Abstract
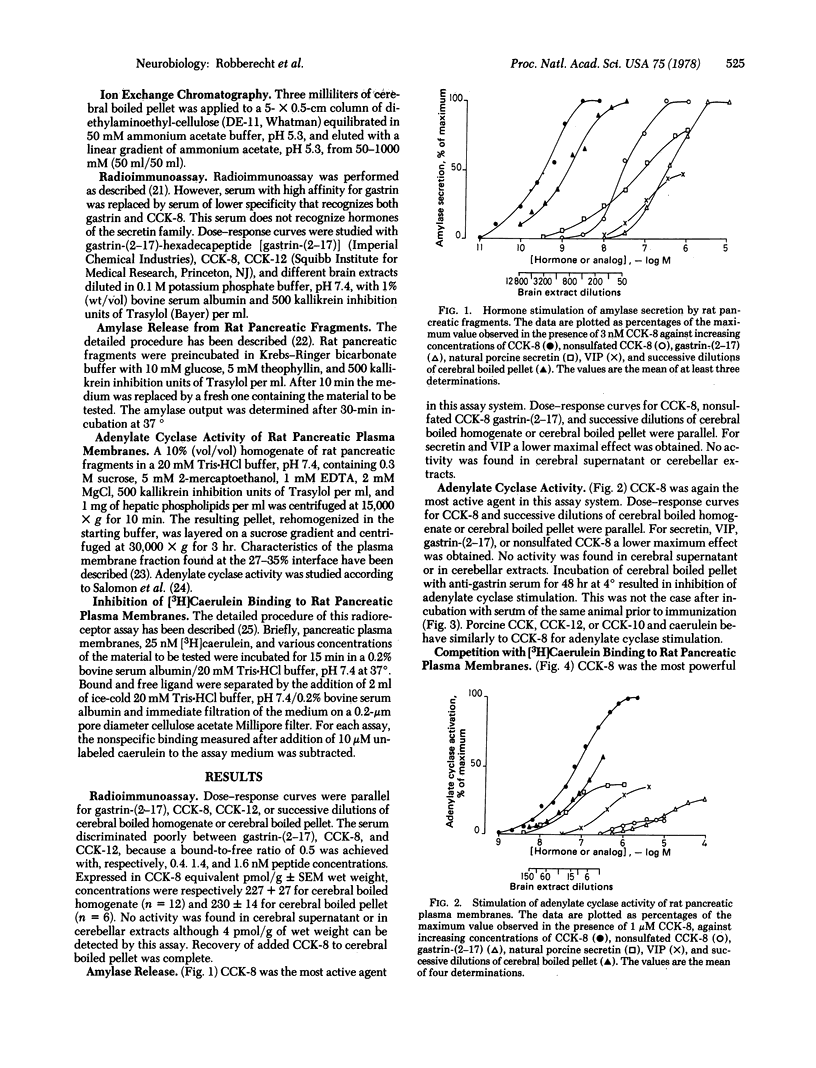
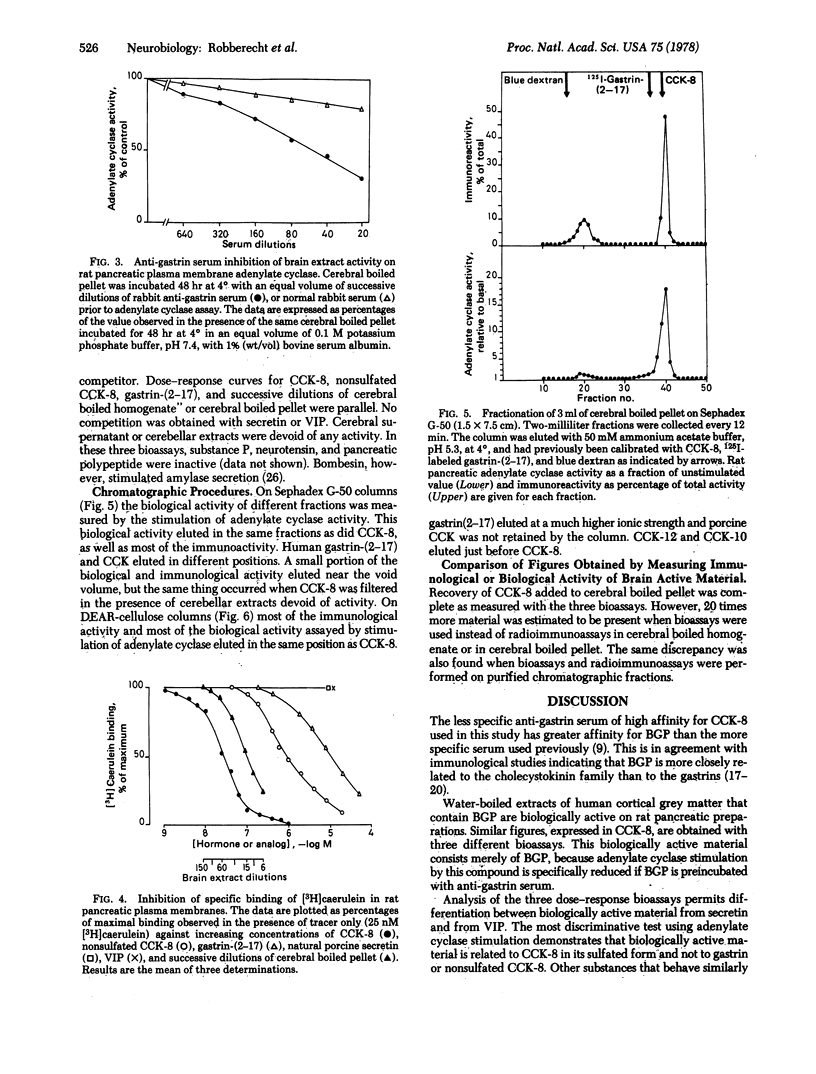
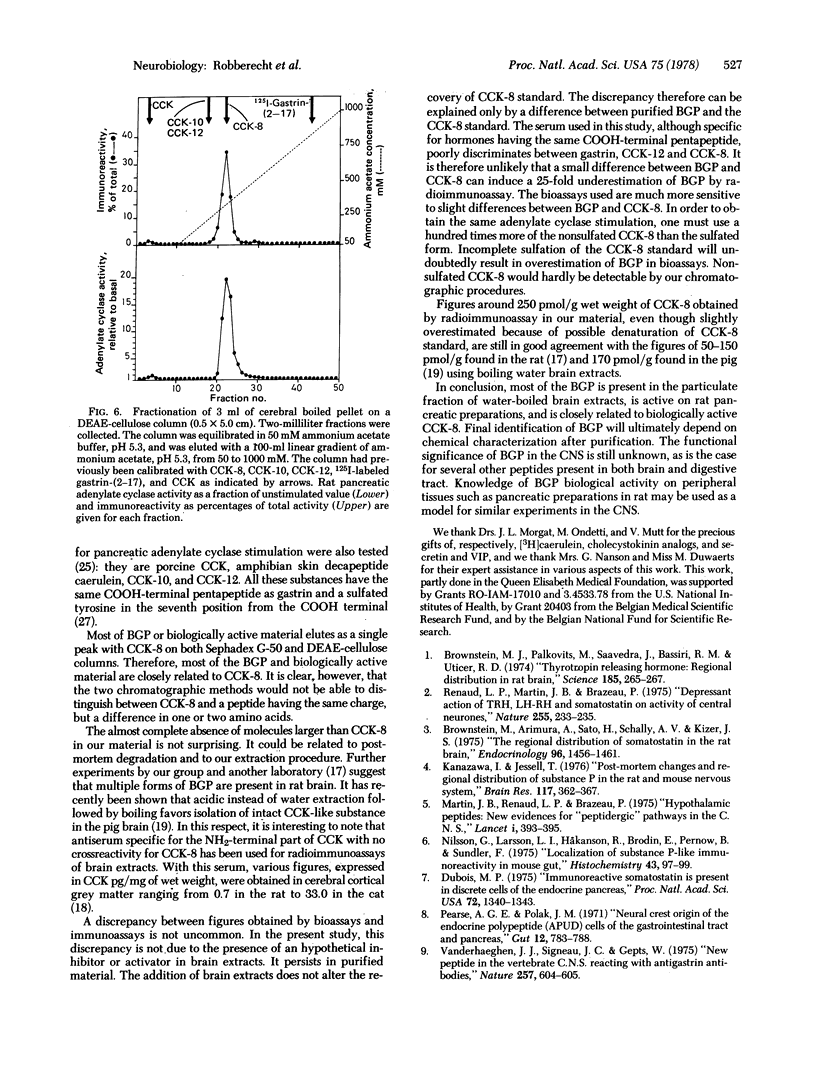
The previously described peptide material that reacts with antibodies to gastrin and is found in the central nervous system of various vertebrates is present in only the 100,000 X g pellet of postmortem human cerebral cortical grey matter. This immunoreactive material, extractable in boiling water, is biologically active on rat pancreatic preparations. On the basis of size, charge, immunological specificity, and patterns of biological activity, most of this material is closely related to the COOH-terminal octapeptide of cholecystokinin in its complete, sulfated biologically active form.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M., Rivier J., Vale W. Bombesin:potent effects on thermoregulation in the rat. Science. 1977 May 27;196(4293):998–1000. doi: 10.1126/science.860130. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Palkovits M., Saavedra J. M., Bassiri R. M., Utiger R. D. Thyrotropin-releasing hormone in specific nuclei of rat brain. Science. 1974 Jul 19;185(4147):267–269. doi: 10.1126/science.185.4147.267. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Arimura A., Sato H., Schally A. V., Kizer J. S. The regional distribution of somatostatin in the rat brain. Endocrinology. 1975 Jun;96(6):1456–1461. doi: 10.1210/endo-96-6-1456. [DOI] [PubMed] [Google Scholar]
- Bryant M. G., Polak M. M., Modlin I., Bloom S. R., Albuquerque R. H., Pearse A. G. Possible dual role for vasoactive intestinal peptide as gastrointestinal hormone and neurotransmitter substance. Lancet. 1976 May 8;1(7967):991–993. doi: 10.1016/s0140-6736(76)91863-8. [DOI] [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine, and stomach. J Biol Chem. 1976 Nov 25;251(22):7045–7052. [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Labrie F., Christophe J. In vitro interactions of gastrointestinal hormones on cyclic adenosine 3':5'-monophosphate levels and amylase output in the rat pancreas. Gastroenterology. 1975 Feb;68(2):318–325. [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Lammens M., Christophe J. In vitro action of bombesin and bombesin-like peptides on amylase secretion, calcium efflux, and adenylate cyclase activity in the rat pancreas: a comparison with other secretagogues. J Clin Invest. 1976 Oct;58(4):891–898. doi: 10.1172/JCI108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Molecular evolution of gut hormones: application of comparative studies on the regulation of digestion. Gastroenterology. 1977 Feb;72(2):344–358. [PubMed] [Google Scholar]
- Dubois M. P. Immunoreactive somatostatin is present in discrete cells of the endocrine pancreas. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1340–1343. doi: 10.1073/pnas.72.4.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa I., Jessell T. Post mortem changes and regional distribution of substance P in the rat and mouse nervous system. Brain Res. 1976 Nov 26;117(2):362–367. doi: 10.1016/0006-8993(76)90748-4. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Schaffalitzky De Muckadell O., Sundler F., Håkanson R., Rehfeld J. R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotstra F., van der Loo W., Gepts W. Are gastrin-cells present in mammalian pancreatic islets? Diabetologia. 1974 Aug;10(4):291–302. doi: 10.1007/BF02627730. [DOI] [PubMed] [Google Scholar]
- Martin J. B., Renaud L. P., Brazeau P. Hypothalamic peptides: New evidence for "peptidergic" pathways in the C.N.S. Lancet. 1975 Aug 30;2(7931):393–395. doi: 10.1016/s0140-6736(75)92901-3. [DOI] [PubMed] [Google Scholar]
- Muller J. E., Straus E., Yalow R. S. Cholecystokinin and its COOH-terminal octapeptide in the pig brain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3035–3037. doi: 10.1073/pnas.74.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G., Larsson L. I., Håkanson R., Brodin E., Pernow B., Sundler F. Localization of substance P-like immunoreactivity in mouse gut. Histochemistry. 1975;43(1):97–99. doi: 10.1007/BF00490158. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M. Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut. 1971 Oct;12(10):783–788. doi: 10.1136/gut.12.10.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Bloom S. R., Sullivan S. N., Facer P., Pearse A. G. Enkephalin-like immunoreactivity in the human gastrointestinal tract. Lancet. 1977 May 7;1(8019):972–974. doi: 10.1016/s0140-6736(77)92277-2. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Martin J. B., Brazeau P. Depressant action of TRH, LH-RH and somatostatin on activity of central neurones. Nature. 1975 May 15;255(5505):233–235. doi: 10.1038/255233a0. [DOI] [PubMed] [Google Scholar]
- Said S. I., Rosenberg R. N. Vasoactive intestinal polypeptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science. 1976 May 28;192(4242):907–908. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Straus E., Muller J. E., Choi H. S., Paronetto F., Yalow R. S. Immunohistochemical localization in rabbit brain of a peptide resembling the COOH-terminal octapeptide of cholecystokinin. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3033–3034. doi: 10.1073/pnas.74.7.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda M., Robberecht P., Camus J., Deschodt-Lanckman M., Christophe J. Subcellular distribution and response to gastroinetstinal hormones of adenylate cyclase in the rat pancreas. Partial purification of a stable plasma membrane preparation. Eur J Biochem. 1976 Oct 1;69(1):185–193. doi: 10.1111/j.1432-1033.1976.tb10872.x. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]



