- 10.Williamson RT, Marquez BL, Gerwick WH, Kover KE. Magn Reson Chem. 2000;38:265. [Google Scholar]
- 11.a) Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J Am Chem Soc. 1991;113:4092. [Google Scholar]; b) Seco JM, Quinoa E, Riguera R. Chem Rev. 2004;104:17. doi: 10.1021/cr2003344. [DOI] [PubMed] [Google Scholar]
- 12.VanReen V, Kelly RC, Cha DY. Tetrahedron Lett. 1976;17:1973. [Google Scholar]
- 13.a) Schröder J, Magg C, Seifert K. Tetrahedron Lett. 2000;41:5469. [Google Scholar]; b) Pérez-García E, Zubía E, Ortega MJ, Carballo JL. J Nat Prod. 2005;68:653. doi: 10.1021/np040237z. [DOI] [PubMed] [Google Scholar]
- 14.a) Stevens RV, Albizati KF. J Org Chem. 1985;50:632. [Google Scholar]; b) Choudary BM, Chowdari NS, Kantam M. Tehtrahedron. 2000;56:7291. [Google Scholar]; c) Mosettig E, Duvall HM. 1937;59:367. [Google Scholar]; d) Dess DB, Martin JC. J Org Chem. 1983;48:4155. [Google Scholar]; e) Nicolaou KC, Zhong YL, Baran PS. J Am Chem Soc. 2000;122:7596. [Google Scholar]; f) Hirai Y, Doe M, Kinoshita T, Morimoto Y. Chem Lett. 2004;33:136. [Google Scholar]
- 15.Gallagher KA, Fenical W, Jensen PR. Curr Opin Biotechnol. 2010;21:794. doi: 10.1016/j.copbio.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Cho JY, Kwon HC, Williams PG, Jensen PJ, Fenical W. Org Lett. 2006;8:2471. doi: 10.1021/ol060630r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Pathirana C, Jensen PR, Fenical W. Tetrahedron Lett. 33:7663–7666. [Google Scholar]; b) Hardt IH, Jensen PR, Fenical W. Tetrahedron Lett. 2000;41:2073. [Google Scholar]
- 18.Soria-Mercado IE, Prieto-Davó A, Jensen PR, Fenical W. J Nat Prod. 2005;68:904. doi: 10.1021/np058011z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


