Abstract
Purpose of review
This review intends to describe recent studies on pancreatic tumor associated stroma and potential opportunities and limitations to its targeting.
Recent findings
One of the defining features of pancreatic cancer is extensive desmoplasia, or an inflammatory, fibrotic reaction. Carcinoma cells live in this complex microenvironment which is comprised of extracellular matrix (ECM), diffusible growth factors, cytokines and a variety of non-epithelial cell types including endothelial cells, immune cells, fibroblasts, myofibroblasts and stellate cells. In addition to the heterogeneity noted in the non-neoplastic cells within the tumor microenvironment, it has also been recognized that neoplastic cancer cells themselves are heterogeneous, and include a subpopulation of stem-cell like cells within tumors termed cancer stem cells. Due to the failure of current therapeutics to improve outcomes in patients with pancreatic cancer, new therapeutic avenues targeting different components of the tumor microenvironment are being investigated. In this review article, we will focus on recent studies regarding the function of the tumor stroma in pancreatic cancer and therapeutic treatments that are being advanced to target the stroma as a critical part of tumor management.
Summary
Recent studies have shed new light on the contribution of the pancreatic cancer fibroinflammatory stroma to pancreatic cancer biology. Additional studies are needed to better define its full contribution to tumor behavior and how to best understand the optimal ways to develop therapies that counteract its pro-neoplastic properties.
Keywords: pancreatic cancer, tumor microenvironment, cancer associated fibroblasts, stellate cells, stroma, fibroblasts, desmoplasia
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth most common cause of cancer related death, accounting for approximately 34,000 deaths each year in the United States, with an increasing annual incidence rate (1). With an overall five year survival rate of <5%, death rates closely mirror incidence rates, reflecting the ineffectiveness of current therapies and the direness of the disease. A recent report has stated that if current trends continue, pancreatic cancer will become the second leading cause of cancer deaths in the United States by 2020 (2). A small number of systemic therapies including Folfirinox, and a combination of gemcitabine and Abraxane, are reported to have modest clinical benefit over gemcitabine alone in the metastatic setting (3-6). However, the prospects of cure, or even modest long term survival, are essentially non-existent in patients with advanced PDAC. Most adenocarcinomas of the pancreas are characterized by a dense fibrous stroma. Recent studies have been focusing on therapeutic targeting of the stroma to enhance drug penetration. It is becoming increasingly clear, however, that the unyielding stroma of pancreatic tumors does not simply act as a barrier to drug delivery, but as a complex signaling partner promoting tumorigenesis. The focus of the review is to update the reader about recent advances in the understanding of pancreatic cancer associated stroma, where key questions remain, and to better understand the therapeutic implications of stromal targeting in pancreatic cancer.
Cancer associated fibroblasts in stroma
Pancreatic ductal adenocarcinoma (PDAC) is one of the most stroma-rich cancers. Individual tumors show a wide range of growth rates and stromal content (7, 8). PDA stroma is very heterogeneous and comprises cellular and acellular components, such as fibroblasts, myofibroblasts, pancreatic stellate cells (PSC), immune cells, blood vessels, extracellular matrix (ECM), and soluble proteins such as cytokines and growth factors (9). The fibrotic stroma in PDAC forms an environment that promotes cancer progression by enhancing pancreatic tumor growth as well as regional and distant metastasis (10). Furthermore, the stroma has been shown to induce resistance to chemotherapy and radiation therapy (11) and to constitute a barrier to the delivery of therapeutic agents (12). Whether depletion of the stroma would indeed result in regression of patient tumors has not been formally demonstrated. A possibility to consider based on the instructive role of mesenchyme in epithelial-mesenchymal interactions during development is that the mesenchyme (in this case tumor associated stroma) might be secreting factors, that in some instances, might affect the differentiation status of tumor cells, causing the tumor cells to differentiate, tilting the balance towards differentiation as opposed to proliferation. One example comes from a study showing the TGF-β target connective tissue growth factor (CTGF) expressing mesenchyme was associated with more highly differentiated tumors and better prognosis (13).
In order to study the contributing role of the tumor stroma to pancreatic cancer biology, researchers have utilized stellate cells from rat and human pancreata (11, 14-18). Stellate cells of the pancreas have an unknown origin, produce vitamin A droplets in the quiescent state, and develop a myofibroblast type appearance once activated. Whether all the fibroblasts within the stroma are derived from stellate cells has not been established. However, it is reasonable to expect that other components might contribute to the fibroblast population, including peri-vascular fibroblasts that become activated upon tissue injury and possibly bone-marrow derived cells. Stellate cells and other fibroblasts might be activated upon tissue injury and accumulate in the pancreas during carcinogenesis. So far, most studies addressing the functional roles of pancreatic cancer associated fibroblasts (CAFs) have relied mainly on immortalized stellate cells, although one group has successfully cultured primary CAFs for functional studies as well (16). These cells have been shown to enhance tumor growth, metastasis, and inhibit the effects of chemotherapy and radiation therapy on tumor cells. (11, 16-18). Interestingly, a subset of pancreatic CAFs have been shown to express the surface marker CD10, thus constituting a distinct subpopulation. CD10 expressing stellate cells were shown to induce an invasive phenotype in pancreatic cancer cells more extensively than cells lacking CD10 expression (14). Despite this interesting finding, the mechanism by which CD10 expressing cells enhanced tumorigenesis was not interrogated. Overall, these studies suggest that stroma can enhance tumor growth and invasion.
Mesenchymal stem cells in stroma
Mesenchymal stem cells (MSCs) have attracted great interest because their presence has recently been identified in human tumors and they have been shown to have the ability to migrate and engraft to areas of tumor development (19). However, the role of MSCs during tumor growth appears to be exquisitely tissue-specific, with tumor-promoting or tumor-inhibiting effects observed in different settings. Recent studies in solid tumors of lung, stomach and ovary has identified MSCs associated with cancer and have described their tumor enhancing properties (20-24). MSCs have so far not been identified in pancreatic cancer, and therefore no information is available as to their potential role in the context of this disease.
Mesenchymal stem cells drive cancer stem cells
Histologically, pancreatic cancer shows immense cellular heterogeneity within the same tumor, indicating a complex regulation of cancer progression. Our lab has previously identified and characterized pancreatic cancer stem cells (CSC), and has demonstrated that the capacity of a tumor to grow and propagate is dependent on this small subset of cells (25). A key question in the field has been whether these CSCs are a fixed population or if they might exhibit some plasticity and be responsive to cues from the microenvironment. To that end, a recent study has identified the contributions of bone marrow derived MSC’s in regulation of EMT and in maintenance of a pancreatic tumor initiating cell population (26). MSCs treated with TGF-beta showed enhanced ability to induce EMT and sphere formation in tumor initiating cells. Furthermore, these effects by TGF-beta treated MSC’s were dependent on Notch signaling activity. It remains to be ascertained whether these results have relevance in the context of an intact pancreatic cancer microenvironment.
Hedgehog and Stroma
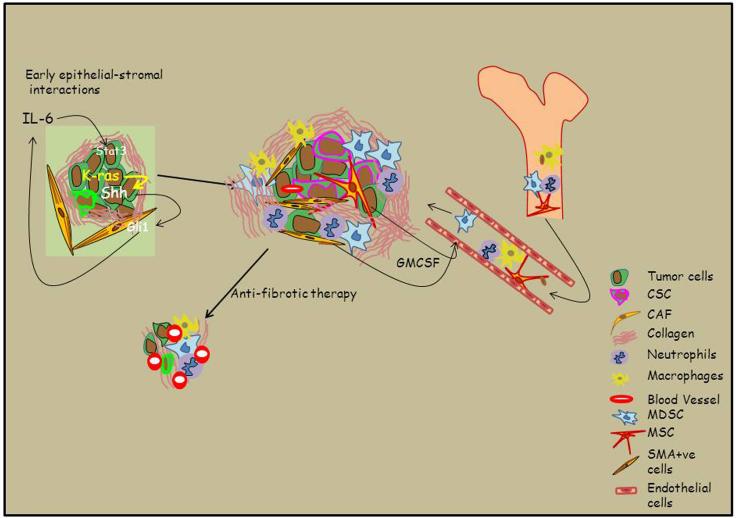
Hedgehog signaling is absent in the normal adult pancreas, but is induced during PanIN formation and PDAC progression (27). Three mammalian hedgehog genes have been identified as ligands: Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh). These ligands can bind to the receptors Patched-1 (Ptch-1) and Patched-2 (Ptch-2). Hh ligands elicit their effects by antagonizing Patched activity. Hh binds Ptch and relieves Ptch inhibition of a transmembrane protein called Smoothened (Smo). Once Smo is activated, it acts through a protein complex to block proteolytic processing of a zinc finger transcription factor family called Gli. The proteolyzed form of Gli is a potent transcriptional repressor, but upon stabilization by Hh signals, full-length Gli translocates to the nucleus, where it binds and activates Hh target genes (28). There are 3 Gli zinc finger transcription factors: Gli1, Gli2, and Gli3. Gli1 is completely dependent on Hh activity for its expression and primarily functions as an Hh activator (29-34). While Gli2 exhibits both activator and repressor activities, Gli3 is primarily a transcriptional repressor although transcriptional activator properties have been described (34-36) Hh signaling in pancreatic cancer occurs in a paracrine mechanism with expression and secretion of Hh ligand in cancer cells and activation of the canonical signaling pathway in the adjacent stromal cells (37, 38). This activation of Hh signaling in the stromal cells in turn supports tumor growth and metastasis. Recent reports show that Hh inhibition resulted in a decrease in the fibrous stroma. Hh signaling was inhibited using anti-Shh 5E1 antibody in orthotopic xenografts derived from pancreatic cancer cell lines which showed a decrease in desmoplasia, as measured by a decrease in smooth muscle actin (SMA) positive cells and reduced collagen deposition (39). In a second report, Hh signaling was inhibited using the smoothened inhibitor (IPI-926, Infinity Pharmaceuticals) in a genetically engineered mouse model of PDAC. This study demonstrated not only a decrease in the fibrous stroma but an increase in the uptake and anticancer effects of gemcitabine (12). These results lead to a Phase II clinical trial using IPI-926, however the study was stopped early due to increased mortality in the treatment arm [NCT01130142 (clinicaltrials.gov)]. The reason for these discordant results in the genetically engineered mouse model and patients remains to be elucidated. In contrast to these studies, another study found that inhibition of Hh signaling using the anti-Shh 5E1 antibody in primary patient derived pancreatic cancer xenografts at orthotopic sites did not result in attenuation of the stroma (40). These differences may be due to different levels of SHH production in the different models used, or alternatively it is possible that targeting different components of the Hh pathway may account for different effects of the tumor and its associated stroma (40). Recently an elegant study detailed the role of Gli1 in Kras induced carcinogenesis (41). Loss of Gli1 in the pancreatic epithelium blocked the progression of Kras induced PanIN lesions to pancreatic cancer. This effect was shown to be mediated by Gli1 regulation of IL-6. It was also shown that Kras induced Shh expression in tumor cells lead to activation of Gli1 in the stroma. Once activated, Gli1 was shown to bind to IL-6 promoter, thereby increasing its expression. IL-6 from the stroma then induced Stat3 activation in the tumor cells (41). This study shows a clear mechanistic link between SHH production by neoplastic epithelial cells and the induction of stromagenesis in the pancreas by an IL-6/Stat3 related mechanism (Figure 1).
Figure 1.
Model of tumor-stromal interactions: Kras induced Hedgehog signaling promotes the early steps of tumor development leading to the characteristic unyielding pancreatic desmoplastic reaction
Immunosuppressive cells in stroma
Immune cells are a significant part of pancreatic tumor associated stroma and can be seen early during PDAC development (9). PDAC creates a highly immunosupperessive tumor microenvironment through the production of inhibitory cytokines and recruitment of immunomodulatory cells (9). Recent reports have focused on the mechanism of recruitment of immunosuppressive cells to the site of tumor growth and the origin of immunosuppressive cells, also known as myeloid derived suppressor cells (MDSC). The increased presence of MDSC in bone marrow and peripheral circulation of patients with PDAC has been correlated with disease stage. Furthermore, targeting MDSCs with zoledronic acid (a potent aminobisphosphonate that is known to target MDSC) in a genetically engineered mouse model of pancreatic cancer improved the host anti-tumor response, delayed tumor growth rate, prolonged median survival, and increased recruitment of T cells to the tumor, suggesting it’s therapeutic potential (42). Two recent papers have demonstrated that Kras induced GM-CSF production from tumor cells leads to recruitment of MDSC to tumor microenvironment. GM-CSF was shown to be necessary and sufficient in recruiting and in driving the development of MDSC (43) (44). Another group has shown that pancreatic cancer associated stellate cells induce differentiation of MDSC in a Stat3 dependent manner via IL-6 signaling pathway (45). Therefore, both the tumor and its associated stroma in PDAC cooperate in suppressing the host immune response. As a result, targeting the immune compartment of the tumor microenvironment may be beneficial in enhancing the anti-tumor response.
Oncogenic Kras and the pancreatic cancer stroma
The use of genetically engineered mouse models of pancreatic cancer has opened the way to studying the initial steps of pancreatic tumorigenesis in the context of an intact microenvironment. The models that best represent the human disease rely on tissue-specific expression of an oncogenic form of Kras, KrasG12D, which is the most common mutation in human pancreatic cancer (7, 8). The most commonly used model combines the Kras mutation with a mutant form of the tumor suppressor p53, and is frequently referred to as KPC mouse (46). Analysis of the KPC mouse has shown that the accumulation of the fibrotic stroma and infiltrating immune cells accompanies the formation of pancreatic cancer from its early stages, represented by the precursor lesions known as PanINs (Pancreatic Intraepithelial Neoplasia) (47). Accumulation of the fibrotic stroma and the establishment on an immunosuppressive environment might be prerequisites for tumor progression. How these characteristics are established and maintained is still a matter of debate. Recent insight indicates that epithelial cells expressing oncogenic Kras provide signals that lead to accumulation of the stroma, proliferation of its components, and maintenance of the stroma over time. The recently described iKras* mice (48) express KrasG12D in a tissue-specific manner. In contrast with KPC mice, KrasG12D expression in these animals is inducible and reversible. Thus, oncogenic Kras expression can be inactivated at any time, and the effects of this inactivation both on the epithelial cells and the surrounding stroma can be evaluated. Similarly to KPC mice, PanIN formation in iKras* mice is accompanied by the accumulation of a fibroinflammatory stroma. Intriguingly, though, inactivation of Kras at the PanIN stage or in tumors is rapidly followed by suppression of proliferation within the stroma compartment, return of the fibroblasts to a quiescent state (characterized by loss of smooth muscle actin expression), and eventually remodeling of the extracellular matrix and elimination of the fibroblasts. In addition, the inflammatory infiltrates gradually decrease over time. The exact mechanisms mediating the relation between the oncogenic Kras-expressing tumor cells and the surrounding fibroblasts and immune cells is not fully elucidated. However, it is to be noted that Shh expression in the epithelial cells returns to basal (undetectable) levels upon Kras inactivation. It is however likely that multiple signaling pathways contribute to the changes in the stroma. In fact, the use of a reporter allele for the Hedgehog target gene Gli1 indicated that only a small subset of the fibroblasts within the stroma, and an even smaller subset of the immune cells, have active hedgehog signaling. Thus, Hedgehog signaling is not likely to be the only,nor the prevalent, signal mediating epithelial-mesenchymal interactions in pancreatic cancer. The evidence that Kras inhibition results in remodeling of the stroma implies that it might be possible to target the stroma though targeting the tumor cells. Another aspect to consider is that the signals produced by the stroma and necessary for tumor growth are largely unknown; if identified, those signals might constitute other potential therapeutic targets.
Stromal markers and prognosis
Stromal prognostic markers that may predict disease recurrence have recently received attention. The activated stroma index, defined as the ratio of myofibroblasts over collagen deposition, was shown to be an independent prognostic marker of survival in pancreatic cancer patients. A high stromal index as shown by increased number of myofibroblast cells correlated with poor survival, while in contrast, high collagen deposition was correlated with significantly better survival (49). These findings are intriguing, and suggest the possibility that the amount of stromal deposition may influence tumor behavior in a way not previously expected. Further functional studies will be needed to validate the relevance of this finding in an independent patient cohort.
The presence of tumor associated macrophages (TAM) and neutrophils (TAN) cells in the tumor microenvironment has been shown to be a poor prognostic marker (50, 51). Both TAM and TAN promote tumor cell invasiveness by breaking down ECM in PDAC (52). While some studies have focused on enumeration of different cell types within the pancreatic tumor micorenvironment to identify potential prognostic and therapeutic markers, others have focused on the proteins expressed in these different cell types. One such study identified expression of fibroblast associated protein (FAP) in tumor adjacent myofibroblasts cells and correlated it with poor prognosis (53). More recently, expression of the kindlin-2 focal adhesion protein in peritumoral cancer associated fibroblasts was associated with poor prognosis (54). Additional analysis will be needed to discern the utility of specifically targeting individual cell types and/or their products as part of emerging treatment regimens.
Current therapeutic stromal targets
While our understanding of tumor biology has facilitated the development of new cancer treatments, based on the growing body of data implicating a tumor-promoting role of the stroma, new therapeutic regimens including agents to target the stroma have been developed. In a recent paper by Provensano et. al., the authors found that targeting hyalouronidase decreased the high interstitial pressure present in tumors and improved chemotherapy delivery with significant inhibition of tumor growth in the KPC model. A similar observation was made by Jacobetz et, al.(55, 56). In addition, an anti-fibrotic drug, pirfenidone known to exert antifibrotic effects by inhibiting fibroblasts and the production of TGF-β, PDGF, and collagen type I and currently approved for treatment of patients with pulmonary fibrosis has recently been reported to inhibit tumor growth using a mouse model of PDAC (57). Further, recent studies in humans have demonstrated that treatment with nab paclitaxel (also known as Abraxane), thought to deplete the stroma through effects on SPARC inhibition, inhibited tumor growth in PDAC patients (5). A large, multi-center randomized Phase III prospective clinical trial was reported at ASCO GI this year showing that gemcitabine and Abraxane resulted in prolonged patient survival compared to gemcitabine alone (3). Analysis of accompanying tumor samples will provide useful information to confirm if this treatment regimen exerts its anti-tumor effects through SPARC or effects on the stromal content of the tumor.
MSC’s as a therapeutic tool to bypass stroma in pancreatic cancer
Cell based therapy is a novel therapeutic strategy for targeting solid tumors. MSCs in particular have shown promise as targeting moieties due to their ability to migrate and engraft to established tumor. Early studies using lentiviral vectors have demonstrated the ability of genetically modified, EGFP lentiviral transduced MSCs to migrate to sites of orthotopic tumors (58). This was followed up by a study demonstrating that intravenous injection of MSCs expressing a CCL5 promoter driven Herpes simplex virus thymidine kinase (HSV-Tk) gene inhibited primary tumor growth by 50% and reduced liver metastasis compared to control lentiviral transduced MSCs(59). In a recent study human pancreas derived MSCs were engineered with TRAIL to study its effects on pancreatic cancer cell lines. This study demonstrated that pancreas derived MSCs possess intrinsic ability to inhibit pancreatic cancer cells which can be potentiated by TRAIL (60).
Conclusions
Our understanding of pancreatic cancer is shifting from a disease of malignant tumor cells only towards a complex interactive tumor microenvironment (Figure 1). This recognition has increased the focus on the fibroinflammatory stroma of PDAC. It is clear that the extensive desmoplastic reaction observed in pancreatic cancer is a key feature of the biology of this disease. New therapeutic regimens are likely to incorporate agents which target components of the tumor microenvironment, including the stroma, along with the neoplastic epithelial compartment of the tumor. Further research is needed to best determine the optimal therapeutic approaches to most effectively target the heterogeneous cell populations in the tumor to eradicate the disease and improve patient survival.
Key points.
Pancreatic cancer is characterized by a dense stroma
The tumor promoting roles of stroma have been documented
Both the tumor and stroma promote immunosuppression
Stromal cells and proteins may serve as prognostic and/or therapeutic biomarkers
The stroma has emerged as a potential therapeutic target
Acknowledgements
We apologize to our colleagues whose work was not cited for reasons of space limitations. The authors do not have any financial conflicts of interest associated with this manuscript.
Funding: This work was funded by NIH grants R01CA1311045 and P50CA130810 (D.M.S.) and the Rogel Family Pancreatic Cancer Fund.
Abbreviations
- CAFS
cancer-associated fibroblasts
- MSCs
mesenchymal stem cells
- ECM
extracellular matrix
- PDAC
pancreatic ductal adenocarcinoma
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2. [accessed May 2013]; http:/seer.cancer.gov/statfacts/html/pancreas.html.
- 3.Drug Combo Effective against Pancreatic Cancer. Cancer Discov. 2013 Apr;3(4):OF8. doi: 10.1158/2159-8290.CD-NB2014-089. [DOI] [PubMed] [Google Scholar]
- 4.Gunturu KS, Yao X, Cong X, Thumar JR, Hochster HS, Stein SM, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol. 2013 Mar;30(1):361. doi: 10.1007/s12032-012-0361-2. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011 Dec 1;29(34):4548–54. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosein PJ, de Lima Lopes G, Jr., Pastorini VH, Gomez C, Macintyre J, Zayas G, et al. A phase II trial of nab-Paclitaxel as second-line therapy in patients with advanced pancreatic cancer. Am J Clin Oncol. 2013 Apr;36(2):151–6. doi: 10.1097/COC.0b013e3182436e8c. [DOI] [PubMed] [Google Scholar]
- 7.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008 Sep 26;321(5897):1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012 Nov 15;491(7424):399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007 Jul 1;101(4):887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 10.Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008 Apr 1;68(7):2085–93. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 11.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008 Feb 1;68(3):918–26. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009 Jun 12;324(5933):1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartel M, Di Mola FF, Gardini A, Zimmermann A, Di Sebastiano P, Guweidhi A, et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg. 2004 Aug;28(8):818–25. doi: 10.1007/s00268-004-7147-4. [DOI] [PubMed] [Google Scholar]
- 14.Ikenaga N, Ohuchida K, Mizumoto K, Cui L, Kayashima T, Morimatsu K, et al. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology. 2010 Sep;139(3):1041–51. 51 e1–8. doi: 10.1053/j.gastro.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 15.Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012 Feb;61(2):172–8. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010 Nov;177(5):2585–96. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, Hamada S, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010 Dec 17;403(3-4):380–4. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 18.Hamada S, Masamune A, Takikawa T, Suzuki N, Kikuta K, Hirota M, et al. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun. 2012 May 4;421(2):349–54. doi: 10.1016/j.bbrc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005 Nov 1;11(21):7749–56. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 20.Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008 Sep 28;269(1):67–77. doi: 10.1016/j.canlet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Lu YR, Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, et al. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008 Feb;7(2):245–51. doi: 10.4161/cbt.7.2.5296. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007 Feb;21(2):304–10. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 23.Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009 Apr;30(4):589–97. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 24.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007 Oct 4;449(7162):557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007 Feb 1;67(3):1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 26.Kabashima-Niibe A, Higuchi H, Takaishi H, Masugi Y, Matsuzaki Y, Mabuchi Y, et al. Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci. 2013 Feb;104(2):157–64. doi: 10.1111/cas.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003 Oct 23;425(6960):851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choy SW, Cheng SH. Hedgehog signaling. Vitam Horm. 2012;88:1–23. doi: 10.1016/B978-0-12-394622-5.00001-8. [DOI] [PubMed] [Google Scholar]
- 29.Marigo V, Johnson RL, Vortkamp A, Tabin CJ. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev Biol. 1996 Nov 25;180(1):273–83. doi: 10.1006/dbio.1996.0300. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997 Jul;124(13):2537–52. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- 31.Platt KA, Michaud J, Joyner AL. Expression of the mouse Gli and Ptc genes is adjacent to embryonic sources of hedgehog signals suggesting a conservation of pathways between flies and mice. Mech Dev. 1997 Mar;62(2):121–35. doi: 10.1016/s0925-4773(96)00648-x. [DOI] [PubMed] [Google Scholar]
- 32.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002 Oct;129(20):4753–61. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999 Sep;126(17):3915–24. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 34.Kogerman P, Grimm T, Kogerman L, Krause D, Unden AB, Sandstedt B, et al. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999 Sep;1(5):312–9. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz i Altaba A. Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development. 1999 Jun;126(14):3205–16. doi: 10.1242/dev.126.14.3205. [DOI] [PubMed] [Google Scholar]
- 36.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999 Mar 19;274(12):8143–52. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 37.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4254–9. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008 Sep 18;455(7211):406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 39.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008 Oct 1;14(19):5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang Q, Foltz WD, Chaudary N, Hill RP, Hedley DW. Tumor-stroma interaction in orthotopic primary pancreatic cancer xenografts during hedgehog pathway inhibition. Int J Cancer. 2013 Jul;133(1):225–34. doi: 10.1002/ijc.28006. [DOI] [PubMed] [Google Scholar]
- 41.Mills LD, Zhang Y, Marler RJ, Herreros-Villanueva M, Zhang L, Almada LL, et al. Loss of the Transcription Factor GLI1 Identifies a Signaling Network in the Tumor Microenvironment Mediating KRAS-Induced Transformation. J Biol Chem. 2013 Mar 12; doi: 10.1074/jbc.M112.438846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012 Sep;61(9):1373–85. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012 Jun 12;21(6):836–47. doi: 10.1016/j.ccr.2012.04.024. This study identified that Kras induces the expression of GMCSF which recruits MDSC to pancreatic cancer microenvironment leading to an immunosuppressive tumor environment
- 44•.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012 Jun 12;21(6):822–35. doi: 10.1016/j.ccr.2012.04.025. This study shows that tumor derived GM-CSF drives the generation of myeloid cells with suppressive activity on T cells and that this suppressive activity favors immune evasion
- 45.Mace TA, Ameen Z, Collins A, Wojcik SE, Mair M, Young GS, et al. Pancreatic cancer associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013 Mar 20; doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005 May;7(5):469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007 Oct 1;67(19):9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 48.Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012 Feb 1;122(2):639–53. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008 Oct;6(10):1155–61. doi: 10.1016/j.cgh.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002 Dec 19-26;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004 Jan;4(1):71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 52.Benson DD, Meng X, Fullerton DA, Moore EE, Lee JH, Ao L, et al. Activation state of stromal inflammatory cells in murine metastatic pancreatic adenocarcinoma. Am J Physiol Regul Integr Comp Physiol. 2012 May;302(9):R1067–75. doi: 10.1152/ajpregu.00320.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008 Aug;37(2):154–8. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 54.Mahawithitwong P, Ohuchida K, Ikenaga N, Fujita H, Zhao M, Kozono S, et al. Kindlin-2 expression in peritumoral stroma is associated with poor prognosis in pancreatic ductal adenocarcinoma. Pancreas. 2013 May;42(4):663–9. doi: 10.1097/MPA.0b013e318279bd66. [DOI] [PubMed] [Google Scholar]
- 55.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013 Jan;62(1):112–20. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012 Mar 20;21(3):418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozono S, Ohuchida K, Eguchi D, Ikenaga N, Fujiwara K, Cui L, et al. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013 Apr 1;73(7):2345–56. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- 58.Kallifatidis G, Beckermann BM, Groth A, Schubert M, Apel A, Khamidjanov A, et al. Improved lentiviral transduction of human mesenchymal stem cells for therapeutic intervention in pancreatic cancer. Cancer Gene Ther. 2008 Apr;15(4):231–40. doi: 10.1038/sj.cgt.7701097. [DOI] [PubMed] [Google Scholar]
- 59.Niess H, Bao Q, Conrad C, Zischek C, Notohamiprodjo M, Schwab F, et al. Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma. Ann Surg. 2011 Nov;254(5):767–74. doi: 10.1097/SLA.0b013e3182368c4f. discussion 74-5. [DOI] [PubMed] [Google Scholar]
- 60•.Moniri MR, Sun XY, Rayat J, Dai D, Ao Z, He Z, et al. TRAIL-engineered pancreas-derived mesenchymal stem cells: characterization and cytotoxic effects on pancreatic cancer cells. Cancer Gene Ther. 2012 Sep;19(9):652–8. doi: 10.1038/cgt.2012.46. This is the first study to use pancreatic mesenchymal stem cells of human origin. This study demonstrated Mesenchymal stem cells cytotoxic effects on pancreatic cancer cell lines