Abstract
Noninvasive prenatal genetic testing is becoming available worldwide—particularly in low- and middle-income countries—but practical and ethical challenges must be overcome.
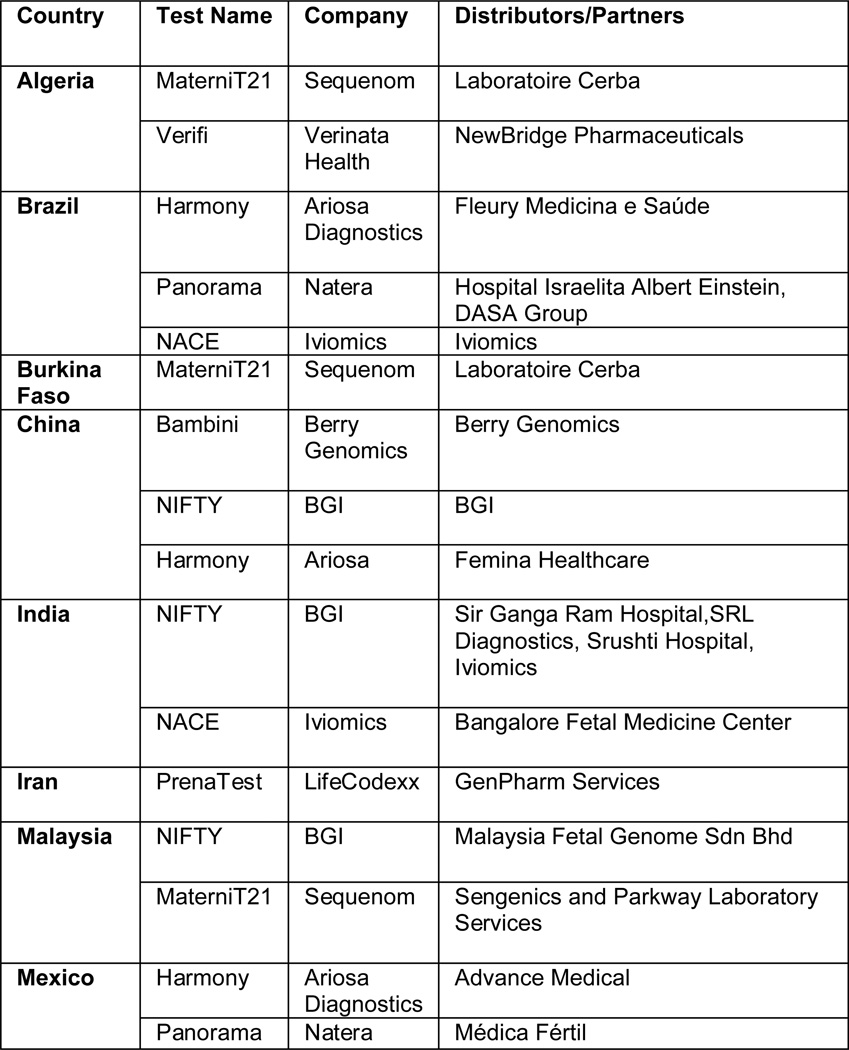
Noninvasive prenatal testing (NIPT) using cell-free fetal DNA (cffDNA) circulating in maternal blood can detect common fetal trisomies, such as Down syndrome, fetal Rhesus D status, sex chromosome disorders, and fetal sex. NIPT is currently marketed in the United States by four commercial providers (Sequenom, Natera, Verinata, and Ariosa), in Europe by LifeCodexx, and in China by two companies (Berry Genomics and BGI). Ongoing research promises to expand the range of conditions that can be tested noninvasively to include microdeletion/duplication syndromes and common Mendelian genetic disorders (1). The commercialization of tests to detect chromosomal aneuploidies has been rapid, and global marketing is steadily increasing. NIPT was introduced in the United States and Western Europe in late 2011, and tests are rapidly becoming available in the Middle East, South America, South and Southeast Asia, and Africa (Fig. 1 and table S1).
Fig. 1. Select global availability of NIPT for chromosomal aneuploidies.
This table shows noninvasive prenatal genetic tests being offered commercially in select developing and developed countries, where NIPT is currently marketed, or where marketing deals have been signed. This list is a subset of 61 countries where NIPT is currently marketed (the full list is available in table S1), generated from Internet searches of press releases, company websites, industry trade reports, and newspapers/popular press articles between 1 January 2012 and 15 March 2014. Searches focused only on commercial offering of noninvasive prenatal tests for chromosomal aneuploidies using cell-free fetal DNA.
The implementation of NIPT in developing countries may offer benefits over standard prenatal genetic testing methods, which are limited to less-accurate serum protein screens or invasive testing (such as sampling amniotic fluid), which carries a small risk of miscarriage. Because NIPT requires only a blood draw, it may reduce the need for trained medical personnel to perform invasive diagnostic procedures and make genetic testing more accessible in resource-poor areas. Early detection of chromosomal and other genetic disorders may also allow better management of pregnancies and the mobilization of scarce financial and/or medical resources required to manage the health of a newborn affected by a genetic abnormality. Early detection may also allow for earlier and safer termination of a pregnancy, including medical termination, in countries where abortion is legally available.
Despite potential benefits, NIPT raises several ethical and practical issues, including the regulation of test dissemination and quality, especially when considering the return of fetal sex information, equity of access, and patient and provider education and understanding needed for appropriate implementation.
REGULATORY GAPS IN PRENATAL TESTING
Many developing countries have had difficulties in regulating new reproductive technologies, such as preimplantation genetic diagnosis or in vitro fertilization, and it is not yet clear how NIPT will be integrated into existing regulatory structures. For example, both China and India forbid returning information about fetal sex to potential parents in order to dissuade sex-based abortion. NIPT can return accurate fetal sex information from 7 weeks of pregnancy (2), which is earlier than with existing methods of determining sex, including most ultrasounds. Companies currently offering NIPT in China and India are aware of these national laws and do not advertise sex testing. However, fetal sex-determination tests that useless-validated methods, such as urine or saliva screening, are already marketed directly to consumers and remain unregulated in many jurisdictions (3).
It is conceivable that new companies may use NIPT technology to launch tests that similarly return fetal sex information directly to consumers. The question of whether the state has an interest in regulating such efforts is of more than academic interest in countries with deeply skewed sex ratios as a result of the practice of sex-based abortion—especially because the effectiveness of these laws in preventing medical personnel from sharing information about fetal sex is already questionable (4, 5). If patients or practitioners have the option to send samples internationally to jurisdictions where such laws do not apply, the ability of governments to control access to prenatal sex information is likely to be compromised even further.
Countries are also struggling to stay ahead of technical innovation when it comes to regulatory validation and approval. In the United States, Ariosa, Natera, Sequenom, and Verinata—the four companies that offer the majority of the commercial testing (table S1)—have performed large-scale validation studies in United States or British populations. All U.S. company laboratories performing NIPT are also compliant with quality-assurance regulations, such as the U.S. Clinical Laboratory Improvement Act, which helps ensure that test quality and reproducibility are maintained. Although the U.S. Food and Drug Administration has thus far exercised its discretionary power in declining to assert regulatory jurisdiction over NIPT, it has indicated that these tests may potentially be considered “high risk” and subject to future regulation (6). In other nations, however, local validation studies have not been conducted, and there is little, if any, regulatory control of either test content or quality. Because some prenatal tests offered in other countries are through licensed purveyors of U.S.-based tests, U.S.-based companies rely on local regulatory agencies to enforce quality and reliability.
The Chinese FDA announced recently that it was freezing the practice of clinical sequencing in China until the tests currently in use could be validated for safety and accuracy. Although the ban applies to all clinical tests, noninvasive prenatal genetic testing constitutes the largest portion of DNA-sequencing tests marketed in China, and some commentators speculate that the ban is targeted to prenatal genetic testing offered by Berry Genomics and BGI (7). BGI has confirmed that it has ceased all sequencing-based prenatal testing in China (www.genomeweb.com/sequencing/bgi-suspends-clinical-ngs-based-trisomy-testing-china). However, it is unclear whether this ban applies to testing that BGI performs in facilities outside China, such as in Europe or Hong Kong, where samples from China and other countries could potentially be processed.
COST AND ACCESS
Another concern is access to noninvasive tests and the information they provide, especially in low- and middle-income countries (LMICs), where large disparities in access to prenatal genetic services already exist (8). Out-of-pocket costs for NIPT in the United States are high, especially for the uninsured. Even if commercial providers offer substantial discounts in LMICs, NIPT will remain unaffordable for many families. For instance, according to a Gallup poll (www.gallup.com/poll/166211/worldwide-median-household-income-000.aspx#2), the 2013 median household income reported in China was approximately US$515. When available, NIPT was offered in China for approximately 2800 to 3600 yuan or US$457 to $587. Amniocentesis, meanwhile, costs approximately 2000 yuan or US$326 and is covered by most state and national programs (www.babytree.com/ask/detail/8223572 [link in Chinese]). In Brazil, the reported monthly income in 2013 was US$626. NIPT is sold in Brazil for 3500 Real or US$1492, whereas amniocentesis averages 1000 Real or US$426.
In both China and Brazil, NIPT tests are not covered by private or state insurance, so customers must pay the entire cost out-of-pocket. Furthermore, in many developing countries, these tests are offered through private specialty institutions—for example, in in vitro fertilization (IVF) clinics in large metropolitan cities that cater to a select and highly affluent client base. Health care systems in many of these countries already rely heavily on subsidies from employer-provided private health insurance or on state welfare programs, and infrastructure challenges result in less-than-adequate prenatal care for many women. In regions where state programs are weak or nonexistent and private health insurance is the purview of the wealthy, differential use of these new technologies may exacerbate inequities in prenatal care already observed in rural and resource-poor areas.
To date, commercial interest has focused on developing NIPT for genetic conditions for which there is high demand, such as screening for Down syndrome. National agencies, however, need comprehensive genetic services that can address common Mendelian genetic conditions as well, such as thalassemias or sickle cell disease, which have sizeable public health consequences (9). This will affect how countries prioritize resources; choose tests and NIPT technologies; integrate NIPT into prenatal screening programs; and, ultimately, decide the cost of implementing NIPT into health care systems. If cost effectiveness is not established, public health agencies may dismiss NIPT as unnecessary or expensive. The absence of parallel public sector initiatives for implementing NIPT could be a lost opportunity for capacity building in genetic medicine and for reducing disparities in access to prenatal care.
INTERPRETING RESULTS AND INFORMED DECISION-MAKING
Educating both patients and providers about the potential advantages and, more importantly, the limitations of noninvasive prenatal testing may prove even more challenging in LMICs than in developed nations. It is not currently clear, for example, whether companies selling tests in many developing countries (table S1) are offering genetic counseling services to recipients or leaving that to the physicians ordering the tests. The lack of trained genetic counselors and medical geneticists combined with low genetic literacy may diminish informed decision-making during prenatal testing (8). Embedded cultural frameworks that emphasize familial consent over individual informed consent may further complicate this problem. Moreover, strong cultural and familial norms, such as favoring male children and stigmas against disabilities, combined with overt or tacit restrictions on the number of children in some jurisdictions, may affect decision-making and even render families vulnerable to targeting by unethical or fraudulent “providers” and financial exploitation.
These are issues that require immediate attention as noninvasive prenatal tests become available globally. Although research on stakeholder experiences and best practices is emerging in the United States and Europe (10), there is a need to solicit the views of stakeholders from developing countries. Empirical research in these countries is necessary to understand how commercial NIPT is being disseminated and its impact on prenatal care. Decisions by companies, local and national governments, nongovernmental organizations, professional societies, regulatory agencies, and international agencies will all affect how effectively and ethically NIPT is implemented in LMICs. It is in the best interests of everyone—patients, providers, regulators, and states—to consider these issues and begin a dialogue as soon as possible.
Supplementary Material
Acknowledgments
We thank R. Cook-Deegan and L. Dame for helpful comments. Funding: S.C. is supported by R01HG007074. S.C. and M.A.M. are supported by P50HG03391. All authors acknowledge support from the Duke University Bass Connections initiative.
Footnotes
Supplementary materials
Methods
Table S1. Global availability of noninvasive prenatal genetic testing for chromosomal aneuploidies.
References and Notes
- 1.Agarwal A, Sayres LC, Cho MK, Cook-Deegan R, Chandrasekharan S. Commercial landscape of noninvasive prenatal testing in the United States. Prenat. Diagn. 2013;33:521–531. doi: 10.1002/pd.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devaney SA, Palomaki GE, Scott JA, Bianchi DW. Noninvasive fetal sex determination using cell-free fetal DNA: A systematic review and meta-analysis. JAMA. 2011;306:627–636. doi: 10.1001/jama.2011.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javitt GH. Pink or blue? The need for regulation is black and white. Fertil. Steril. 2006;86:13–15. doi: 10.1016/j.fertnstert.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Nie JB. Non-medical sex-selective abortion in China: ethical and public policy issues in the context of 40 million missing females. Br. Med. Bull. 2011;98:7–20. doi: 10.1093/bmb/ldr015. [DOI] [PubMed] [Google Scholar]
- 5.Jain A. Sex selection and abortion in India. BMJ. 2013;346:f1957. doi: 10.1136/bmj.f1957. [DOI] [PubMed] [Google Scholar]
- 6.Weaver C. Tough Calls on Prenatal Tests. [accessed March 2014];WSJ. 2013 Apr 3; available at http://online.wsj.com/news/articles/SB10001424127887324883604578398791568615644. [Google Scholar]
- 7.Chen JSC. China Cracks Down on DNA Testing. [accessed March 2014];Forbes. 2014 Mar 3; available at www.forbes.com/sites/shuchingjeanchen/2014/03/03/china-cracks-down-on-dna-testing-2. [Google Scholar]
- 8.Community Genetics Services. Geneva, Switzerland: World Health Organization; 2010. Sep 13 to 14, Report of a WHO Consultation on community genetics in low- and middle-income countries. [Google Scholar]
- 9.Lench N, Barrett A, Fielding S, McKay F, Hill M, Jenkins L, White H, Chitty LS. The clinical implementation of noninvasive prenatal diagnosis for single-gene disorders: Challenges and progress made. Prenat. Diagn. 2013;33:555–562. doi: 10.1002/pd.4124. [DOI] [PubMed] [Google Scholar]
- 10.Allyse MA, Sayres LC, Havard M, King JS, Greely HT, Hudgins L, Taylor J, Norton ME, Cho MK, Magnus D, Ormond KE. Best ethical practices for clinicians and laboratories in the provision of noninvasive prenatal testing. Prenat. Diagn. 2013;33:656–661. doi: 10.1002/pd.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.