Abstract
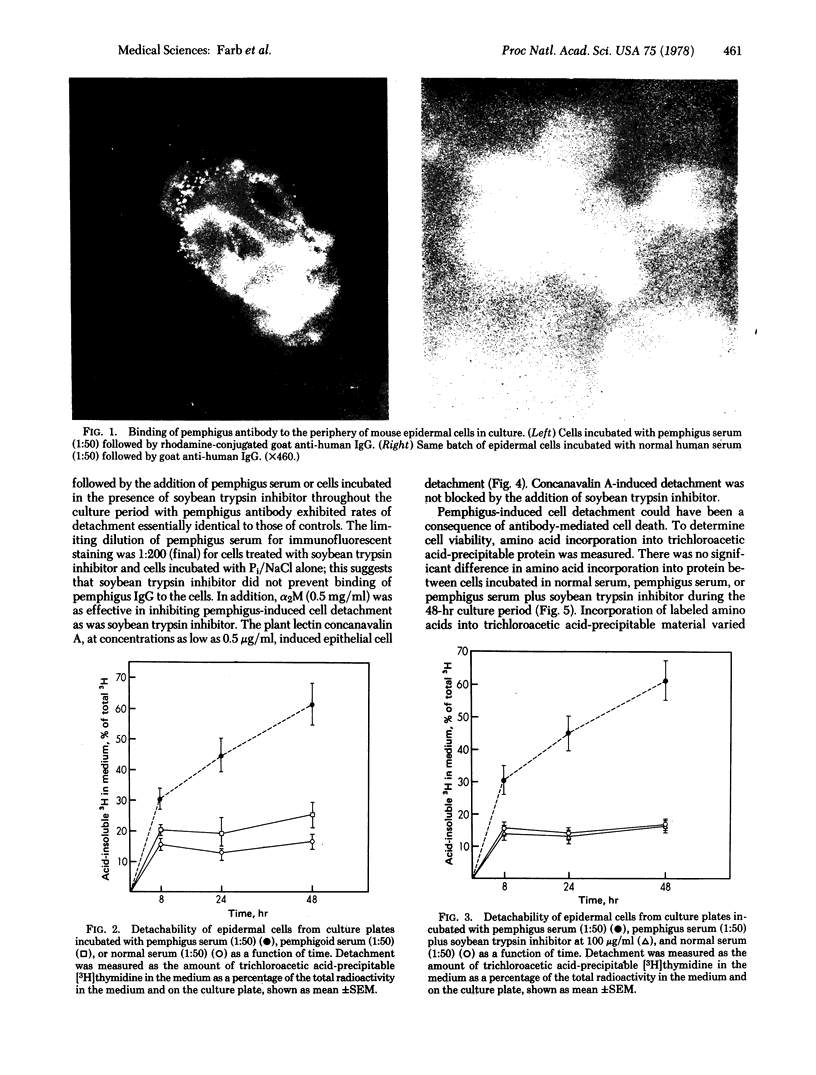
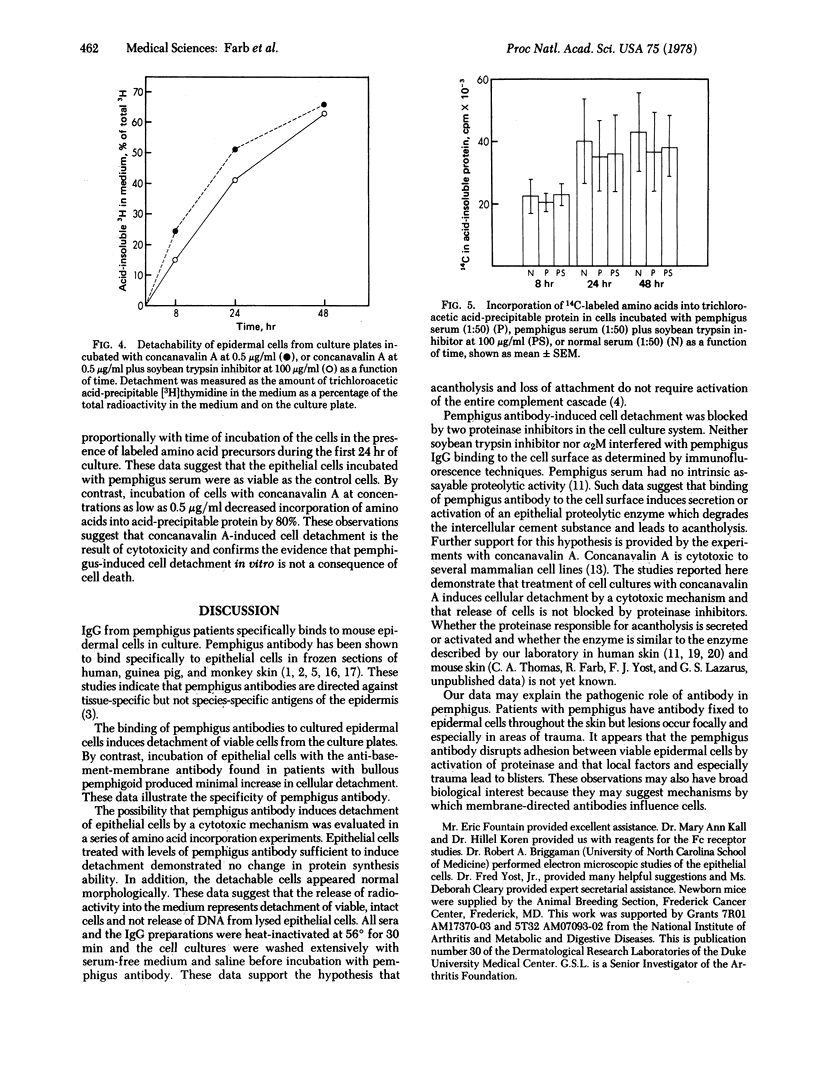
Immunoglobulin from pemphigus patients binds to the surface of mouse epidermal cells in culture. Cells incubated with the pemphigus antibody are easily detached from culture plates whereas cells incubated with serum from normal patients remain on the plate. Pemphigus antibody-mediated cell detachment is blocked by the addition of the proteinase inhibitors soybean trypsin inhibitor and alpha2-macroglobulin to the culture media. Detachable cells are viable, and activation of the complement cascade is not necessary for cell detachment. The anti-cell-surface antibody of pemphigus appears to disrupt adhesion between viable epidermal cells by activation of proteinase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTNER E. H., JORDON R. E. DEMONSTRATION OF SKIN ANTIBODIES IN SERA OF PEMPHIGUS VULGARIS PATIENTS BY INDIRECT IMMUNOFLUORESCENT STAINING. Proc Soc Exp Biol Med. 1964 Nov;117:505–510. doi: 10.3181/00379727-117-29622. [DOI] [PubMed] [Google Scholar]
- BEUTNER E. H., LEVER W. F., WITEBSKY E., JORDON R., CHERTOCK B. AUTOANTIBODIES IN PEMPHIGUS VULGARIS: RESPONSE TO AN INTERCELLULAR SUBSTANCE OF EPIDERMIS. JAMA. 1965 May 24;192:682–688. doi: 10.1001/jama.1965.03080210026006. [DOI] [PubMed] [Google Scholar]
- Barnett M. L., Beutner E. H., Chorzelski T. P. Organ culture studies of pemphigus antibodies. II. Ultrastructural comparison between acantholytic changes in vitro and human pemphigus lesions. J Invest Dermatol. 1977 May;68(5):265–271. doi: 10.1111/1523-1747.ep12494207. [DOI] [PubMed] [Google Scholar]
- Deng J. S., Beutner E. H., Shu S., Chorzelski T. P. Pemphigus antibody action on skin explants: kinetics of acantholytic changes and stability of antigens in tissue cultures of normal monkey skin explants. Arch Dermatol. 1977 Jul;113(7):923–926. doi: 10.1001/archderm.113.7.923. [DOI] [PubMed] [Google Scholar]
- Elgjo K., Hennings H., Michael D., Yuspa S. H. Natural synchrony of newborn mouse epidermal cells in vitro. J Invest Dermatol. 1976 May;66(5):292–296. doi: 10.1111/1523-1747.ep12482235. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Yost F. J., Jr, Thomas C. A. Polymorphonuclear leukocytes: possible mechanism of accumulation in psoriasis. Science. 1977 Dec 16;198(4322):1162–1163. doi: 10.1126/science.929193. [DOI] [PubMed] [Google Scholar]
- Levine N., Hatcher V. B., Lazarus G. S. Proteinases of human epidermis; a possible mechanism for polymorphonuclear leukocyte chemotaxis. Biochim Biophys Acta. 1976 Dec 8;452(2):458–467. doi: 10.1016/0005-2744(76)90196-0. [DOI] [PubMed] [Google Scholar]
- Sams W. M., Jr, Jordon R. E. Pemphigus antibodies: their role in disease. J Invest Dermatol. 1971 Jun;56(6):474–479. doi: 10.1111/1523-1747.ep12261412. [DOI] [PubMed] [Google Scholar]
- Schiltz J. R., Michel B. Production of epidermal acantholysis in normal human skin in vitro by the IgG fraction from pemphigus serum. J Invest Dermatol. 1976 Aug;67(2):254–260. doi: 10.1111/1523-1747.ep12513454. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Ellman L., Davie J. M., Green I. L2C Guinea pig lymphatic leukemia: a "B" cell leukemia. Blood. 1972 Jan;39(1):1–12. [PubMed] [Google Scholar]
- Shevach E. M., Stobo J. D., Green I. Immunoglobulin and theta-bearing murine leukemias and lymphomas. J Immunol. 1972 May;108(5):1146–1151. [PubMed] [Google Scholar]
- Takigawa M., Imamura S. Experimental production of rabbit anti-guinea-pig epidermal cell sera. Comparison to pemphigus antibodies. J Invest Dermatol. 1977 May;68(5):259–264. doi: 10.1111/1523-1747.ep12506651. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Yost F. J., Jr, Snyderman R., Hatcher V. B., Lazarus G. S. Cellular serine proteinase induces chemotaxis by complement activation. Nature. 1977 Oct 6;269(5628):521–522. doi: 10.1038/269521a0. [DOI] [PubMed] [Google Scholar]
- Wickerhauser M., Hao Y. L. Large scale preparation of macroglobulins. Vox Sang. 1972 Jul-Aug;23(1):119–125. [PubMed] [Google Scholar]