Abstract
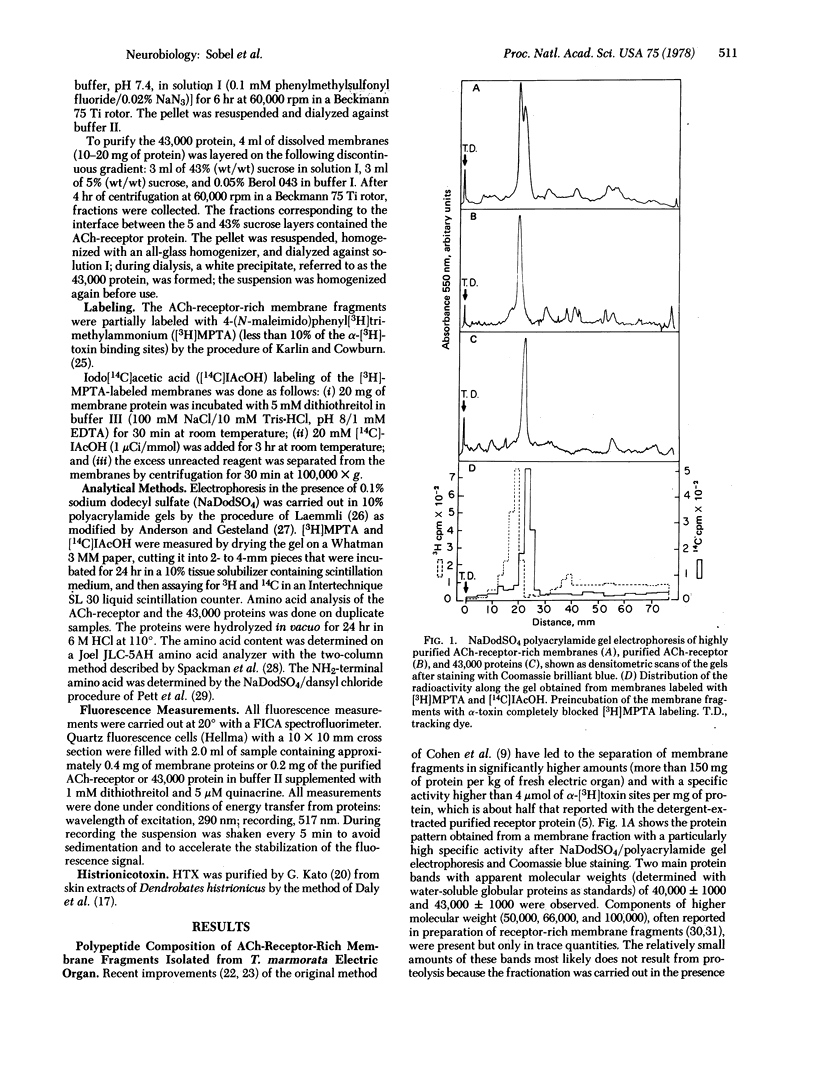
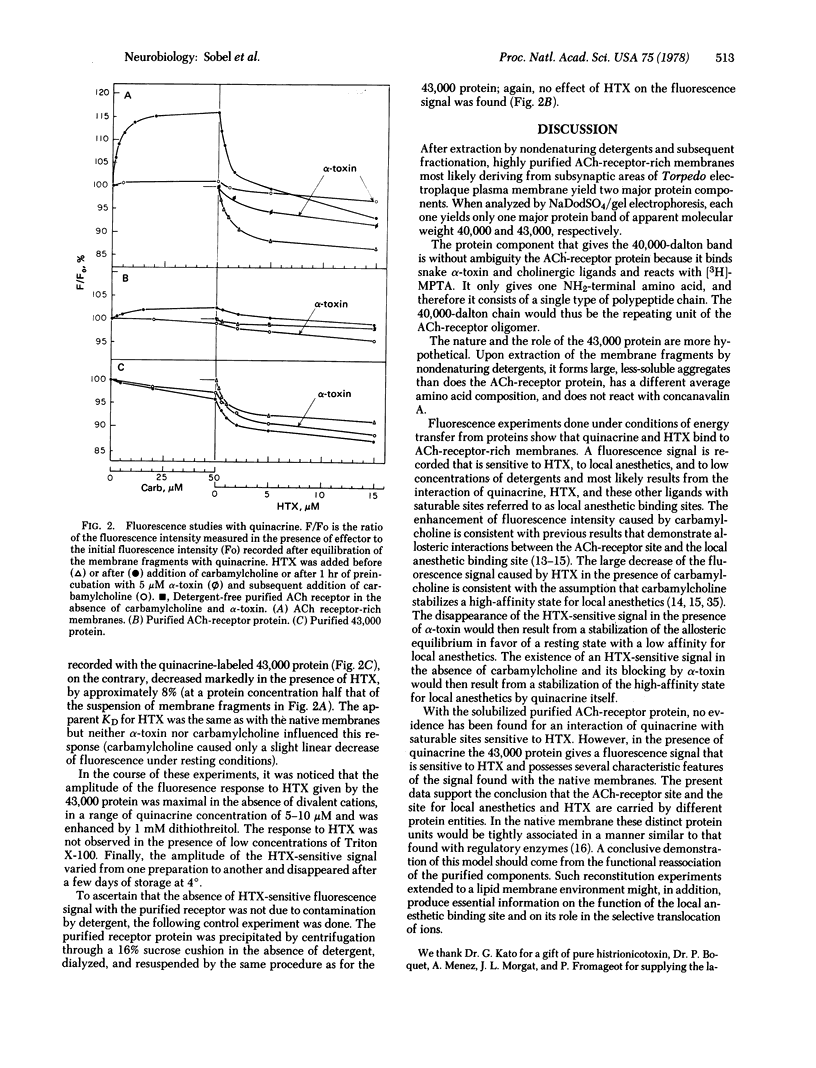
Highly purified subsynaptic membrane fragments prepared from Torpedo marmorata electric organ (specific activity, greater than 4 mumol of Naja nigricollis alpha-[3H]toxin per mg of protein) exhibit, on sodium dodecyl sulfate/polyacrylamide gel electrophoresis, two major protein bands of apparent molecular weight 40,000 and 43,000, respectively. Dissolution of these membranes by the nondenaturing detergents Triton X-100 and Berol 043 followed by standard fractionation yielded (i) the 9S acetylcholine-receptor protein which still binds the alpha-[3H]toxin and after further purification yielded, in the presence of sodium dodecyl sulfate, the 40,000-dalton component, covalently labeled by the affinity reagent 4-(N-maleimido)phenyl[3H]trimethylammonium; only serine was found as the NH2-terminal amino acid of this protein; and (ii) a high molecular weight aggregate named 43,000 protein which was resolved in denaturing gels almost exclusively as the 43,000-dalton band, In the absence of detergents, the 43,000 protein binds compounds known to interact with the acetylcholine ionophore: a fluorescent local anesthetic quinacrine and histrionicotoxin (apparent dissociation constant, 7 +/- 1 X 10(-7) M). The regulation of quinacrine fluorescennce by carbamylcholine, observed in the intact membrane, no longer occurs with the isolated 43,000 component.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Barnard E. A., Chiu T. H., Lapa A. J., Dolly J. O., Jansson S. E., Daly J., Witkop B. Acetylcholine receptor and ion conductance modulator sites at the murine neuromuscular junction: evidence from specific toxin reactions. Proc Natl Acad Sci U S A. 1973 Mar;70(3):949–953. doi: 10.1073/pnas.70.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Kuba K., Daly J. Effect of histrionicotoxin on the ionic conductance modulator of the cholinergic receptor: a quantitative analysis of the end-plate current. J Pharmacol Exp Ther. 1974 May;189(2):513–524. [PubMed] [Google Scholar]
- Anderson C. W., Gesteland R. F. Pattern of protein synthesis in monkey cells infected by simian virus 40. J Virol. 1972 May;9(5):758–765. doi: 10.1128/jvi.9.5.758-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson A., Devaux P. F., Changeux J. P. Effet anesthésique local de plusieurs composés liposolubles sur la réponse de l'électroplaque de Gymnote à la carbamylcholine et sur la liaison de l'acétylcholine au récepteur cholinergique de Torpille. C R Acad Sci Hebd Seances Acad Sci D. 1975 May 12;280(18):2153–2156. [PubMed] [Google Scholar]
- Cohen J. B., Weber M., Huchet M., Changeux J. P. Purification from Torpedo marmorata electric tissue of membrane fragments particularly rich in cholinergic receptor protein. FEBS Lett. 1972 Oct 1;26(1):43–47. doi: 10.1016/0014-5793(72)80538-6. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Karle I., Myers C. W., Tokuyama T., Waters J. A., Witkop B. Histrionicotoxins: roentgen-ray analysis of the novel allenic and acetylenie spiroalkaloids isolated from a Colombian frog, Dendrobates histrionicus. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1870–1875. doi: 10.1073/pnas.68.8.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolly J. O., Albuquerque E. X., Sarvey J., Mallick B., Barnard E. A. Binding of perhydro-histrionicotoxin to the postsynaptic membrane of skeletal muscle in relation to its blockage of acetylcholine-induced depolarization. Mol Pharmacol. 1977 Jan;13(1):1–14. [PubMed] [Google Scholar]
- Eldefrawi A. T., Eldefrawi M. E., Albuquerque E. X., Oliveira A. C., Mansour N., Adler M., Daly J. W., Brown G. B., Burgermeister W., Witkop B. Perhydrohistrionicotoxin: a potential ligand for the ion conductance modulator of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1977 May;74(5):2172–2176. doi: 10.1073/pnas.74.5.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T. Purification and molecular properties of the acetylcholine receptor from Torpedo electroplax. Arch Biochem Biophys. 1973 Nov;159(1):362–373. doi: 10.1016/0003-9861(73)90462-1. [DOI] [PubMed] [Google Scholar]
- Grünhagen H. H., Changeux J. P. Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. V. Qualitative correlation between pharmacological effects and equilibration processes of the cholinergic receptor protein as revealed by the structural probe quinacrine. J Mol Biol. 1976 Sep 25;106(3):517–535. doi: 10.1016/0022-2836(76)90250-3. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Changeux J. P. Reconstitution of a chemically excitable membrane. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1479–1483. doi: 10.1073/pnas.71.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Cowburn D. The affinity-labeling of partially purified acetylcholine receptor from electric tissue of Electrophorus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3636–3640. doi: 10.1073/pnas.70.12.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Weill C. L., McNamee M. G., Valderrama R. Facets of the structures of acetylcholine receptors from Electrophorus and Torpedo. Cold Spring Harb Symp Quant Biol. 1976;40:203–210. doi: 10.1101/sqb.1976.040.01.022. [DOI] [PubMed] [Google Scholar]
- Kato G., Changeux J. P. Studies on the effect of histrionicotoxin on the monocellular electroplax from Electrophorus electricus and on the binding of (3H)acetylcholine to membrane fragments from Torpedo marmorata. Mol Pharmacol. 1976 Jan;12(1):92–100. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y. Elapid neurotoxins and their mode of action. Clin Toxicol. 1970 Sep;3(3):457–472. doi: 10.3109/15563657008990119. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Menez A., Morgat J. -L., Fromageot P., Ronseray A. -M., Boquet P., Changeux J. -P. Tritium labelling of the alpha-neurotoxin of Naja nigricollis. FEBS Lett. 1971 Oct 1;17(2):333–335. doi: 10.1016/0014-5793(71)80180-1. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Sealock R., Olsen R., Changeux J. P. Purification and properties of the cholinergic receptor protein from Electrophorus electricus electric tissue. Eur J Biochem. 1974 Jun 15;45(2):371–394. doi: 10.1111/j.1432-1033.1974.tb03563.x. [DOI] [PubMed] [Google Scholar]
- PODLESKI T. R., BARTELS E. DIFFERENCE BETWEEN TETRACAINE AND D-TUBOCURARINE IN THE COMPETITION WITH CARBAMYLCHOLINE. Biochim Biophys Acta. 1963 Nov 29;75:387–396. doi: 10.1016/0006-3002(63)90626-7. [DOI] [PubMed] [Google Scholar]
- Pett D. M., Vanaman T. C., Joklik W. K. Studies on the amino and carboxyl terminal amino acid sequences of reovirus capsid polypeptides. Virology. 1973 Mar;52(1):174–186. doi: 10.1016/0042-6822(73)90407-8. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Sugiyama H., Changeux J. P. Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. II. The permeability response of the receptor-rich membrane fragments to cholinergic agonists in vitro. J Mol Biol. 1976 Sep 25;106(3):469–483. doi: 10.1016/0022-2836(76)90247-3. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Vandlen R. L., Reed K. L., Lee T. Characterization of Torpedo californica acetylcholine receptor: its subunit composition and ligand-binding properties. Cold Spring Harb Symp Quant Biol. 1976;40:193–202. doi: 10.1101/sqb.1976.040.01.021. [DOI] [PubMed] [Google Scholar]
- Sobel A., Changeux J. P. Purification and characterization of the cholinergic receptor protein in its membrane-bound and detergent-soluble forms from the electric organ of Torpedo marmorata. Biochem Soc Trans. 1977;5(2):511–514. doi: 10.1042/bst0050511. [DOI] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Weber M., Changeux J. P. Binding of Naja nigricollis (3H)alpha-toxin to membrane fragments from Electrophorus and Torpedo electric organs. 3. Effects of local anaesthetics on the binding of the tritiated alpha-neurotoxin. Mol Pharmacol. 1974 Jan;10(1):35–40. [PubMed] [Google Scholar]



