Abstract
One of the endemic fungi, Blastomyces dermatitidis, can cause epidemics of infection with multiple persons involved in a point source outbreak but more commonly causes sporadic cases of infection within the areas of endemicity. Blastomycosis can present as an acute pneumonia which is often misdiagnosed as acute pneumococcal pneumonia or the infection may present as a chronic pneumonia along with weight loss, night sweats, hemoptysis, and a lung mass suggesting tuberculosis or carcinoma of the lung. Extrapulmonary infection with B. dermatitidis is protean with many different manifestations. Most commonly, skin or subcutaneous lesions are found with either a verrucous or warty appearance or in an ulcerative form. Cases have been misidentified as keratoacanthoma, pyoderma gangrenosum, carcinoma, or as Weber-Christian panniculitis if there are nodular subcutaneous lesions. Essentially any site or organ can have lesions of disseminated blastomycosis. In our series, cases of laryngeal carcinoma, adrenal insufficiency, thyroid nodules, granulomatous hypercalcemia, abnormal mammograms thought to represent breast carcinoma, otitis media with cranial extension, immune thrombocytopenic purpura, and hemolytic anemia of unknown cause have been misdiagnosed and blastomycosis subsequently identified as the cause. This infection causes manifestations which mimic many other more commonly diagnosed conditions and must always be considered by clinicians practicing in the endemic region.
INTRODUCTION
Many infections can present with signs and symptoms that suggest more common etiologies. A famous clinicopathological conference published in the New England Journal of Medicine was one of the case records from Massachusetts General Hospital in 1963 (1); it discussed a 75-year-old widow with epistaxis. She had enjoyed good health and was afebrile. She was a world traveler who had recently returned from Europe; 2 months after arrival home, she presented with petechial hemorrhages on the trunk. Thrombocytopenia and anemia were discovered and prednisone was administered. The prednisone dose was reduced but she returned 2 months later with quadriceps muscle atrophy and fever to 102°F. Her platelet count and leukocyte count had dropped substantially; she developed continued fever despite penicillin and tetracycline and died on the ninth hospital day. The differential diagnosis discussion of her illness focused on her cytopenias, which was thought to be a primary reticulosis and possibly lymphosarcoma with an associated immunopancytopenia. Dr Benjamin Castleman presented the findings of the autopsy which revealed miliary tuberculosis with tubercles in the bone marrow, lung, and liver as well as other organs. The patient had untreated tuberculosis. For many years thereafter, many infectious diseases experts and other physicians have thought, and continue to think, that this case was actually Eleanor Roosevelt, the wife of President Franklin Roosevelt, and that she had been misdiagnosed and had untreated miliary tuberculosis which caused her death. Dr Barron Lerner was able to investigate the medical files of Mrs Roosevelt when they were unsealed 25 years after her death. The case in the Clinical Pathological Conference had very similar features but was from a different hospital (Yale/New Haven rather than Columbia-Presbyterian) and the patient described was 3 years younger than Mrs Roosevelt at her death. Lerner described from his review of her chart that tuberculosis was indeed considered during her hospitalization and that she had been started on anti-tuberculous therapy for the last 6 weeks of her life. It was after her death that the organism grew and was found to be resistant to the two drugs for tuberculosis that she had been administered. Our presiding President of the American Clinical and Climatological Association for this meeting, Dr Philip A. Mackowiak, published a book entitled Diagnosing Giants: Solving the Medical Mysteries of Thirteen Patients Who Changed the World 19 days before the date of this presentation (2). His final chapter of the work was entitled “Too Busy to be Sick,” and in it he updated the story of Mrs Roosevelt's life, illness, and death.
Blastomycosis is another infectious disease that often is initially misdiagnosed. It usually is a disease of “normal” persons, unlike many other fungal pathogens which cause opportunistic infections in immunocompromised patients. This infection occurs both in epidemics and sporadically as one of the endemic mycoses throughout regions of the South Central, Southeastern, and Upper Midwestern regions of the United States (1). The reported incidence of blastomycosis depends on recognition of the clinical diagnosis because there are no good or reliable markers of previous infection such as the skin test for tuberculosis, histoplasmosis, or coccidioidomycosis.
CASE REPORT
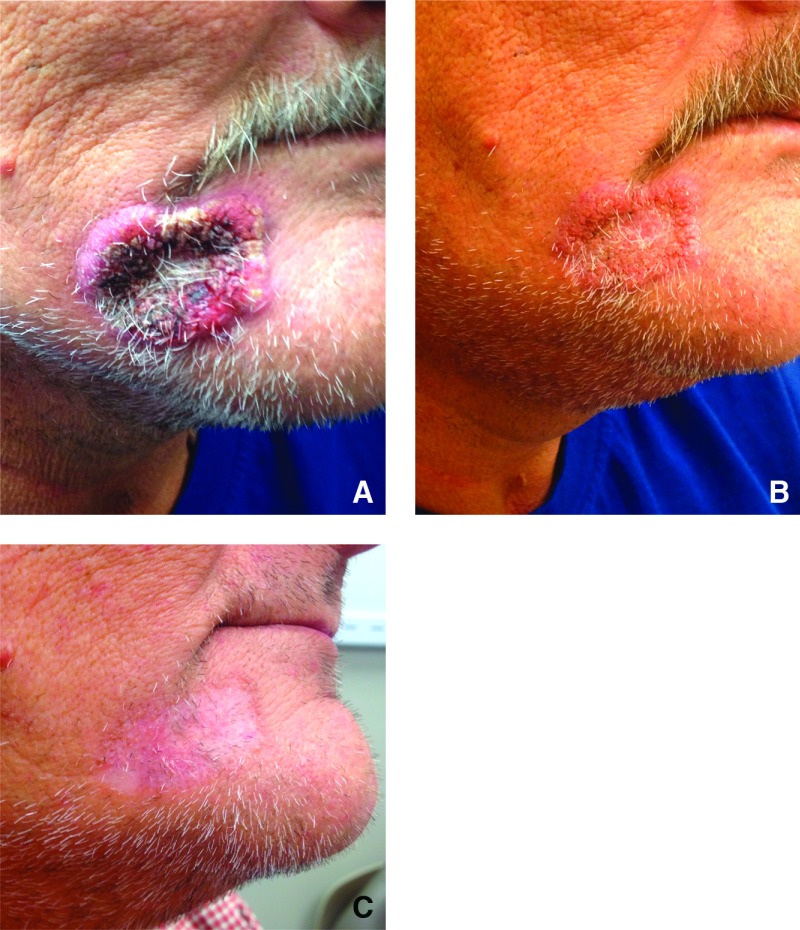
A 52-year-old man presented to a dermatology clinic for a 3-month progressive lesion on his right chin which started as a papule and rapidly progressed in size (Figure 1A). He had no pain at the site but it would intermittently bleed when abraded by clothes or touch. The lesion was thought to be a neoplasm and a biopsy was performed. Broad-based budding yeast was identified as presumptive Blastomyces dermatitidis which was confirmed by culture when reviewed by the infectious disease physicians. Additional history revealed that he had a lung nodule evaluated by mediastinoscopy 4 years earlier which had ruled out cancer by biopsy, but neither cultures nor special stains were performed. He was treated with itraconazole with rapid resolution of the skin lesion over 6 weeks and total resolution by 3 months (Figures 1B and 1C). The total course of the antifungal was 6 months. It is presumed that the lung nodule was the initial manifestation of blastomycosis and the cutaneous lesion was a late relapse of the untreated infection.
Fig. 1.
(A) Lesion on chin of patient with blastomycosis. Culture and potassium hydroxide preparation slide confirmed the diagnosis. (B) Lesion of blastomycosis after 2 weeks of oral itraconazole. (C) Healed lesion of blastomycosis after 3 months of oral itraconazole.
EPIDEMIOLOGY
Gilchrist first described blastomycosis in Baltimore in the 1890s as a skin infection caused by a protozoan organism (3), and the illness was known for a time as Gilchrist's disease. However, blastomycosis is not common in the areas surrounding Baltimore and cutaneous lesions occur secondarily rather than primarily. Gilchrist was the first to refute portions of his own description of protozoa when he isolated and named the fungus B. dermatitidis (4). Because skin manifestations of blastomycosis are prominent, the initial cases were perceived as mainly dermatologic (5). The concept of primary pulmonary blastomycosis was not recognized until other more common fungi were similarly described and pathologic descriptions allowed the pathophysiologic mechanisms to be delineated (6). Almost all cases of blastomycosis are considered to originate from a pulmonary portal of entry (5).
Unlike several other fungal infections which are opportunistic, blastomycosis is usually diagnosed in “normal” hosts and has a similar epidemiology as other endemic mycoses, i.e., histoplasmosis and coccidioidomycosis. Therefore, greater details are understood about the epidemiology of the former two diseases than blastomycosis. For example, histoplasmosis was once considered to be a rare but uniformly fatal infection. However, with specific skin testing, it was discovered that, in endemic areas, many persons without a clinical history of histoplasmosis had been infected with the fungus (7).
The vast majority of patients with blastomycosis who have been reported in the medical literature in the past have had clinically diagnosed disease and are located within a fairly well-defined geographical area of the South Central United States. In the last two decades, there have been more reports of cases in Illinois (8) and Wisconsin (9). Likewise, in the Kenora region of upper Ontario and in Manitoba, there have been a large number of cases of blastomycosis reported (10). Following the report of point source epidemics of infection at Eagle River (11) and at Tomorrow River (12), both in Minnesota, it has become clear that areas can be hyperendemic for the disease (13). Factors which allow for the microecology that promotes this hyperendemicity are being elucidated (14). Molecular techniques are being used to look at genetic differences detected by polymerase chain reaction of components of the organism recovered from patients and from nature (15).
Many of these patients with blastomycosis have a history of recreational or occupational exposure to wooded areas and often to bodies of water, such as lakes or rivers. For the most part, the incidence of blastomycosis depends on the reporting of clinically diagnosed cases of infection because there are no simple and reliable markers of previous infection such as the histoplasmin skin test for histoplasmosis. However, it is becoming increasingly apparent that blastomycosis is similar to histoplasmosis as more and more patients with subclinical infection are being discovered.
CLINICAL PRESENTATION
General
The clinical presentations of blastomycosis are highly variable. Nonspecific complaints, including weight loss, fever, malaise, and fatigue, are common but offer little diagnostic help. The typical patient is a male between 25 and 50 years of age who either works in or visits outdoor areas. Because dogs are infected in the same way as humans, a clinical clue to the diagnosis of blastomycosis is the history of a pet dog diagnosed with the fungus. Other than from a bite wound, blastomycosis is not contagious from the animal to the human. The observation merely represents the fact that the dog has been in the same environment and acts as a herald for human illness (16). In an outbreak of blastomycosis, children and women are as likely as males to be infected. Aside from an epidemic, it is very rare for children to be diagnosed with blastomycosis (17). The male-to-female ratio has been reported to be from 4:1 to 15:1 in series of endemic cases (18). However, some of these studies were from Veterans Administration medical centers, which conspicuously add bias to the male ratio. During a 13-year period, 78 male patients and 57 female patients were referred to me for therapy of blastomycosis. Of these, 47% had extrapulmonary manifestations and 53% had only lung involvement. Women accounted for only 30% of the extrapulmonary cases whereas 47% of the pneumonia cases were in women.
Pulmonary
The presentation of clinical blastomycosis for most patients is pneumonia with an alveolar or mass-like infiltrate by radiography. In two large series, 80% and 90% of patients had this type of radiographic appearance, respectively (19,20). Many blastomycosis patients who have mass lesions on radiography are initially thought to have lung cancer. Miliary or reticulonodular patterns on radiographs are the next most frequent pattern. Cavitary disease is distinctly uncommon compared to chronic pulmonary histoplasmosis or tuberculosis. Pleural disease has been stated to be distinctly unusual in this infection; however, in two series, 26% and 42% of patients had evidence of pleural effusion with blastomycosis, respectively (19, 21). In summary, none of the radiographic patterns are diagnostic for this fungal infection.
Clinically, patients with pneumonia due to blastomycosis have an acute presentation mimicking pneumococcal pneumonia, a more chronic picture similar to tuberculosis or cancer, or no pulmonary symptoms at all (19). Blastomycosis patients with pulmonary infiltrates may be discovered by routine radiographs with subsequent denial of pulmonary complaints even after extensive questioning. In one group of our patients, 2 were asymptomatic, 16 had a chronic pneumonia picture, and 8 initially had acute pneumonia (19).
Cutaneous
Following pneumonia, cutaneous lesions are the next most common manifestation of blastomycosis. The skin lesions are either verrucous or ulcerative. The verrucous form has a raised, irregular border, often with crusting and some drainage above an abscess in the subcutaneous tissue. These lesions show histologic evidence of papillomatosis, downward proliferation of the epidermis with intraepidermal abscesses, and inflammatory cells in the dermis (5,18). Cutaneous ulcers due to blastomycosis borders which are usually sharp, heaped-up, and the base commonly contains exudate. These ulcers of blastomycosis originate from subcutaneous pustular lesions that spontaneously drain. On biopsy, microabscesses are typically present, even in those patients with little inflammation clinically apparent in the ulcer. Subcutaneous localization without either ulceration or the verrucous appearance may be found. Aspiration of the subcutaneous mass or biopsy will reveal organisms on microscopy and culture.
Osseous
Osteomyelitis due to B. dermatitidis infection is reported in up to a quarter of extrapulmonary cases and is most often misdiagnosed clinically as a neoplasm. This frequency is higher than my experience. Granuloma, suppuration, or necrosis may be found in the bone biopsy. The vertebrae, pelvis, sacrum, skull, ribs, or long bones have been reported most frequently but essentially any bone may be involved (6). The radiographic appearance of blastomycosis is not specific and cannot be discriminated from that of other fungal, bacterial, or neoplastic disease. Debridement may be required for cure but most bone lesions resolve with antifungal therapy alone.
Genitourinary
The genitourinary system follows lung, skin, and bone in frequency of involvement, and because men are more likely to have extrapulmonary disease, prostatitis and epididymo-orchitis have been reported most commonly (5). Urine collected after prostatic massage will improve the detection of genitourinary involvement. As occurs with skin or bone infection, B. dermatitidis will present at the same time in the lung as in the prostate or testicle; therefore, chest radiographs should be performed in every case of blastomycosis, even if the patient does not have pulmonary complaints.
Central Nervous System
Blastomycosis involves the nervous system in 5% to 10% of cases of disseminated disease either as meningitis or cranial abscesses (22). Computed tomography should direct the surgeon for biopsy of the cranial abscesses, but identification of blastomycosis as the cause of meningitis is more problematic. Evaluation of spinal fluid usually does not reveal the organism so that ventricular fluid may be required for a positive culture.
Other Clinical Manifestations
Lesions of blastomycosis may show up in virtually any organ. Widely disseminated or miliary blastomycosis may occur with adult respiratory distress syndrome as the presenting feature. One patient reported by Evans et al had an ulcer found at the carina on bronchoscopy, prompting the speculation that a subcarinal lymph node ruptured into the trachea, spilling enough organisms into the lungs to cause adult respiratory distress syndrome (23). A large portion of patients with this pattern of diffuse infiltrates, noncardiac pulmonary edema, and refractory hypoxemia die very quickly. Three patients in one series died with this manifestation despite intensive medical and antifungal therapy (24). Another report suggested that survival is possible if the diagnosis is quickly made and therapy is promptly begun (25).
Abscesses are most common in the subcutaneous tissue but, as already noted, they may be found in the brain, skeletal system, prostate, or any other organ including lesions in the myocardium, pericardium, orbit, sinuses, pituitary, adrenal, or other organs as reviewed by Witorsch and Utz (6). The reticuloendothelial system has been involved with reports of several cases of lymph node or hepatic involvement (5,18,26) and cases of splenic abscesses have been documented. Surgical drainage of splenic abscesses is required, just as it is for the majority of large abscesses secondary to this fungus.
Lesions in the mouth and oropharyngeal area occur but are not found with the same frequency as the lesions found in disseminated histoplasmosis. One exception is the larynx (27). Witorsch and Utz refuted the hypothesis that the larynx might become infected primarily rather than by hematogenous dissemination from a pulmonary focus (6). Laryngeal biopsy reveals histologic features similar to those in the skin. The hyperplasia, acanthosis, and fungating appearance of the larynx may be confused with squamous cell carcinoma. Fixation of the vocal cords secondary to fibrosis has led, in some cases, to radiation therapy or total laryngectomy due to an incorrect diagnosis of neoplasm rather than blastomycosis.
Specific endocrine abnormalities may appear in patients with blastomycosis (6). Adrenal insufficiency from gland destruction, diabetes insipidus, thyroid infection, and hypercalcemia (as seen with other granulomatous diseases) have been reported (18).
Although blastomycosis may cause infections in immunocompromised patients, other fungal infections such as disseminated histoplasmosis or cryptococcal meningitis are much more likely to be opportunistic than blastomycosis. Immunosuppressed patients usually develop infection after exposure in the environment or through subsequent reactivation, similar to immunocompetent patients. Unlike similar fungi, B. dermatitidis has been reported to be a significant pathogen after infection with human immunodeficiency virus (HIV) in only a relatively small number of cases (28).
Unusual Manifestations
Because blastomycosis can be rarely found in essentially any organ, a number of unusual manifestations have been described. We reported the cases of two women with blastomycosis who had abnormal mammograms with a strong clinical impression of breast carcinoma (29). One had a computerized tomography scan which revealed partial destruction of a vertebral body consistent with metastatic disease. Breast biopsy on both revealed B. dermatitidis on microscopy and subsequent culture. The mass in each woman resolved with antifungal therapy. Other cases of blastomycosis of the breast have been reported (30). The skin lesions of blastomycosis may be confused with a number of alternative diagnoses, including basal cell carcinoma, squamous cell carcinoma, or pyoderma gangrenosum. We described one patient with what appeared to be condyloma acuminatum surrounding his anus (26). Only after postoperative suppurative drainage occurred was the histology reviewed and B. dermatitidis found. When blastomycosis is found at sites where other diseases are more common, misdiagnosis is frequent.
One patient was diagnosed with giant keratoacanthoma based on biopsy of nasal and maxillary area skin and soft tissue (26). Treatment with isotretinoin was unsuccessful. Two months later, a chest radiograph showed miliary infiltrates and a new pustular lesion developed on his ankle. Biopsy of lung and skin revealed B. dermatitidis. He responded to itraconazole but required skin grafts and plastic surgery to repair fungal-induced cicatricial ectropion of his eyelid. Blastomycosis is frequently found on the face for reasons which are not clear.
Ocular involvement may be discovered. In one case series (31), a patient with a mass on the iris, uveitis, and a choroidal mass was described. Systemic and local antifungal therapy was associated with improvement of the mass lesions and vision. Review of the literature by the authors revealed 10 other cases of ocular blastomycosis, including iritis, uveitis, endophthalmitis, and choroidal lesions (31). The rarity of ocular disease with blastomycosis in humans is in contradistinction to canine blastomycosis in which ocular findings, including endophthalmitis in particular, are very common. The reason for the difference in human and canine cases is unclear but may relate to an even longer delay in diagnosis in dogs than humans, allowing more systemic spread of the infection.
The particular frequency of laryngeal blastomycosis has been discussed above. Other manifestations of blastomycosis in the ear, nose, and throat area are not common. Although ulcerative bronchitis is found with pulmonary blastomycosis, tracheal localization is uncommon (32). We reported two cases of otitis media with cranial extension due to this fungus (33). Both were cured with amphotericin B. One patient was described with infection in a presumed branchial cleft cyst infection (26). Surgical removal showed lymphadenopathy and both suppurative and granulomatous inflammation with B. dermatitidis organisms.
Peripheral lymphadenopathy is not particularly frequent in systemic blastomycosis. In the series of 135 study patients referred to me over a 13-year period, 63 had extrapulmonary blastomycosis with 5 who had lymphadenopathy, which was one case more than those with prostatic blastomycosis (18). One of those patients had amyloid deposition in the node by microscopy and staining. After therapy, no other evidence of amyloidosis remained. Another patient, who was not part of those included in the study, also ultimately had a diagnosis of amyloidosis. Her case was indirectly reported by Schutze et al (34) when they described a patient with perinatal blastomycosis. The mother, at the time of delivery, had a cutaneous ulcer but refused further evaluation or therapy. Her newborn son developed overwhelming pulmonary blastomycosis at day 18 of life; he ultimately died of the infection despite aggressive therapy. The mother subsequently reported total recovery with complete healing of the large cutaneous ulcer. Two years later, after a brief course of corticosteroids for presumed ulcerative colitis, this patient developed adult respiratory distress syndrome secondary to blastomycosis along with dissemination of the infection. She died of progressive infection within 3 days of beginning amphotericin B. At autopsy, she had extensive disease due to blastomycosis, including bone, joint, uterine, and ovarian abscesses. She had dramatic lymph node and renal amyloidosis. Likely, she had low-grade or smoldering blastomycosis and development of systemic amyloidosis. With the immunosuppression of corticosteroids, the infection became florid and caused her death.
Several cases of blastomycosis have been reported during pregnancy (30), including one mother who transmitted blastomycosis via intrauterine transfer of the organisms. The patient described with amyloidosis above had uterine and ovarian abscesses and could have likewise transmitted the infection in the peripartum period. The infection has been transmitted in the other direction, that is, into the uterus from sexual contact with a man with blastomycosis on the penis (35).
As discussed, most with blastomycosis are immunocompetent. Immunocompromised patients have been reported, including patients with AIDS, transplantation, sarcoidosis, or treatment with corticosteroids. A recent publication reported a patient with concurrent pulmonary blastomycosis and Hodgkin's disease; the fungal infection was not discovered until dissemination occurred after chemotherapy (36). Pappas et al described a number of patients in these categories with blastomycosis (37). They found an increased percentage of cases of blastomycosis in immunosuppressed patients from 1978 through 1991 compared to the cases from 1956 through 1977. This could have been from a bias in referral patterns of patients, but they speculated that the continually enlarging population of patients with complicated immune-compromising illnesses has lived in the endemic area for this fungus and, thereby, became infected (37). Therapy with only azole antifungal agents was followed by a higher relapse rate in this retrospective review compared to those who received either amphotericin B alone or amphotericin B followed by an azole agent (37).
Blastomycosis may occur simultaneously with other infections or other illnesses. Diabetes mellitus is listed as a risk factor for this infection, but epidemiologic studies to compare the two illnesses are difficult to perform because of the relative lack of serologic or other markers of subclinical infection. Blastomycosis has been described in association with tuberculosis, histoplasmosis, and coccidioidomycosis (30). I have treated blastomycosis in one patient who presented with idiopathic thrombocytopenic purpura and in another patient who presented with hemolytic anemia of unknown cause (18). Both hematologic conditions were treated with corticosteroids while the blastomycosis was treated with first amphotericin B followed by itraconazole. Corticosteroids were rapidly tapered and the hematologic conditions did not recur as the blastomycosis was cured. Whether the infection caused the thrombocytopenia and anemia is not clear. Another patient previously reported (26) with both sarcoidosis and blastomycosis was treated with both corticosteroids and itraconazole with cure of the fungal infection. It appears that as long as effective antifungal chemotherapy is being used, corticosteroid therapy may not have the deleterious result that has been described in blastomycosis when the infection is not recognized and, therefore, untreated. This observation has previously been made in similar infectious diseases, particularly tuberculosis.
DIAGNOSIS
Microscopic examination of stained histopathology or cytology specimens and culture of secretions or tissues remains the cornerstone of diagnosis for blastomycosis. When clinical suspicion is high, direct microscopic examination of sputum, skin, aspirates of abscess fluid, or body fluid following either digestion of human cells with 10% potassium hydroxide or staining with calcofluor white or Papanicolaou stain is the most rapid and effective means for diagnosis (5,30). The organism can be visualized in tissue specimens stained with methenamine silver or periodic acid-Schiff stains. Culture should be performed and is positive in almost all cases. At room temperature, the organism will grow as a mold in 2 to 4 weeks on a wide range of culture media, including Sabouraud agar (30). Skin tests are not helpful and are not available (5). Serologic studies using a variety of antigens and methods have likewise been unreliable for diagnosis. An assay to detect B. dermatitidis antigens in urine is now available commercially and may be helpful in diagnosis, although significant cross-reactivity has been noted with histoplasmosis, paracoccidioidomycosis, and penicilliosis.
TREATMENT
The vast majority of patients coming to medical attention require antifungal chemotherapy. Oral itraconazole at a dose of 200 mg to 400 mg daily is the recommended drug for all but the most severe cases (38). Amphotericin B was the drug of choice for all patients in the past and continues to be initial therapy for those with life-threatening blastomycosis or central nervous system (CNS) infection (22). The deoxycholate formulation is given intravenously at a dose of 0.7 to 1mg/kg daily. Alternatively, the liposomal formulation, which is preferred for CNS disease, can be given at 3 to 5 mg/kg daily with fewer side effects, but at greater cost. Amphotericin formulations are typically given for 1 to 2 weeks (4 to 6 weeks for CNS disease), until improvement occurs, after which therapy can be completed with oral itraconazole. Non−life-threatening infections can be treated for the entire course with itraconazole alone. Itraconazole achieves cure rates of approximately 95% in those completing therapy. It also has fewer adverse effects than amphotericin B. Fluconazole has lower success rates in treating blastomycosis and requires much higher doses than itraconazole (39). A few cases have been treated with voriconazole or posaconazole.
SUMMARY
In conclusion, blastomycosis commonly causes pneumonia and cutaneous ulcers or verrucous skin lesions but often the diagnosis is delayed. Bone, prostatic, and CNS blastomycosis are fairly common presentations of this infection. Other organ system involvement is not common but may occur. The disease is often misdiagnosed, particularly as malignancy. Search for the organism by microscopy and culture should be considered, even in those with a classic presentation of other illnesses. History of occupational or recreational exposure to this fungus in endemic areas should be obtained to aid in the prompt diagnosis of blastomycosis.
ACKNOWLEDGMENTS
The author thanks Dr Katrina Coulter, fellow in Infectious Diseases, for the photographs.
Footnotes
Potential Conflicts of Interest: None disclosed
DISCUSSION
Hochberg, Baltimore: Excellent clinical presentation. In the last 15 years, what proportion of the cases that you've seen are on TNF inhibition for treatment of immune-mediated diseases?
Bradsher, Little Rock: Thank you. Actually, your being from Baltimore, I should say that the organism was first isolated in Baltimore by Dr Gilchrist in 1894. He only got three things wrong of the three things that he said. He thought that it was a parasite, that it was cutaneous, and that it was near Baltimore; all three were wrong, but he was the first.
Hochberg, Baltimore: It was before my time.
Bradsher, Little Rock: It was before your time, right. Mine too. So how many have TNF blockers? Not very many. Not nearly the number of histoplasma patients that we see. Most of the folks that we see with blasto typically are immunocompetent. We have seen some that have been on TNF, and typically we treat those for the rest of their life.
Bryan, Columbia: Two of your slides — one with the large ulcer, and the other with the lesion at the venous puncture site — were eerily reminiscent of a case that I published last year on pyoderma gangrenosum. I felt the differential in this frightening case with systemic reaction was either pyoderma gangrenosum or blasto despite multiple negative fungal studies. They sent the patient to a tertiary care center — we won't say where — where the infectious disease people gave amphotericin B and an extremely academic dermatologist missed a diagnosis of pyoderma gangrenosum. Tragically, the patient later died from an immense pathergy reaction after undergoing cardiopulmonary bypass surgery. Do you have any comment about differential diagnosis with pyoderma gangrenosum?
Bradsher, Little Rock: Yes, it is very difficult. We've seen the opposite many times; people that have been said to have pyoderma and actually have blasto have been treated with steroids that, kind of, was a steroid challenge test just like our lady from the CPC with TB. There is a urine blasto antigen that's done now by Joe Wheat at MiraVista Diagnostics, and it could be useful in that kind of case. The majority of patients I see, it's pretty easy to find the organism of blasto if you look very carefully for it.
Bryan, Columbia: You can read my published case report. It screamed pyoderma gangrenosum.
Patterson, San Antonio: Thanks, Bob, for the nice presentation. I know the diagnosis is difficult, but as clinicians we think about typical risk factors; you know, foresters, workers, and the beavers have got a bad rap in this. I wonder if you might comment to the audience about the outbreak, and your thoughts, of the group in Wisconsin recently published that didn't seem to have any risk factors. This outbreak happened in the middle of the winter and was especially in Vietnamese patients.
Bradsher, Little Rock: Right, and that was a real interesting outbreak with the Hmong population having a 25- to 50-fold higher incidence than the typical Wisconsin person, and I think we just don't know yet. I am sure that there are going to be subtle immunological factors that we don't know about yet that would be similar to coccidioidomycosis in the Philippine population.
Neilson, Chicago: We still see blasto in Chicago and it doesn't seem to be related to Rockford. It raises the question, where does it come from in a stable environment? It seems like there is some relationship to excavation, building, and turning over earth, those sorts of things. Do you have an opinion about that?
Bradsher, Little Rock: Well there have only been — unlike histoplasma or cocci, which can be grown pretty regularly from soil and from outbreaks — a couple of times when blastomycosis has been isolated from outbreaks, one with tail-vein inoculation into some mice from an Eagle River outbreak in the 1990s. It's thought, though, to be around soil that is moist, that gets unearthed, either by recreation or occupational, or perhaps, excavational types of things. There is not quite the same similarity of association with excavation as with coccidioidomycosis or histoplasmosis with blasto, and maybe because it's just less common in the environment.
Goodenberger, St. Louis: My brother is a vet, and he tells me that blasto is common in dogs.
Bradsher, Little Rock: It's very common. In fact the epidemiology — that curve of the United States that I showed — really we have no other markers except for clinical infection. So those maps were drawn based on patients and dogs that had blastomycosis.
Goodenberger, St. Louis: So is there any relationship between dog blastomycosis and human blastomycosis?
Bradsher, Little Rock: The major one is that if you have a patient who you ask, “Do you have a dog?” “Well he died with blasto not long ago.” Maybe ought to make you think that may be a diagnosis here. So they probably get it in the same manner, just by inhaling the spores. There is no human-to-human or dog-to-human transmission with the exception of veterinarians or others that might have been bitten by a dog that had lesions in its mouth.
REFERENCES
- 1.Anonymous. Weekly clinicopathological exercises: case 121963. N Engl J Med. 1963;268:378–85. [Google Scholar]
- 2.Mackowiak PA. Diagnosis Giants: Solving the Medical Mysteries of Thirteen Patients Who Changed the World. New York: Oxford University Press; 2013. pp. 193–212. [Google Scholar]
- 3.Gilchrist TC. Protozoan dermatitis. J Cutan Gen Dis. 1894;12:496–9. [Google Scholar]
- 4.Gilchrist TC, Stokes WR. Case of pseudo-lupus vulgaris caused by blastomyces. J Exp Med. 1898;3:53–78. doi: 10.1084/jem.3.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarosi GA, Davies SF. Blastomycosis. State of the art. Am Rev Respir Dis. 1979;120:911–38. doi: 10.1164/arrd.1979.120.4.911. [DOI] [PubMed] [Google Scholar]
- 6.Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47:169–200. doi: 10.1097/00005792-196805000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Christie A, Peterson JC. Pulmonary calcifications in negative reactors to tuberculin. Am J Public Health. 1945;35:1131–47. doi: 10.2105/ajph.35.11.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dworkin MS, Duckro AN, Proia L, Semel JD, Huhn G. The epidemiology of blastomycosis in Illinois and factors associated with death. Clin Infect Dis. 2005;41:e107–11. doi: 10.1086/498152. [DOI] [PubMed] [Google Scholar]
- 9.Baumgardner DJ, Steber D, Glazier R. Geograhic information system analysis of blastomycosis in northern Wisconsin, USA: waterways and soil. Med Mycol. 2005;43:117–25. doi: 10.1080/13693780410001731529. [DOI] [PubMed] [Google Scholar]
- 10.Morris SK, Brophy J, Richardson SE, et al. Blastomycosis in Ontario, 1994–2003. Emerg Infect Dis. 2006;12:274–9. doi: 10.3201/eid1202.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529–34. doi: 10.1056/NEJM198602273140901. [DOI] [PubMed] [Google Scholar]
- 12.Klein BS, Vergeront JM, DiSalvo AF, et al. Two outbreaks of blastomycosis along rivers in Wisconsin: isolation of blastomyces dermatitidis from riverbank soil and evidence of its transmission along waterways. Am Rev Respir Dis. 1987;136:1333–38. doi: 10.1164/ajrccm/136.6.1333. [DOI] [PubMed] [Google Scholar]
- 13.Baumgardner DJ, Buggy BP, Mattson BJ, Burdick JS, Ludwig D. Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin. Clin Infect Dis. 1993;15:629–35. doi: 10.1093/clind/15.4.629. [DOI] [PubMed] [Google Scholar]
- 14.Reed KD, Meece JK, Archer JR, et al. Ecologic niche modeling of blastomyces dermatitidis in Wisconsin. PLoS ONE. 2008:e2034. doi: 10.1371/journal.pone.0002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meece JK, Anderson JL, Klein BS, et al. Genetic diversity in Blastomyces dermatitidis: implications for PCR detection in clinical and environmental samples. Med Mycol. 2010;48:285–90. doi: 10.1080/13693780903103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarosi GA, Eckman MR, Davies SF, Laskey WK. Canine blastomycosis as a harbinger of human disease. Ann Intern Med. 1983;98:48–9. doi: 10.7326/0003-4819-91-5-733. [DOI] [PubMed] [Google Scholar]
- 17.Steele RW, Abernathy RS. Systemic blastomycosis in children. Pediatr Infect Dis. 1983;2:304–7. doi: 10.1097/00006454-198307000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Bradsher RW. Clinical features of blastomycosis. Semin Respir Infect. 1997;12:229–34. [PubMed] [Google Scholar]
- 19.Bradsher RW, Rice DC, Abernathy RS. Ketoconazole therapy of endemic blastomycosis. Ann Intern Med. 1985;103:872–9. doi: 10.7326/0003-4819-103-6-872. [DOI] [PubMed] [Google Scholar]
- 20.Halvorsen RA, Duncan JD, Merten DF, Gallis HA, Putman CE. Pulmonary blastomycosis. Radiology. 1984;150:1–5. doi: 10.1148/radiology.150.1.6689749. [DOI] [PubMed] [Google Scholar]
- 21.Kinasewitz GT, Penn RL, George RB. The spectrum and significance of pleural disease in blastomycosis. Chest. 1984;86:580–584. doi: 10.1378/chest.86.4.580. [DOI] [PubMed] [Google Scholar]
- 22.Bariola JR, Perry P, Pappas PG, et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis. 2010;50:797–804. doi: 10.1086/650579. [DOI] [PubMed] [Google Scholar]
- 23.Evans ME, Haynes JB, Atkins JB, Atkinson JB, Delvaux TC, Jr, Kaiser AB. Blastomyces dermatitidis and the adult respiratory distress syndrome. Am Rev Respir Dis. 1982;126:1099–1102. doi: 10.1164/arrd.1982.126.6.1099. [DOI] [PubMed] [Google Scholar]
- 24.Meyer KC, McManus EJ, Maki DG. Overwhelming pulmonary blastomycosis associated with the adult respiratory distress syndrome. N Engl J Med. 1993;329:1231–6. doi: 10.1056/NEJM199310213291704. [DOI] [PubMed] [Google Scholar]
- 25.Skillrud DM, Douglas WW. Survival in ARDS caused by blastomycosis infection. Mayo Clin Proc. 1985;60:266–9. doi: 10.1016/s0025-6196(12)60320-6. [DOI] [PubMed] [Google Scholar]
- 26.Bradsher RW, Martin MR, Wilkes TD, Waltman C, Bolyard K. Unusual presentations of blastomycosis: ten case summaries. Infect Med. 1990;7:10–9. [Google Scholar]
- 27.Suen JY, Wetmore SJ, Wetzel WJ. Blastomycosis of the larynx. Ann Otol. 1980;89:563–6. doi: 10.1177/000348948008900616. [DOI] [PubMed] [Google Scholar]
- 28.Pappas PG, Pottage JC, Powderly WG, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1992;116:847–53. doi: 10.7326/0003-4819-116-10-847. [DOI] [PubMed] [Google Scholar]
- 29.Farmer C, Stanley MW, Bardales RH, et al. Mycoses of the breast: diagnosis by fine needle aspiration. Diagn Cytopathol. 1995;12:51–5. doi: 10.1002/dc.2840120112. [DOI] [PubMed] [Google Scholar]
- 30.Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin N Am. 2003;17:21–40. doi: 10.1016/s0891-5520(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 31.Lopez R, Mason JO, Parker JS, Pappas PG. Intraocular blastomycosis: case report and review. Clin Infect Dis. 1994;18:805–7. doi: 10.1093/clinids/18.5.805. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman J. Tracheal blastomycosis. Chest. 1988;93:424–5. doi: 10.1378/chest.93.2.424. [DOI] [PubMed] [Google Scholar]
- 33.Istorico LJ, Sanders M, Jacobs RF, Gillian S, Glasier C, Bradsher RW. Otitis media due to blastomycosis. Clin Infect Dis. 1992;14:335–8. doi: 10.1093/clinids/14.1.355. [DOI] [PubMed] [Google Scholar]
- 34.Young L, Schutze GE. Perinatal blastomycosis: the rest of the story. Pediatr Infect Dis J. 1995;14:83. [PubMed] [Google Scholar]
- 35.Farber ER, Leahy MS, Meadows TR. Endometrial blastomycosis acquired by sexual contact. Obstetr Gynecol. 1968;32:195–9. [PubMed] [Google Scholar]
- 36.Winquist EW, Walmsley SL, Berinstein NL. Reactivation and dissemination of blastomycosis complicating Hodgkin's disease. Am J Hematol. 1993;43:129–32. doi: 10.1002/ajh.2830430211. [DOI] [PubMed] [Google Scholar]
- 37.Pappas PG, Threlkeld MG, Bedsole GD, Cleveland KO, Gelfand MS, Dismukes WE. Blastomycosis in immunocompromised patients. Medicine. 1993;72:311–25. doi: 10.1097/00005792-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Chapman SW, Dismukes WE, Proia AL, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801–12. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 39.Pappas PG, Bradsher RW, Kaufmann CA, et al. Treatment of blastomycosis with higher doses of fluconazole. Clin Infect Dis. 1997;25:200–5. doi: 10.1086/514539. [DOI] [PubMed] [Google Scholar]