Summary
This study demonstrates that wild tobacco Nicotiana attenuata plants accumulate the phytoalexin scopoletin to defend against the necrotrophic fungus Alternaria alternata in a JA signalling-dependent manner.
Key words: Alternaria alternata, feruloyl-CoA 6ʹ -hydroxylase 1 (F6ʹH1), jasmonic acid (JA), MYC2, Nicotiana attenuata, scopoletin, virus-induced gene silencing (VIGS).
Abstract
Alternaria alternata (tobacco pathotype) is a necrotrophic fungus causing severe losses in Nicotiana species by infection of mature leaves. Similar to what has been observed in cultivated tobacco, N. tabacum, young leaves of wild tobacco, N. attenuata, were more resistant to A. alternata than mature leaves, and this was correlated with stronger blue fluorescence induced after infection. However, the nature of the fluorescence-emitting compound, its role in defence, and its regulation were not clear. Silencing feruloyl-CoA 6ʹ-hydroxylase 1 (F6ʹH1), the gene encoding the key enzyme for scopoletin biosynthesis, by virus-induced gene silencing (VIGS) revealed that the blue fluorescence was mainly emitted by scopoletin and its β-glycoside form, scopolin. Further analysis showed that scopoletin exhibited strong antifungal activity against A. alternata in vitro and in vivo. Importantly, jasmonic acid (JA) levels were highly elicited in young leaves but much less in mature leaves after infection; and fungus-elicited scopoletin was absent in JA-deficient plants, but was largely restored with methyl jasmonate treatments. Consistent with this, plants strongly impaired in JA biosynthesis and perception were highly susceptible to A. alternata in the same way scopoletin/scopolin-depleted VIGS F6ʹH1 plants. Furthermore, silencing MYC2, a master regulator of most JA responses, reduced A. alternata-induced NaF6ʹH1 transcripts and scopoletin. Thus, it is concluded that JA signalling is activated in N. attenuata leaves after infection, which subsequently regulates scopoletin biosynthesis for the defence against A. alternata partly through MYC2, and higher levels of scopoletin accumulated in young leaves account for their strong resistance.
Introduction
In many plant–pathogen systems, the resistance of plants usually depends on the developmental stage at which the plant is infected. Plants are generally more susceptible to disease in early than in late phases, including rice against Xanthomonas oryzae, tobacco to Phytophthora parasitica, and Arabidopsis to Pseudomonas syringae (Century et al., 1999; Hugot et al., 1999; Kus et al., 2002; Develey-Riviere and Galiana, 2007). However, this is not true in the Nicotiana tabacum–Alternaria alternata interaction (Cheng and Sun, 2001; Zhang et al., 1998).
Alternaria alternata (tobacco pathotype) is a necrotrophic fungus causing brown spot disease in N. tabacum (LaMondia, 2001). The disease usually occurs in mature leaves (Zhang et al., 1998; Cheng and Sun, 2001). It has been shown that young leaves of tobacco are highly resistant to A. alternata, while they gradually lose their resistance as leaves become mature (Zhang et al., 1998; Cheng and Sun, 2001). However, the reason for this age-dependent susceptibility is not clear. It may involve regulation of R genes, activation of different phytohormone signalling pathways, and accumulation of antifungal chemicals (Develey-Riviere and Galiana, 2007).
By using wild tobacco, Nicotiana attenuata, as a model plant, it was also observed that young source–sink transition leaves are more resistant to A. alternata than all fully expanded leaves, which is correlated with less abscisic acid (ABA) accumulated in older leaves (Sun et al., 2014). This ABA distribution pattern at least partially contributed to the observed age-dependent susceptibility, since ABA-dependent stomatal closure is required for the resistance to A. alternata in wild tobacco (Sun et al., 2014).
Interestingly, strong blue fluorescence was observed around the infection zone of N. attenuata leaves under UV light in this study, suggesting that some specific secondary metabolites were accumulated after infection. Whether this A. alternata-induced blue fluorescence is due to scopoletin, a blue autofluorescent compound accumulating in Nicotiana species after pathogen attack (Chong et al., 2002; El Oirdi et al., 2010), is not known. More importantly, the intensity of the blue fluorescence was 2- to 3-fold higher in source–sink transition leaves than in fully expanded leaves, indicating that this accumulation pattern may account for the age-dependent susceptibility. However, the nature of this fluorescence-emitting compound, its role in defence, and its regulation were not known.
Phytoalexins are antimicrobial substances of low molecular weight produced by plants in response to pathogen attack, including camalexin, the major substance in Arabidopsis, kauralexin and zealexin in maize, and scopoletin and capsidiol in N. tabacum (Ahuja et al., 2012).
Scopoletin, a phenolic coumarin deriving from the phenylpropanoid pathway with strong blue fluorescence under UV light (Kai et al., 2006, 2008), can be isolated from many plant species (Murray et al., 1982), and was proposed as an important phytoalexin against microbial pathogens (Gnonlonfin et al., 2012). Using T-DNA insertion mutants of caffeoyl CoA O-methyltransferase1 and feruloyl-CoA 6ʹ-hydroxylase 1 (F6ʹH1) in Arabidopsis, Kai et al. (2008) demonstrated that scopoletin biosynthesis is strongly dependent on F6ʹH1, and feruloyl-CoA is the key precursor. In addition, scopoletin levels are also influenced by the levels of scopoletin glucosyltransferase in tobacco (Chong et al., 2002; Gachon et al., 2004; Simon et al., 2014).
In good agreement with its role in defence, scopoletin increases its level dramatically after fungal challenge, and exhibits fungitoxicity in vitro (Goy et al., 1993; Garcia et al., 1995; Valle et al., 1997; Churngchow and Rattarasarn, 2001; Silva et al., 2002; Carpinella et al., 2005; El Oirdi et al., 2010; Gnonlonfin et al., 2012). Moreover, the resistance of some plant species against fungal pathogens is correlated with the rapidity and intensity of scopoletin accumulation (Goy et al., 1993; Garcia et al., 1995; Churngchow and Rattarasarn, 2001; El Oirdi et al., 2010). These results represent a very important step in evaluating the defensive function of scopoletin; however, important experiments using scopoletin-depleted plants are still lacking. Ideally, the benefits of a putative defence trait should be determined in plants differing only in a single gene that controls the resistance trait and are otherwise identical (Bergelson et al., 1996). In this study, virus-induced gene silencing (VIGS) of F6ʹH1 was used to manipulate the production of scopoletin in order to investigate its role in the resistance of N. attenuata to A. alternata.
Here it is reported that A. alternata-induced blue fluorescence in N. attenuata leaves was mainly due to scopoletin and scopolin. Scopoletin possessed antifungal activity against the fungus in vitro and in vivo, and its production was demonstrated to be dependent on jasmonic acid (JA) signalling. Higher levels of this JA-dependent scopoletin in young leaves were one of the main reasons for their strong resistance.
Materials and methods
Plant and fungal materials
Seeds of the 31st generation of an inbred line of N. attenuata were used as the wild-type (WT) genotype. Stably transformed lines of irNaAOC (Kallenbach et al., 2012), irNaCOI1 (Paschold et al., 2007), and irNaMPK4 (Hettenhausen et al., 2012) were used as plants that were silenced in the expression of N. attenuata allene oxide cyclase (NaAOC; the gene encoding the key enzyme of JA biosynthesis), NaCOI1 (encoding the JA-Ile receptor), and NaMPK4. Seed germination and plant growth were conducted as described by Krügel et al. (2002).
Alternaria alternata were grown and inoculated as described by Sun et al. (2014).
Isolation of NaF6ʹH1
The 1275bp cDNA sequence of NaF6ʹH1 (accession no. KF771989) was amplified by primers Js144_F, 5ʹ-ACAAAAATG CCTACTACAGTCTCA-3ʹ; and Js145_R, 5ʹ- TCCAGTCCAAAAT TCAGACACCT-3ʹ, the design of which was based on the sequence similarity to the tobacco expressed sequence tag (EST) EB446976, the EST FS420915, and AtF6ʹH1 (At3g13610). Subsequently the cDNA fragment was cloned into a pMD18-T vector (Takara, www.takara.com.cn) and sequenced.
Generation of VIGS plants
A 235bp fragment of the NaF6ʹH1 cDNA sequence, amplified by primers JS138_F, 5ʹ- CAAAGAGGATTAACGTTAACTACTA-3ʹ; and JS140_R, 5ʹ- CTCTTGTATCGTCCATTGCTCATTAT-3ʹ was cloned into pTV00 (Ratcliff et al., 2001), and Agrobacterium tumefaciens (strain GV3101) carrying these constructs were inoculated into N. attenuata, generating NaF6ʹH1-silenced plants (VIGS NaF6ʹH1).The A. tumefaciens-mediated transformation procedure was performed as described previously (Saedler and Baldwin, 2004; Wu et al., 2008). To monitor the progress of VIGS, phytoene desaturase (PDS) was silenced; this is a gene that oxidizes and cyclizes phytoene to α- and β-carotene, compounds that are converted into the xanthophylls of the antenna pigments of the photosystems of plants. Silencing PDS eventually results in the visible bleaching of green tissues (Saedler and Baldwin, 2004; Wu et al., 2008) ~2–3 weeks after the inoculation. When the leaves of PDS-silenced plants began to bleach and plants started bolting, the youngest rosette leaves of VIGS NaF6ʹH1 and empty vector-inoculated plants (EV plants) were selected for further experiments as source–sink transition leaves were hard to distinguish in VIGS plants. Around 40 plants were inoculated with EV and VIGS NaF6ʹH1 constructs, five biological replicates per genotype were used for experiments, and all VIGS experiments were repeated twice.
VIGS NaMYC2 plants were generated as described by Woldemariam et al. (2013)
Bioassays for the inhibition of A. alternata growth by scopoletin in vitro and in vivo
The inhibition of A. alternata mycelium growth by scopoletin in vitro was tested in Petri dishes by subculturing a mycelium plug of 3mm diameter on PDA (potato dextrose agar) medium containing various concentrations of scopoletin for 6 d in the dark at 25 °C. A 50mg aliquot of scopoletin (Sigma) was dissolved in 5ml of methanol, and then added to the PDA medium at concentrations of 0, 48, 96, 240, and 480 μg ml–1. PDA plates supplied with 1% methanol served as controls. The area of mycelium growth was recorded every 2 d.
To test the role of scopoletin in planta, fully expanded +3 leaves (Sun et al., 2014) were supplied through petiole feeding for 7h with 0, 100, and 500 μM scopoletin and then used for infection. These leaves usually had less fungus-induced blue fluorescence and were more susceptible to A. alternata. As indicators of the severity of disease symptoms, the diameters of the developing lesions at 5 days post-infection (dpi) were recorded.
Quantification of blue fluorescence intensity, scopoletin, and scopolin
Leaf samples of ~0.1g were harvested from the inoculation site, and were ground in liquid nitrogen. A 1ml aliquot of 70% methanol with 1000ng ml–1 internal standard 4-methylumbelliferone was added to each sample. Supernatants were collected after vortexing and centrifugation at 15 000 g for 20min. Samples were then subjected to analysis by microplate reader and high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS; Thermo Scientific TSQ Quantum Access MAX).
The blue fluorescence intensities were determined by a microplate reader (Tecan infinite M200 PRO) at an excitation wavelength of 320nm and an emission wavelength of 420nm by comparing them with a standard curve of scopoletin (Sigma)
The levels of scopoletin and scopolin were determined by HPLC-MS/MS according to a method modified from Kai et al. (2006). Samples were separated by a Hyperil gold C18 column (Thermo), with H2O containing 0.1% (v/v) formic acid as solvent A and methanol containing 0.1% (v/v) formic acid as solvent B, at a flow rate of 0.2ml min–1. Elution was started with isocratic conditions of 15% solvent B for 2min, followed by a linear gradient flow up to 55% within 18min. The detection of scopoletin was set at m/z 193/133, scopolin at m/z 355/193, and 4-methylumbelliferone at m/z 177/77. The levels of scopoletin and scopolin were quantified by comparing their peak area with those of the internal standard.
Analysis of JA
JA was extracted and quantified by LC-MS/MS as described by Wu et al. (2008).
Real-time PCR
Total RNA was extracted from ground leaf samples using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. For quantitative PCR analysis, five replicate biological samples were used. cDNA was synthesized from 500ng of total RNA with reverse transcriptases (Thermo Scientific, http://www.thermoscientificbio.com). Real-time PCR was performed as described (Sun et al., 2014) on a Biorad CFX Connect qPCR System (Biorad, http://www.bio-rad.com) with iTaq Universal SYBR Green Supermix (Biorad) and gene-specific primers (Supplementary Table S1 available at JXB online) according to the manufacturer’s instructions.
Results
Alternaria alternata-induced blue fluorescence and NaF6ʹH1 transcripts in N. attenuata
Previously, it was reported that young source–sink transition leaves (0 leaves) of wild tobacco N. attenuata are more resistant to A. alternata than all the fully expanded leaves including +3 leaves (Sun et al., 2014). In addition to ABA signalling-mediated stomatal immunity (Sun et al., 2014), it was hypothesized that young source–sink transition leaves may accumulate higher amounts of antifungal chemicals than fully expanded leaves do after infection, and this accumulation pattern may account for the age-dependent susceptibility.
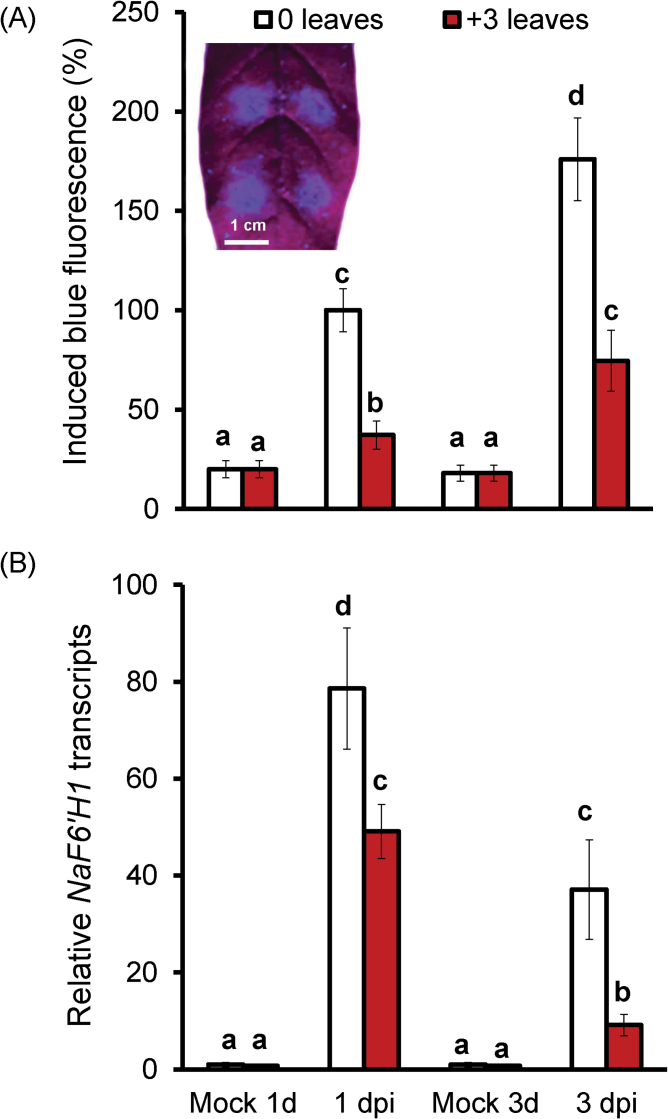
Strong blue fluorescence was observed around the infection zones in 0 leaves at 1 dpi, and the fluorescence intensity was even higher at 3 dpi (Fig. 1A). However, in fully expanded +3 leaves, which are more susceptible to A. alternata (Sun et al., 2014), only around one-third of the blue fluorescence intensity that accumulated in 0 leaves was observed at both 1 and 3 dpi (Fig. 1A), indicating that some compounds capable of emitting blue fluorescence under UV light were highly induced after fungal challenge, and these chemicals may contribute to the resistance to A. alternata.
Fig. 1.
Alternaria alternata-induced blue fluorescence and NaF6′H1 transcripts in N. attenuata. (A) Relative blue fluorescence intensity induced by A. alternata in four replicates of 0 and +3 leaves at 1 and 3 dpi. The mean (±SE) level of the intensity in 0 leaves at 1 dpi was arbitrarily set as 100%. Inset: one 0 leaf was inoculated with four agar plugs with active A. alternata mycelium for 1 d, and then agar plugs were removed in order to photograph the leaves under UV light. Strong blue fluorescence was observed around the four inoculation sites. (B) Mean (±SE) NaF6ʹH1 transcripts were measured by real-time PCR in five replicates of 0 and +3 leaves infected with A. alternata at 1 and 3 dpi. The level of NaF6ʹH1 transcripts in 0 leaves with mock 1 d treatment was arbitrarily set as 1. Leaves inoculated with an PDA agar plug only (without A. alternata) for 1 and 3 d served as controls. Different letters indicated significant differences between each treatment group (one-way ANOVA, P<0.05).
Kai et al. (2008) demonstrated that scopoletin biosynthesis is strongly dependent on F6ʹH1 in Arabidopsis roots. A cDNA which displayed a high sequence similarity to F6ʹH1 in Arabidopsis was cloned in N. attenuata, and it is referred to as NaF6ʹH1 (GenBank accession no. KF771989; Supplementary Fig. S1 at JXB online). NaF6ʹH1 transcripts were dramatically induced after infection (Fig. 1B); young 0 leaves at 1 dpi accumulated ~80 times more NaF6ʹH1 transcripts than did mock controls. Transcripts of NaF6ʹH1 were also highly induced in +3 leaves, but its levels were significantly lower than those of 0 leaves at both 1 and 3 dpi.
Silencing NaF6ʹH1 reduces scopoletin levels and plant resistance
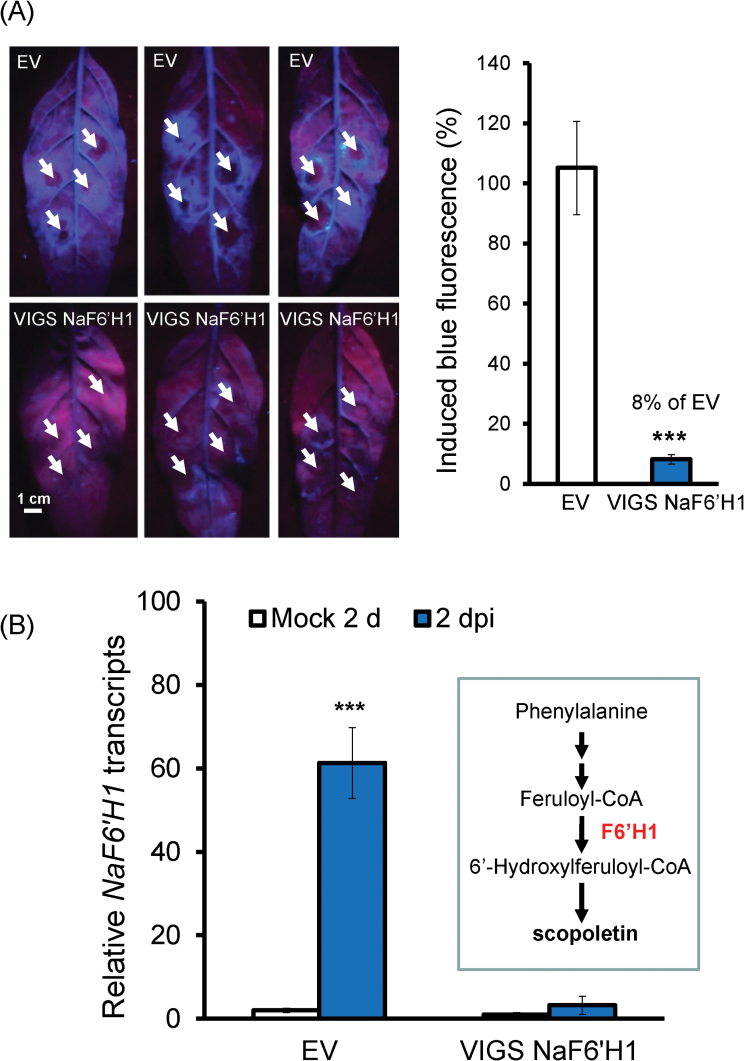
To understand whether the A. alternata-induced blue fluorescence was emitted by scopoletin, NaF6ʹH1, the candidate key enzyme gene for scopoletin biosynthesis, was silenced (Fig. 2B) to generate scopoletin-depleted N. attenuata plants by VIGS.
Fig. 2.
Alternaria alternata-elicited blue fluorescence and NaF6ʹH1 transcripts in EV and VIGS NaF6′H1 plants. (A) Left: three independent young leaves from EV and VIGS NaF6ʹH1 plants were infected with A. alternata. Photographs were taken from their underside under UV light at 5 dpi; strong blue fluorescence was emitted around the four inoculation sites (indicated by white arrows) of each leaf in EV but not in VIGS NaF6ʹH1 plants. Right: quantification of blue fluorescence intensity induced by A. alternata at 5 dpi in four replicates of leaves of EV and VIGS NaF6ʹH1 plants. The level of the intensity in EV plants was arbitrarily set as 100%. Asterisks indicate the level of significant differences between EV and VIGS NaF6ʹH1 plants (Student’s t-test: ***P<0.005). (B) Mean (±SE) relative A. alternata-induced NaF6ʹH1 transcript levels as measured by real-time PCR in four replicates of leaves of EV and VIGS NaF6′H1 plants at 2 dpi. Inset: schematic depiction of the scopoletin biosynthetic pathway. Asterisks indicate the level of significant differences between mock and infected samples in EV plants (Student’s t-test: ***P<0.0001).
At 5 dpi, strong blue fluorescence was observed around the infection zone of young leaves in plants transformed with the empty vector (EV plants), but only 8% of the fluorescence intensity of EV plants was detected in plants transformed with the NaF6ʹH1-silenced construct (VIGS NaF6ʹH1 plants; Fig. 2A). The transcriptional levels of NaF6ʹH1 in VIGS NaF6ʹH1 plants did not increase significantly after elicitation, and were reduced by 95% when compared with EV plants at 2 dpi (Fig. 2B), indicating that silencing of NaF6ʹH1 was effective and the fungus-elicited fluorescence was dependent on NaF6ʹH1.
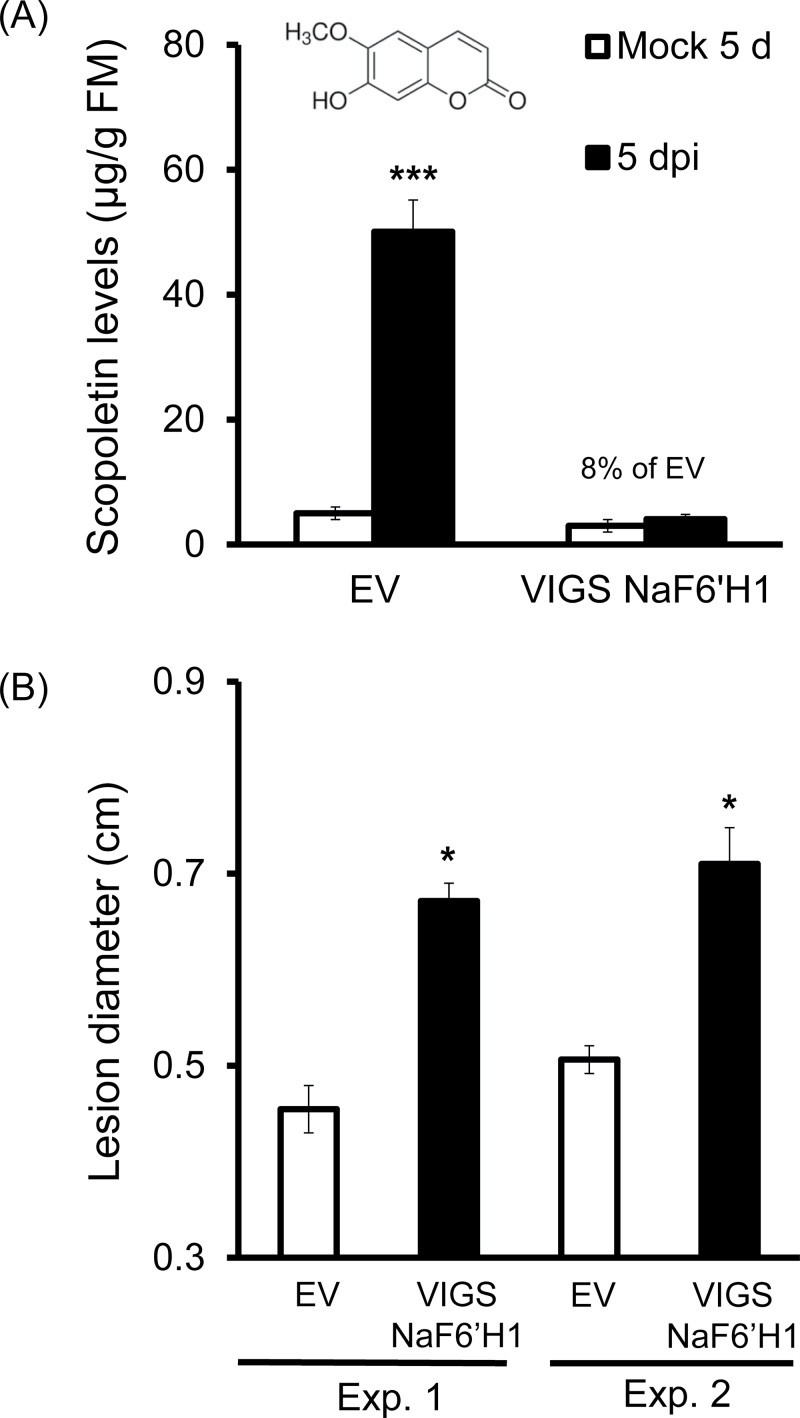
The amounts of fungus-elicited scopoletin and its β-glycoside scopolin, both of which are capable of emitting blue fluorescence, were quantified by LC-MS/MS. About 50 μg of scopoletin g–1 fresh leaf mass were detected in EV leaves at 5 dpi; in contrast, only ~4 μg g–1 fresh leaf mass were found in VIGS NaF6ʹH1 leaves with the same treatment (Fig. 3A). At the same time, scopolin accumulated to 72.5 μg g–1 fresh leaf mass in EV leaves but only one-fifth of these levels were detected in VIGS NaF6ʹH1 leaves (Supplementary Fig. S2A at JXB online). The total blue fluorescence intensity was also quantified by a microreader, and the results revealed that the total fluorescence intensity was equivalent to the amount of chemicals emitted by ~110 μg g–1 fresh leaves of scopoletin, suggesting that the A. alternata-induced blue fluorescence was mainly due to scopoletin and scopolin.
Fig. 3.
Silencing NaF6ʹH1 reduced A. alternata-induced scopoletin levels and plant resistance. (A) Mean (±SE) scopoletin levels were determined by LC-MS/MS in five replicates of young leaves of EV and VIGS NaF6ʹH1 infected with A. alternata for 5 d. Asterisks indicate the level of significant differences between mock and infected samples in EV plants (Student’s t-test: ***P<0.005). (B) Mean (±SE) diameter of necrotic lesions in four replicates of young leaves of EV and VIGS NaF6ʹH1 infected with A. alternata for 7 d. Two independent VIGS experiments are presented showing similar results. The asterisk indicates the level of significant difference between EV and VIGS NaF6ʹH1 leaves (Student’s t-test: *P<0.05).
Importantly, two independent VIGS experiments showed that pathogen-induced necrotic lesions were significantly larger in VIGS NaF6ʹH1 plants than in EV plants when the youngest rosette leaves were infected with A. alternata (Fig. 3B).
All these results strongly indicated that the blue fluorescence induced by A. alternata was NaF6ʹH1 dependent, and was mainly due to scopoletin and scopolin, and that these compounds are important for the resistance against A. alternata.
The inhibition of A. alternata growth in vitro and in vivo by scopoletin
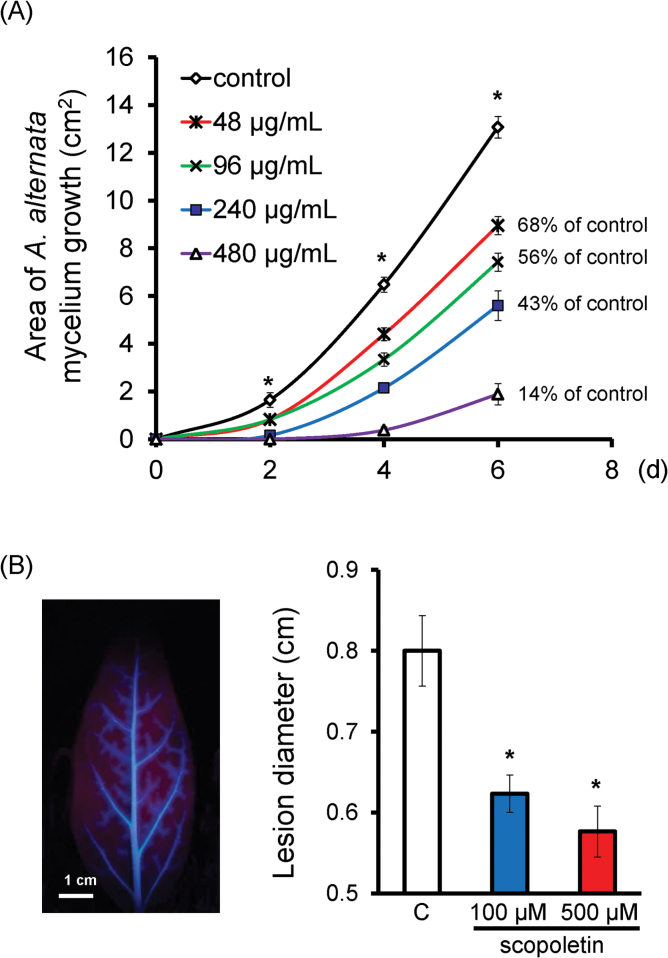
To confirm further the role of scopoletin in the resistance of N. attenuata to A. alternata, it was next tested whether it could restrict fungal growth on PDA medium and in planta. Mycelium growth of A. alternata on PDA plates with 48 μg ml–1 or 96 μg ml–1 scopoletin, concentrations more or less equal to physiological levels, was decreased to 68% or 56% of that of control plates, and it was reduced further to 43% and 14% in plates supplied with 240 μg ml–1 and 480 μg ml–1 scopoletin, respectively (Fig. 4A), confirming that scopoletin has a large impact on fungal growth in vitro, as reported (Garcia et al., 1995; Valle et al., 1997; Churngchow and Rattarasarn, 2001; Silva et al., 2002; Carpinella et al., 2005). When +3 leaves, which accumulate less scopoletin (Fig. 1A) and are more susceptible to A. alternata (Sun et al., 2014), were fed with 100 μM or 500 μM scopoletin through the petioles for 7h and then used for infection, they developed significantly smaller lesions than control leaves (Fig. 4B), indicating that exogenously supplied scopoletin enhanced plant resistance to A. alternata.
Fig. 4.
Inhibition of A. alternata growth in vitro and in vivo. (A) Area of A. alternata mycelium growth on PDA supplied with 0, 48, 96, 240, or 480 μg ml–1 scopoletin; data were collected every 2 d. (B) Left panel: a detached +3 leaf was fed with 500 μM scopoletin for 7h, and then the photograph was taken under UV light. Right panel: mean diameter (±SE) of necrotic lesions in four replicates of +3 leaves at 5 dpi, which were pre-treated with water or with 100 μM or 500 μM scopoletin for 7h before inoculation. Asterisks indicate the level of significant difference between control and treated samples (Student’s t-test: *P<0.05).
Alternaria alternata-induced scopoletin biosynthesis is strongly dependent on JA but not ABA signalling
Previously it was shown that ABA signalling plays an important role in the resistance of N. attenuata to A. alternata through stomatal immunity (Sun et al., 2014). Feeding with 10 μM ABA for 4h, which is effective in inducing stomatal closure responses and resistance to A. alternata (Sun et al., 2014), had little effect on A. alternata-elicited scopoletin levels (Supplementary Fig. S3A at JXB online). The fungus-induced blue fluorescence intensity at 1 dpi was slightly reduced in 0 leaves but not in +3 leaves in irNaMPK4 (ABA-insensitive) plants (Supplementary Fig. S3B). These results suggested that ABA signalling had a minor effect on the A. alternata-elicited scopoletin production.
It is commonly believed that plant defence responses to necrotrophic pathogens are regulated by JA signalling (Glazebrook, 2005). Thus pathogen-induced JA levels were measured by LC-MS/MS, and JA mutants generated previously by RNA interference (RNAi) in N. attenuata were also selected to verify the involvement of JA signalling in scopoletin production and defence against A. alternata.
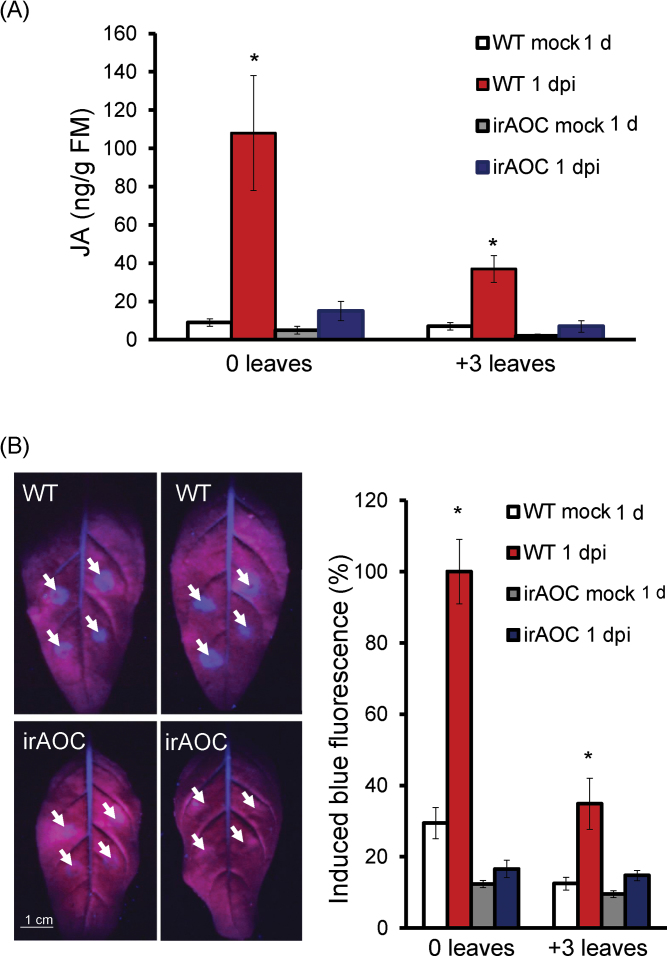
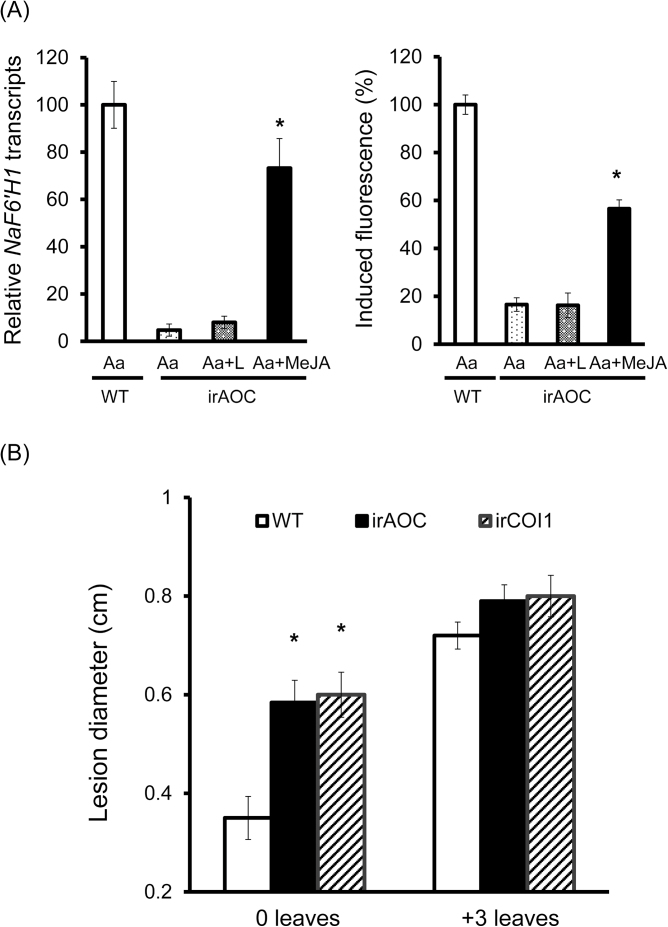
In response to A. alternata infection at 1 dpi, N. attenuata plants increased their JA levels dramatically in 0 leaves (increased from 10ng g–1 to 100ng g–1 fresh leaves; Fig. 5A), but much less in +3 leaves, where JA levels were also significantly elicited but were only around one-third of those of 0 leaves (Fig. 5A). However, the induction of JA was almost abolished in plants silenced in AOC (irAOC; Fig. 5A), which has been demonstrated to be the gene encoding the key enzyme leading to the formation of JA (Kallenbach et al., 2012). Importantly, A. alternata-elicited blue fluorescence at 1 dpi was completely abolished in irAOC plants (Fig. 5B), indicating that the fungus-induced scopoletin production was dependent on JA. Indeed, not only the levels of blue fluorescence but also NaF6ʹH1 transcripts were not elicited at all in irAOC plants (Fig. 6A). However, when irAOC plants were treated with methyl jasmonate (MeJA), which was rapidly metabolized to JA by methyl jasmonate esterase in planta (Wu et al., 2008), the levels of both A. alternata-elicited blue fluorescence and NaF6ʹH1 transcripts were largely restored (Fig. 6A). These results demonstrated that A. alternata-induced JA production was indeed very important for scopoletin biosynthesis.
Fig. 5.
Alternaria alternata-induced JA is required for the fungus-elicited scopoletin production. (A) Mean (±SE) JA levels elicited by A. alternata at 1 dpi determined by LC-MS/MS in five replicates of 0 and +3 leaves of WT and irAOC plants. (B) Left: two 0 leaves from WT and irAOC plants were infected with A. alternata for 1 d. Photographs were taken from their underside under UV light; strong blue fluorescence was emitted around four inoculation sites (indicated by white arrows) of each leaf in WT plants but not in irAOC plants. Right: mean (±SE) relative blue fluorescence intensity induced by A. alternata at 1 dpi in five replicates of 0 leaves of WT or irAOC plants. The level of intensity in 0 leaves of WT plants was arbitrarily set as 100%. Asterisks indicate the level of significant differences between mock and infected samples (Student’s t-test: *P<0.05).
Fig. 6.
MeJA largely restores A. alternata-elicited responses in JA-deficient plants, and both JA biosynthesis and perception are required for plant resistance. (A) Left: mean (±SE) NaF6ʹH1 transcripts as measured by real-time PCR in four replicates of 0 leaves of WT and irAOC plants infected with A. alternata for 1 d. Right: mean (±SE) relative blue fluorescence intensity induced by A. alternata at 1 dpi in five replicates of leaves of WT and irAOC plants. The levels of NaF6′H1 transcripts and blue fluorescence intensity in 0 leaves of WT plants was arbitrarily set as 100%. All leaves were inoculated with A. alternata (Aa), five leaves of irAOC were additionally treated with lanolin (Aa+L), and another five leaves were treated with 150 μg of MeJA in lanolin paste (Aa+MeJA). Asterisks indicate the level of significant difference between lanolin and MeJA-treated leaves (Student’s t-test: *P<0.05). (B) Mean (±SE) diameter of necrotic lesions of five replicates of 0 and +3 leaves of WT, irCOI1, and irAOC plants at 5 dpi. Asterisks indicate the level of significant differences between WT and irCOI1 or irAOC plants (Student’s t-test: *P<0.05).
To confirm further the role of JA signalling for fungus-induced scopoletin, plants silenced with COI1 previously generated by Paschold et al. (2007) were also tested. As expected, the fungus-elicited scopoletin levels in irCOI1 were the same as those in irAOC plants at 1 dpi, which were similar to the levels in WT mock controls (Supplementary Fig. S2B at JXB online), suggesting that the perception of JA signalling is also very important for the induction of scopoletin.
Consistent with the role of scopoletin in the resistance of A. alternata, irAOC and irCOI1 plants were also shown to be more susceptible to the fungus by developing larger lesions in 0 leaves (Fig. 6B), where more JA and scopoletin were elicited. In contrast, lesion diameters were almost similar in +3 leaves (Fig. 6B), most probably because JA signalling was less induced and overall scopoletin levels were lower in these leaves.
MYC2 is involved in the regulation of scopoletin biosynthesis
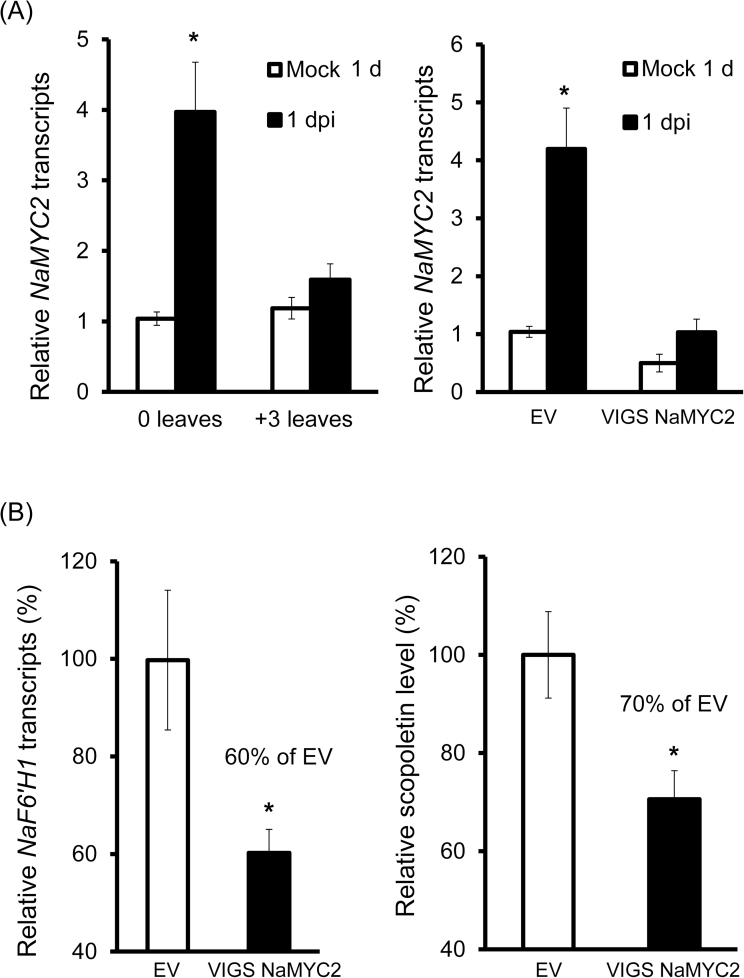
MYC2, a member of the basic helix–loop–helix (bHLH) family of transcription factors, participates in the regulation of many JA-dependent defences against insect herbivores and pathogens (Lorenzo et al., 2004; Dombrecht et al., 2007; Woldemariam et al., 2013). Consistent with the induction of JA at 1 dpi, the levels of MYC2 transcripts were increased to 4-fold in 0 leaves compared with mock control, but remained unchanged in +3 leaves (Fig. 7A, left panel).
Fig. 7.
Silencing NaMYC2 affects A. alternata-elicited levels of NaF6ʹH1 transcripts and scopoletin. (A) Left: mean (±SE) NaMYC2 transcripts were measured by real-time PCR in four replicates of 0 and +3 leaves of the WT at 1 dpi. Right: mean (±SE) NaMYC2 transcripts were measured by real-time PCR in four replicates of the youngest rosette leaves of EV and VIGS NaMYC2 plants at 1 dpi. (B) Left: mean (±SE) NaF6ʹH1 transcripts were measured by real-time PCR in four replicates of the youngest rosette leaves of EV and VIGS NaMYC2 plants at 1 dpi. Right: relative mean (±SE) scopoletin levels were determined by LC-MS/MS in five replicates of the youngest rosette leaves of EV and VIGS NaMYC2 plants at 1 dpi. The level in EV plants was arbitrarily set as 100%. Asterisks indicate the level of significant differences between mock and infected plants at 1 dpi or between EV and VIGS NaMYC2 plants (Student’s t-test: *P<0.05; ***P<0.0001).
To investigate whether MYC2 was involved in the regulation of scopoletin biosynthesis, MYC2 was also silenced by VIGS in this study. Alternaria alternata infection increased MYC2 transcripts significantly by 4-fold as expected in EV plants, but not in VIGS MYC2 plants at 1 dpi (Fig. 7A, right panel), suggesting that the MYC2 gene was successfully silenced in VIGS MYC2 plants. Meanwhile, NaF6ʹH1 transcripts were reduced by 40%, and scopoletin levels were also reduced by 30% in VIGS MYC2 plants when compared with EV plants at 1 dpi (Fig. 7B). These results indicated that MYC2 was involved in the regulation of scopoletin biosynthesis.
Discussion
Alternaria alternata is a necrotrophic fungus causing brown spot disease in N. tabacum (LaMondia, 2001) and wild tobacco N. attenuata (Sun et al., 2014). Similar to cultivated tobacco (Zhang et al., 1998; Cheng and Sun, 2001), young leaves of N. attenuata are more resistant to A. alternata than mature leaves (Sun et al., 2014). Previous work has demonstrated that ABA signalling-mediated stomata-based defence partially contributes to this age-dependent susceptibility (Sun et al., 2014), and now the new data presented here reveal that a higher level of scopoletin accumulated in young leaves is another main reason for this.
Scopoletin, a phenolic coumarin, has been proposed to have an important role in defending against fungal pathogens (Goy et al., 1993; Garcia et al., 1995; Valle et al., 1997; Churngchow and Rattarasarn, 2001; Silva et al., 2002; Carpinella et al., 2005; El Oirdi et al., 2010; Gnonlonfin et al., 2012). However, important evidence regarding whether scopoletin/scopolin-depleted plants were more susceptible was lacking. The present data strongly indicate that N. attenuata plants accumulate scopoletin and scopolin as defence compounds against A. alternata, since their levels were dramatically increased after A. alternata infection (Figs 1A, 3A); 0 leaves accumulated more and they were more resistant (Figs 1A, 6B); scopoletin exhibited fungal toxicity to A. alternata in planta and in vitro (Fig. 4); and, more importantly, scopoletin/scopolin-depleted plants, which were generated by silencing NaF6ʹH1, were more susceptible to A. alternata (Fig. 3B).
The results of silencing NaF6ʹH1 also indicated that the A. alternata-elicited blue fluorescence was due mainly to scopoletin and scopolin. However, further analysis is needed to exclude the possibility of the involvement of other NaF6ʹH1-based courmarins including esculetin and esculin, which are also capable of emitting blue fluorescence under UV light. Because there is no direct evidence of an effect of scopolin on the restriction of fungal growth, whether scopolin plays a role in the resistance against A. alternata is still not known. However, it may be converted back to scopoletin in planta. In tobacco, silencing or overexpression of a scopoletin glucosyltransferase affects scopoletin and scopolin levels and also plant resistance to Tobacco mosaic virus (Chong et al., 2002; Gachon et al., 2004). It will be interesting to find the de-glucosylation enzyme responsible for the conversion of scopolin to scopoletin, and to test its role in defence.
The formation of scopoletin is induced by various stress or phytohormone treatments (Gnonlonfin et al., 2012). Induction of cytokinin biosynthesis in tobacco enhances resistance to the hemibiotrophic pathogen Pseudomonas syringae pv tabaci by increasing scopoletin and capsidiol independent of JA and salicylic acid (SA) signalling (Grosskinsky et al., 2011). However, whether cytokinin is also involved in the regulation of scopoletin in N. attenuata against A. alternata needs further investigation. In Arabidopsis, the levels of scopoletin in the shoots increase after treatment with 2,4-dichlorophenoxyacetic acid (2,4-D), whereas other plant hormones such as SA, MeJA, and kinetin have no effects (Kai et al., 2006). Consistent with this, the present results also showed that MeJA alone cannot elicit scopoletin production (Supplementary Fig. S5 at JXB online), but these findings contradict what has been observed in tobacco suspension culture (Sharan et al., 1998), suggesting that MeJA-induced responses in intact plants differ from those in cell cultures.
Compared with defence responses induced by SA, JA-regulated genes are more involved in the resistance against necrotrophic pathogens (Glazebrook, 2005); for example, JA-insensitive coi1 mutants of Arabidopsis are highly susceptible to the necrotrophic fungi Alternaria brassicicola and Botrytis cinerea (Spoel et al., 2007; Rowe et al., 2010). To a large extent, B. cinerea-elicited camalexin is controlled by JA signalling (Rowe et al., 2010). However, whether JA plays a role in the regulation of scopoletin in the resistance of N. attenuata to A. alternata is not known.
In wild tobacco N. attenuata, JA biosynthesis starts with the release of trienoic fatty acids [e.g. α-linolenic acid (18:3)] from membrane lipids in the chloroplast, which are oxidized by 13-lipoxygenases (NaLOX3) to form (13S)-hydroperoxy-18:3 (Halitschke and Baldwin, 2003). This molecule is the substrate for allene oxide synthase and subsequently AOC (Kallenbach et al., 2012). 12-Oxophytodienoic acid (OPDA) is formed and transported into the peroxisome where it is oxidized, forming JA. In the cytosol, JA-Ile, the bioactive form of jasmonate, is produced by the conjugation of JA to isoleucine by JASMONATE RESISTANT (JAR) (Kang et al., 2006; Wang et al., 2007, 2008). JA-Ile activates the SCFC°I1–JAZ complex (Chini et al., 2007; Thines et al., 2007), leading to the release of transcription factors such as MYC2, which is repressed by JAZ in the absence of JA (Lorenzo et al., 2004; Dombrecht et al., 2007; Woldemariam et al., 2013).
The present data strongly support the idea that JA signalling plays an important role in N. attenuata in defending against the necrotrophic fungus A. alternata by regulating NaF6ʹH1-dependent scopoletin biosynthesis. JA production was increased after fungal challenge (Fig. 5A); 0 leaves, which were more resistant to A. alternata, accumulated more JA and more scopoletin (Figs 1A, 5A); and plants strongly impaired in JA biosynthesis (irAOC) and perception (irCOI1) could not respond to A. alternata infection by increasing scopoletin production (Fig. 5B; Supplementary Fig. S2B at JXB online), and these plants were highly susceptible to A. alternata (Fig. 6B). In addition, exogenous MeJA can partially restore the levels of A. alternata-induced NaF6ʹH1 transcripts and scopoletin production in JA-deficient irAOC plants (Fig. 6A).
MYC2 is a member of bHLH family of transcription factors, and has emerged as a master regulator of most aspects of the JA signalling pathway in Arabidopsis and N. attenuata (Lorenzo et al., 2004; Dombrecht et al., 2007; Woldemariam et al., 2013), by binding to the G-box motif and its variants found in MYC2 target promoters (Godoy et al., 2011; Kazan and Manners, 2013). In Arabidopsis, two major branches of the JA signalling pathway are recognized: the MYC2 branch and the ERF (ethylene response factor) branch. In general, the ERF branch is associated with enhanced resistance to necrotrophic pathogens (Berrocal-Lobo et al., 2002; Lorenzo et al., 2003), whereas the MYC2 branch is associated with the wound response and defence against insect herbivores (Lorenzo et al., 2004; Kazan and Manners, 2008;Verhage et al., 2010). The MYC2-regulated branch antagonizes the ERF branch, and accordingly the myc2 mutant increased resistance to B. cinerea (Lorenzo et al., 2004). In N. attenuata, a T/G-box 5ʹ-AACGTG-3ʹ, one of the motifs recognized by MYC2 in the promoter region of many JA-responsive genes (Boter et al., 2004; Godoy et al., 2011), was also detected in the NaF6ʹH1 promoter (Supplementary Fig. S4 at JXB online). Consequently, silencing MYC2 in N. attenuata affected fungus-induced NaF6ʹH1 transcripts and scopoletin production (Fig. 7), indicating that unlike in Arabidopsis, NaMYC2 plays a role in the resistance of N. attenuata to the necrotrophic fungus A. alternata by regulating scopoletin biosynthesis. However, silencing NaMYC2 did not abolish fungus-induced scopoletin, suggesting that some other unknown transcriptional factors dependent on JA are also involved.
Previous work has shown that ABA is involved in the resistance of N. attenuata to A. alternata, and, like JA, higher levels of ABA were also detected in young leaves (Sun et al., 2014). Whether fungus-induced ABA cross-talks with JA needs further investigation. In Arabidopsis, ABA has been proposed to have an important role in the activation of defences against the oomycete Pythium irregulare by affecting JA biosynthesis (Adie et al., 2007). It will be interesting to test whether A. alternata-elicited JA levels are influenced in ABA-deficient N. attenuata plants.
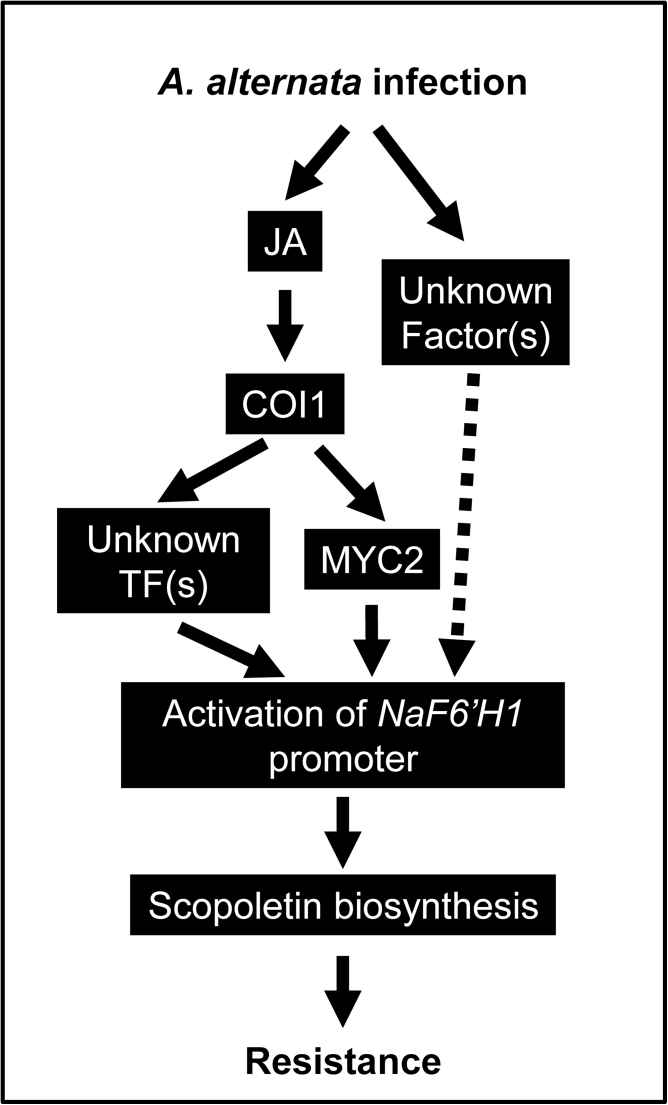
Taken all together, the data strongly suggests that when infected with A. alternata, N. attenuata plants increase JA production, activate JA signalling through COI1, and then stimulate scopoletin biosynthesis for defence possibly by binding of MYC2 and some unknown transcriptional factors to the promoter region of NaF6ʹH1 (Fig. 8). However, without A. alternata elicitation, MeJA treatment itself could not induce scopoletin production (Supplementary Fig. S5 at JXB online), indicating that another signal produced by fungal challenge is also needed for the induction. More efforts are required to understand the complex system for the regulation of scopoletin biosynthesis.
Fig. 8.
Working model of the regulation of A. alternata-induced scopoletin production by JA signalling. After A. alternata infection, the JA signalling pathway is activated and NaF6ʹH1, coding for the key enzyme in scopoletin biosynthesis, is trans-activated by the binding of MYC2 and some other unknown proteins to the T/G-box of the NaF6ʹH1 promoter; the biosynthesis of scopoletin around the infection sites finally affects the resistance of N. attenuata to A. alternata. However, without A. alternata elicitation, MeJA treatment itself could not induce scopoletin production, indicating that another JA-independent signal is also needed for NaF6ʹH1 activation.
Supplementary data
Figure S1. Alignment of NaF6ʹH1 and AtF6ʹH1.
Figure S2. Induction of scopolin in VIGS NaF6ʹH1 plants and of scopoletin in irCOI1 and irAOC plants.
Figure S3. Effect of ABA signalling on scopoletin accumulation.
Figure S4. T/G box in the NaF6ʹH1 promoter.
Figure S5. MeJA alone cannot induce scopoletin.
Table S1. Gene-specific primers used for real-time PCR.
Acknowledgements
We thank Professor Ian T. Baldwin (Max-Planck Institute for Chemical Ecology) for providing N. attenuata seeds silenced for NaAOC, NaCOI1, and NaMPK4, and for discussion. This project is supported by the Chinese Academy of Sciences, the Key Project of Applied Basic Research Program of Yunnan, and the Scientific Research Foundation of the Ministry of Education of China for Returned Scholars to Jinsong Wu.
References
- Adie BA, Perez-Perez J, Perez-Perez MM, Godoy M, Sanchez-Serrano JJ, Schmelz EA, Solano R. 2007. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis . The Plant Cell 19, 1665–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja I, Kissen R, Bones AM. 2012. Phytoalexins in defense against pathogens. Trends in Plant Science 17, 73–90 [DOI] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A, Solano R. 2002. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. The Plant Journal 29, 23–32 [DOI] [PubMed] [Google Scholar]
- Bergelson J, Purrington CB, Palm CJ, Lopez-Gutierrez JC. 1996. Costs of resistance: a test using transgenic Arabidopsis thaliana . Proceedings of the Royal Society B: Biological Sciences 263, 1659–1663 [DOI] [PubMed] [Google Scholar]
- Boter M, Ruiz-Rivero O, Abdeen A, Prat S. 2004. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis . Genes and Development 18, 1577–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinella MC, Ferrayoli CG, Palacios SM. 2005. Antifungal synergistic effect of scopoletin, a hydroxycoumarin isolated from Melia azedarach L. fruits. Journal of Agricultural and Food Chemistry 53, 2922–2927 [DOI] [PubMed] [Google Scholar]
- Century KS, Lagman RA, Adkisson M, Morlan J, Tobias R, Schwartz K, Smith A, Love J, Ronald PC, Whalen MC. 1999. Short communication: developmental control of Xa21-mediated disease resistance in rice. The Plant Journal 20, 231–236 [DOI] [PubMed] [Google Scholar]
- Cheng J, Sun W. 2001. Study on variety resistance of tobacco developing period to brown spot and the intergrated management techniques. Acta Phytopathologica Sinica 28, 44–48 [Google Scholar]
- Chini A, Fonseca S, Fernandez G, et al. 2007. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666–671 [DOI] [PubMed] [Google Scholar]
- Chong J, Baltz R, Schmitt C, Beffa R, Fritig B, Saindrenan P. 2002. Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. The Plant Cell 14, 1093–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churngchow N, Rattarasarn M. 2001. Biosynthesis of scopoletin in Hevea brasiliensis leaves inoculated with Phytophthora palmivora . Journal of Plant Physiology 158, 875–882 [Google Scholar]
- Develey-Riviere MP, Galiana E. 2007. Resistance to pathogens and host developmental stage: a multifaceted relationship within the plant kingdom. New Phytologist 175, 405–416 [DOI] [PubMed] [Google Scholar]
- Dombrecht B, Xue GP, Sprague SJ, et al. 2007. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis . The Plant Cell 19, 2225–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oirdi M, Trapani A, Bouarab K. 2010. The nature of tobacco resistance against Botrytis cinerea depends on the infection structures of the pathogen. Environmental Microbiology 12, 239–253 [DOI] [PubMed] [Google Scholar]
- Gachon C, Baltz R, Saindrenan P. 2004. Over-expression of a scopoletin glucosyltransferase in Nicotiana tabacum leads to precocious lesion formation during the hypersensitive response to tobacco mosaic virus but does not affect virus resistance. Plant Molecular Biology 54, 137–146 [DOI] [PubMed] [Google Scholar]
- Garcia D, Sanier C, Macheix JJ, Dauzac J. 1995. Accumulation of scopoletin in Hevea brasiliensis infected by Microcyclus ulei (Henn, P.) V. ARX and evaluation of its fungitoxicity for 3 leaf pathogens of rubber trees. Physiological and Molecular Plant Pathology 47, 213–223 [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43, 205–227 [DOI] [PubMed] [Google Scholar]
- Gnonlonfin GJB, Sanni A, Brimer L. 2012. Review scopoletin—a coumarin phytoalexin with medicinal properties. Critical Reviews in Plant Sciences 31(1): 47–56 [Google Scholar]
- Godoy M, Franco-Zorrilla, Perez-Perez J, Oliveros JC, Lorenzo O, Solano R. 2011. Improved protein-binding microarrays for the identification of DNA-binding specificities of transcription factors. The Plant Journal 66, 700–711 [DOI] [PubMed] [Google Scholar]
- Goy PA, Signer H, Reist R, Aichholz R, Blum W, Schmidt E, Kessmann H. 1993. Accumulation of scopoletin is associated with the high disease resistance of the hybrid Nicotiana glutinosa×Nicotiana debneyi . Planta 191, 200–206 [Google Scholar]
- Grosskinsky DK, Naseem M, Abdelmohsen UR, et al. 2011. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiology 157, 815–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Baldwin IT. 2003. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata . The Plant Journal 36, 794–807 [DOI] [PubMed] [Google Scholar]
- Hettenhausen C, Baldwin IT, Wu J. 2012. Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiology 157, 759–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot K, Aime S, Conrod S, Poupet A, Galiana E. 1999. Developmental regulated mechanisms affect the ability of a fungal pathogen to infect and colonize tobacco leaves. The Plant Journal 20, 163–170 [DOI] [PubMed] [Google Scholar]
- Kai K, Mizutani M, Kawamura N, Yamamoto R, Tamai M, Yamaguchi H, Sakata K, Shimizu B. 2008. Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana . The Plant Journal 55, 989–999 [DOI] [PubMed] [Google Scholar]
- Kai K, Shimizu B, Mizutani M, Watanabe K, Sakata K. 2006. Accumulation of coumarins in Arabidopsis thaliana . Phytochemistry 67, 379–386 [DOI] [PubMed] [Google Scholar]
- Kallenbach M, Bonaventure G, Gilardoni PA, Wissgott A, Baldwin IT. 2012. Empoasca leafhoppers attack wild tobacco plants in a jasmonate-dependent manner and identify jasmonate mutants in natural populations. Proceedings of the National Academy of Sciences, USA 109, E1548–E1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Wang L, Giri A, Baldwin IT. 2006. Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid–isoleucine-mediated defenses against Manduca sexta . The Plant Cell 18, 3303–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Manners JM. 2008. Jasmonate signaling: toward an integrated view. Plant Physiology 146, 1459–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Manners JM. 2013. MYC2: the master in action. Molecular Plant 6, 686–703 [DOI] [PubMed] [Google Scholar]
- Kus JV, Zaton K, Sarkar R, Cameron RK. 2002. Age-related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae . The Plant Cell 14, 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. 2002. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12, 177–183 [Google Scholar]
- LaMondia JA. 2001. Outbreak of brown spot of tobacco caused by Alternaria alternata in Connecticut and Massachusetts. Plant Disease 85, 230–230 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R. 2004. Jasmonate-insensitive1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. The Plant Cell 16, 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. 2003. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. The Plant Cell 15, 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RDH, Mendez J, Brown SA. 1982. The natural coumarins: occurrence, chemistry and biochemistry. Chichester, UK: John Wiley and Sons [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT. 2007. Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. The Plant Journal 51, 79–91 [DOI] [PubMed] [Google Scholar]
- Ratcliff F, Martin-Hernandez AM, Baulcombe DC. 2001. Technical advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. The Plant Journal 25, 237–245 [DOI] [PubMed] [Google Scholar]
- Rowe HC, Walley JW, Corwin J, Chan EK, Dehesh K, Kliebenstein DJ. 2010. Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathogenesis 6, e1000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler R, Baldwin IT. 2004. Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata . Journal of Experimental Botany 55, 151–157 [DOI] [PubMed] [Google Scholar]
- Sharan M, Taguchi G, Gonda K, Jouke T, Shimosaka M, Hayashida N, Okazaki M. 1998. Effects of methyl jasmonate and elicitor on the activation of phenylalanine ammonia-lyase and the accumulation of scopoletin and scopolin in tobacco cell cultures. Plant Science 132, 13–19 [Google Scholar]
- Silva WPK, Deraniyagala SA, Wijesundera RLC, Karunanayake EH, Priyanka UMS. 2002. Isolation of scopoletin from leaves of Hevea brasiliensis and the effect of scopoletin on pathogens of H. brasiliensis . Mycopathologia 153, 199–202 [DOI] [PubMed] [Google Scholar]
- Simon C, Langlois-Meurinne M, Didierlaurent L, et al. 2014. The secondary metabolism glycosyltransferases UGT73B3 and UGT73B5 are components of redox status in resistance of Arabidopsis to Pseudomonas syringae pv. tomato. Plant, Cell and Environment 37, 1114–1129 [DOI] [PubMed] [Google Scholar]
- Spoel SH, Johnson JS, Dong X. 2007. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proceedings of the National Academy of Sciences, USA 104, 18842–18847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Hu X, Ma J, Hettenhausen C, Wang L, Sun G, Wu J, Wu J. 2014. Requirement of ABA signalling-mediated stomatal closure for resistance of wild tobacco to Alternaria alternata . Plant Pathology. doi:10.1111/ppa.12181 [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. 2007. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448, 661–665 [DOI] [PubMed] [Google Scholar]
- Valle T, Lopez JL, Hernandez JM, Corchete P. 1997. Antifungal activity of scopoletin and its differential accumulation in Ulmus pumila and Ulmus campestris cell suspension cultures infected with Ophiostoma ulmi spores. Plant Science 125, 97–101 [Google Scholar]
- Verhage A, van Wees SC, Pieterse CM. 2010. Plant immunity: it’s the hormones talking, but what do they say? Plant Physiology 154, 536–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Allmann S, Wu J, Baldwin IT. 2008. Comparisons of LIPOXYGENASE3- and JASMONATE-RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid–amino acid conjugates play different roles in herbivore resistance of Nicotiana attenuata . Plant Physiology 146, 904–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Halitschke R, Kang JH, Berg A, Harnisch F, Baldwin IT. 2007. Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta 226, 159–167 [DOI] [PubMed] [Google Scholar]
- Woldemariam MG, Inh ST, Oh Y, Gaquerel E, Baldwin IT, Galis I. 2013. NaMYC2 transcription factor regulates a subset of plant defense responses in Nicotiana attenuata . BMC Plant Biology 13, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang L, Baldwin IT. 2008. Methyl jasmonate-elicited herbivore resistance: does MeJA function as a signal without being hydrolyzed to JA? Planta 227, 1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang J, Jia W, Zhao Y, Ma R. 1998. The relationship between maturity or senescence of tobacco leaves and brown spot. Acta Phytopathologica Sinica 28, 49–54 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.