Summary
The fungal cell wall is a dynamic organelle required for cell shape, protection against the environment and, in pathogenic species, recognition by the innate immune system. The outer layer of the cell wall is comprised of glycosylated mannoproteins with the majority of these post‐translational modifications being the addition of O‐ and N‐linked mannosides. These polysaccharides are exposed on the outer surface of the fungal cell wall and are, therefore, the first point of contact between the fungus and the host immune system. This review focuses on O‐ and N‐linked mannan biosynthesis in the fungal pathogen Candida albicans and highlights new insights gained from the characterization of mannosylation mutants into the role of these cell wall components in host–fungus interactions. In addition, we discuss the use of fungal mannan as a diagnostic marker of fungal disease.
Introduction
Candida albicans is an opportunistic fungal pathogen of humans, which is part of the natural flora of the oral, genital and gastrointestinal tracts. The maintenance of colonization over dissemination is achieved through an intricate balance of fungal proliferation and host immune recognition and control. During periods of immune suppression, caused by chemotherapy, trauma, age and cancer, C. albicans is able to overcome the immune system, disseminate and cause life‐threatening systemic disease. The associated mortality rates of systemic fungal disease are reported to be up to 40%, which is higher than that reported for most bacterial infections (Almirante et al., 2005; Klevay et al., 2008; Leroy et al., 2009). It is also a frequent mucosal pathogen, with more than 75 million women suffering from vaginitis each year (Sobel, 2007).
The interplay between C. albicans and the host immune system is largely mediated by components of the fungal cell wall including mannans, β‐glucans and chitin. The structural organization of the fungal cell wall has been extensively reviewed elsewhere (Bowman and Free, 2006; Latgé, 2007; Gow and Hube, 2012), but comprehensive reviews on fungal mannan biosynthesis are limited. This review focuses on O‐ and N‐mannan biosynthesis, the role(s) of mannans in innate immune recognition, and the use of fungal mannan as a diagnostic marker for invasive candidaemia.
The cell wall
The fungal cell wall is a dynamic structure important for maintaining cell shape, protection and stability against environmental stresses and outwardly directed turgor pressure and for immunogenicity. The cell wall must be physically robust, but also flexible enough to permit cell expansion, cell division and morphogenesis. The wall must also be permeable to allow egress of secreted proteins and the import of solutes, but sufficiently impermeable to protect the inner skeletal layer from environmental hydrolases. The cell wall is comprised of three major polysaccharides, chitin, glucans and mannans. In C. albicans, these polysaccharides are organized as two layers: an inner skeletal layer of chitin and β1,3‐linked glucan and an outer layer of β1,6‐glucan and cell wall proteins anchored to the skeletal layer via a glycosylphosphatidylinositol (GPI) remnant. These proteins include cell wall remodelling enzymes involved in cell wall biogenesis (Douglas et al., 1997; Dünkler et al., 2005), modification of the properties of the nascent and mature polysaccharides, and proteins essential for adhesion (Buurman et al., 1998; Hoyer, 2001) and biofilm formation (Nobile et al., 2006; Zhao et al., 2006), all of which influence the pathogenesis of the organism. The cell wall and secreted proteins of C. albicans are highly decorated with elaborate carbohydrate structures comprised of α‐ and β‐linked mannose units referred to as mannoproteins. Mannose sugars are incorporated into three structures: linear O‐linked mannan, highly branched N‐linked mannan and phospholipomannan. Protein mannosylation occurs during protein synthesis in the endoplasmic reticulum (ER) and is further elaborated as the protein is passed through the Golgi apparatus. Initially, sugars (i.e. mannose and glucose) are added to dolichol phosphate acceptors, from which are then incorporated into C‐, N‐, O‐mannosylation, as well as GPI anchors. On the other hand, in the Golgi, the donor of mannosyl residues is GDP‐mannose. Initiation of mannosylation in C. albicans has been reviewed elsewhere (Mora‐Montes et al., 2009), and this review will focus on the transglycosylases involved in the elaboration of O‐ and N‐mannan structures.
C. albicans mannosylation mutants
Studies exploring the role(s) of mannosylation in fungal biology and virulence have been informed by the creation of a series of C. albicans mannosylation mutants with truncations in the normal wild‐type structures of both O‐ and N‐linked mannan. Because these mutants express stably altered mannan structures on their cell surface (Fig. 1), these mutants have been used as tools to explore the importance of specific mannan epitopes on cell function, pathogenesis and immune recognition (Table 1).
Figure 1.

N‐ and O‐linked glycosylation structures of the C. albicans mannosylation mutants. Asterisks highlight structures, which are predicted from comparisons with S. cerevisiae, but have not yet been experimentally determined for C. albicans.
Table 1. Summary of the phenotypes for the C. albicans mannosylation mutants.
| Gene | Activity | Mutant phenotype | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Growth | Cell Wall | Sensitivity | Morphology | Adhesion | Phagocytosis | Virulence | ||||
| O‐mannan | PMT1 | Required for initiation of O‐glycosylation | Biofilm formation reduced. Required for growth with PMT4 | Reduced mannan, increased β1,3‐glucan | Increased sensitivity to Congo red, CFW, SDS, heat stress and antifungals | Hyphal growth reduced | Epithelial adhesion reduced | Reduced in HDC, RHE and oral mucosal models | Timpel et al. (1998); Prill et al. (2005); Rouabhia et al. (2005); Peltroche‐Llacsahuanga et al. (2006); Murciano et al. (2011) | |
| PMT2 | Required for initiation of O‐glycosylation |
Essential for viability, Biofilm formation reduceda |
Increased sensitivity to Congo red, CFW, caffeine, heat stress and antifungalsa | Hyphal growth reduceda | Normala | Reduced in murine systemic infection modela | Prill et al. (2005); Rouabhia et al. (2005); Peltroche‐Llacsahuanga et al. (2006); Corbucci et al. (2007) | |||
| PMT4 | Required for initiation of O‐glycosylation | Biofilm formation reduced. Required for growth with PMT1 | Reduced mannan, increased β1,3‐glucan | Increased sensitivity to antifungals | Hyphal growth reduced | Reduced in RHE and murine systemic infection model | Prill et al. (2005); Rouabhia et al. (2005) | |||
| PMT5 | Required for initiation of O‐glycosylation | Normal | Normal | Normal | Normal | Uptake normal, but cells are not killed by neutrophils | Reduced damage in oral mucosal models | Prill et al. (2005); Rouabhia et al. (2005); Corbucci et al. (2007) | ||
| PMT6 | Required for initiation of O‐glycosylation | Normal | Normal | Normal | Hyphal growth reduced | Epithelial adhesion reduced | Reduced damage in RHE model | Timpel et al. (2000); Prill et al. (2005); Rouabhia et al. (2005) | ||
|
MNT1 MNT2 |
Addition of α1,2‐mannose | Cell separation defect in the double mutant | Reduced O‐mannan in double mutant | Increased sensitivity to CFW (mnt1Δ and double mutant only), double mutant has increased sensitivity to SDS | Reduced hyphal formation in the double mutant | Reduced | Neutrophil Uptake reduced, macrophage uptake increased | Reduced in murine systemic infection model (double mutant only) | Munro et al. (2005); McKenzie et al. (2010); Sheth et al. (2011) | |
| N‐mannan | CWH41 | Removes terminal α1,2 linked glucose from core | Reduced growth rate, increased flocculation | Reduced PM, mannan and glucan, increased chitin and protein | Increased sensitivity to Congo red, CFW, SDS and antifungals | Hyphal growth reduced | Reduced in murine infection model | Mora‐Montes et al. (2007) | ||
| ROT2 | Removes the two α1,3 linked glucose units from core | Reduced growth rate, increased flocculation | Reduced PM, mannan and glucan, increased chitin and protein | Increased sensitivity to Congo red, CFW, SDS and antifungals | Hyphal growth reduced | Reduced in murine infection model | Mora‐Montes et al. (2007) | |||
| MNS1 | Removes α1,2 mannose from core | Reduced growth rate, increased flocculation | Reduced PM | Increased sensitivity to Congo red and CFW | Normal | Macrophage uptake increased | Reduced in murine infection model | Mora‐Montes et al. (2007); McKenzie et al. (2010) | ||
| OCH1 | Addition of initial α1,6‐mannose | Increased cell size, decreased growth rate, cell separation defect |
Reduced PM and mannan, Increased chitin and glucan |
Increased sensitivity to Congo red, CFW, SDS, heat stress and antifungals | Hyphal growth reduced | Epithelial adhesion reduced | Neutrophil uptake reduced | Reduced in murine infection model | Bates et al. (2006); Murciano et al. (2011); Sheth et al. (2011) | |
|
MNN1 MNN12 MNN13 MNN14 MNN15 MNN16 |
Addition of terminal α1,3‐mannan | Normal |
Extended PM chains mnn14Δ only) |
Increased sensitivity to SDS and antifungals (mnn14Δ only) | Reduced hyphal growth in response to pH, temperature and Lee's and spider media (mnn14Δ only) | Reduced in murine infection model (mnn14Δ only) | Bates et al. (2013) | |||
|
MNN2 MNN21 MNN22 MNN23 MNN24 MNN26 |
Addition of initial α1,2‐mannan to α1,6 backbone | Reduced growth rate and increased flocculation in double, triple, quintuple and sextuple mutants | N‐mannan and PM severely truncated in double, triple, quintuple and sextuple mutants. Chitin content increased | Increased sensitivity to Congo red, CFW and SDS, | Delayed hyphal growth (mnn22Δ, quintuple and sextuple mutants) | Reduced in Galleria mellonella and murine infection models | Bai et al. (2006); Hall et al. (2013) | |||
| MNN4 | Positive regulator of MNN6 | Normal | Reduced PM | Normal | Normal | Neutrophil and macrophage uptake reduced | Normal | Hobson et al. (2004); McKenzie et al. (2010); Sheth et al. (2011) | ||
| MNN9 | Elaboration of the α1,6‐mannose backbone | Reduced growth rate, increased flocculation | Reduced mannan content | Increased sensitivity to antifungals | Hyphal growth reduced | Epithelial adhesion reduced | Southard et al. (1999); Murciano et al. (2011) | |||
| BMT1‐9 | Addition of β1,2‐ mannose | Normal | Normal | Normal | Normal | Normal | Mille et al. (2008) | |||
| MNT3‐5 | Addition of α1,2 mannose to outer chain | Normal, increased flocculation in multiple mutant | Reduced PM | Multiple mutant shows increased sensitivity to CFW, SDS and antifungals | Normal | Reduced macrophage uptake | Multiple mutant shows reduced virulence in murine infection model | McKenzie et al. (2010); Mora‐Montes et al. (2010) | ||
| PLM | MIT1 | Addition of α‐mannan to lipid | Normal | No PLM, less β‐mannose in PM | Increased sensitivity to calcium and SDS | Normal | Increased susceptibility to phagocytosis | Reduced in murine infection model | Mille et al. (2004) | |
| Other enzymes | PMR1 | Transport of Ca2+/Mn2+ into Golgi | Normal | Reduced PM and O‐mannan | Increased sensitivity to Congo red, CFW, heat stress and antifungals | Delayed | Normal | Reduced neutrophil and macrophage uptake | Reduced in murine infection model | Bates et al. (2005); McKenzie et al. (2010); Sheth et al. (2011) |
a. Phenotype of the heterozygous mutant.
O‐mannosylation mutants
As discussed above, the C. albicans O‐mannan is a simple linear carbohydrate comprised of a series of α1,2‐linked mannose units (typically, 1–5 residues). The initial α‐mannose residue is attached to the hydroxyl group of serine/threonine residues through the actions of PMT1, PMT2, PMT4, PMT5 and PMT6 (Prill et al., 2005). Mnt1 and Mnt2 are partially redundant α1,2‐mannosyltransferases required for the addition of the first and second α1,2‐mannose units onto the α‐mannose (Munro et al., 2005). Deletion of MNT1 and MNT2 alone, or in combination, results in truncation of the O‐mannan (Buurman et al., 1998; Munro et al., 2005). Recent biochemical characterization of the MNT gene family suggests that MNT1 may be required for further elaboration of the O‐mannan chain (Díaz‐Jiménez et al., 2012). Deletion of the PMT gene family, and MNT1 and MNT2 reduced the capacity for biofilm formation and resulted in increased sensitivity to cell wall perturbing agents such as Calcofluor White, Congo Red and SDS (Table 1), suggesting that O‐mannosylation is important for the general integrity of the cell wall (Timpel et al., 1998; Munro et al., 2005; Prill et al., 2005; Peltroche‐Llacsahuanga et al., 2006). Although a significant amount of redundancy is expected between the PMT family members, PMT2 is the only member that has been shown to be essential for viability (Prill et al., 2005), suggesting that Pmt2 may play additional roles compared to the other family members. Likewise, Pmt1 and Pmt6 are required for the adhesive properties of the fungus to epithelial cells (Timpel et al., 1998; 2000; Murciano et al., 2011). All mutants involved in the biosynthesis of O‐mannan that have been studied show attenuated virulence in the murine systemic infection model, and most also have adhesion defects (Buurman et al., 1998; Timpel et al., 1998; Munro et al., 2005; Rouabhia et al., 2005) confirming the importance of O‐mannan in fungus‐host interactions (Table 1).
N‐mannosylation mutants
N‐mannan core
The core structure of N‐mannan is a dolichol pyrophosphate anchored oligosaccharide comprised of three glucose, nine mannose and two N‐acetylglucosamine residues (Glc3Man9GlcNAc2). After attachment to asparagine residues within the polypeptide chain via the OST complex (Kelleher and Gillmore, 2006), this oligosaccharide is processed in the endoplasmic reticulum by three glycosidases (Cwh41, Rot2 and Mns1). These glycosidases remove the three terminal glucose units and one additional α1,2‐mannose units, forming the mature core (Man8GlcNAc2). The processed core is similar in structure in all eukaryotes, but the pattern of elaboration of the outer N‐mannan chains is fungal specific. Prevention of core processing by deletion of these genes not only affects the structure of the core, but also alters the structure of the outer chain branched N‐mannan (Mora‐Montes et al., 2007), suggesting that these processing steps are key regulators of N‐mannan biosynthesis. Deletion of MNS1, CWH41 and ROT2 results in increased flocculation, decreased growth and lower phosphomannan content (Mora‐Montes et al., 2007). These changes in cell wall composition also result in reduced secretion of pro‐inflammatory cytokines from human monocytes, correlating with attenuated virulence in the murine model of systemic candidiasis (Mora‐Montes et al., 2007). Therefore, full processing of the core N‐mannan is important for virulence (Table 1).
Branched N‐mannan
The outer chain branched mannan is attached to the N‐mannan core through an α1,6‐backbone. Addition of the first α1,6‐mannose is catalysed by a single mannosyltransferase, Och1. Therefore, the N‐mannan of the och1 mutant has no branched outer chain mannan, but the core N‐mannan contains additional mannose residues (Bates et al., 2006; Fig. 1). Deletion of och1 results in significant shortening of the mannan fibrils (Netea et al., 2006), and the activation of the cell salvage pathway, resulting in an elevation in the levels of chitin and glucan, and hence a thickened cell wall (Bates et al., 2006). The α1,6‐mannose backbone is extended by the enzyme complexes mannan polymerase I (M‐Pol I) and mannan polymerase II (M‐Pol II). In Saccharomyces cerevisiae, M‐Pol I is composed of Mnn9 and Van1, while M‐Pol II is composed of Mnn9 and Anp1 (Hashimoto and Yoda, 1997; Jungmann and Munro, 1998). Deletion of the C. albicans Mnn9 orthologue results in a 50% decrease in total mannan levels, and a phenotype characterized by increased flocculation of yeast cells, reduced growth rates, osmotic sensitivity and abnormal morphogenesis (Southard et al., 1999). Therefore, it is likely that Mnn9 is the major contributor to the extension of the α1,6‐backbone in C. albicans. The backbone is then elaborated with extensive branches composed of α1,2‐mannose. In S. cerevisiae, the initial α1,2‐mannose unit is attached to the backbone via the actions of Mnn2, which are then extended with additional α1,2‐mannose units by Mnn5. blast searches of the C. albicans genome identify a family of related genes, which are putative Mnn2 and Mnn5 orthologues (Hall et al., 2013). Bai et al. characterized one of the family members and confirmed that the encoded protein had both α1,2‐ and α1,6‐mannosyltransferase activity, but was unable to complement the S. cerevisiae mnn2Δ mutant, and was hence designated an Mnn5 orthologue (Bai et al., 2006). A more detailed systematic characterization of this gene family suggests that three members have redundant Mnn2 activity, while the other three members display Mnn5‐like activity (Hall et al., 2013). The C. albicans mnn5Δ mutant also showed a reduced ability to synthesize O‐mannan (Bai et al., 2006). Deletion of Mnn2 and Mnn5 orthologues in C. albicans resulted in shortened mannan fibrils protruding from the cell wall, while deletion of all six genes abolished visible mannan fibrils (Fig. 2), with only α1,6‐mannose present in the N‐mannan side‐chain (Hall et al., 2013; Fig. 1). Biochemical evidence suggests that Mnt5 is also required for the addition of the second α1,2‐mannose unit to the outer chains from the N‐linked mannan (Díaz‐Jiménez et al., 2012), suggesting that there may be a degree of functional redundancy in the mannan biosynthetic pathways in C. albicans.
Figure 2.
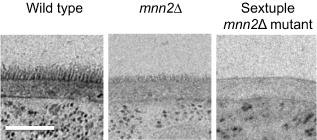
TEM of the cell wall in selected mannosylation mutants. The sextuple mnn2Δ mutant contains deletions in all six MNN2 genes (mnn2Δ/mnn21Δ/mnn22Δ/mnn23Δ/mnn24Δ/mnn26Δ). Uridine auxotrophy was complemented by the integration of the CIp10 plasmid at the RPS1 locus. The scale bar represents 0.2 μm.
The α1,2‐mannnose chains are capped with α1,3‐mannose via the actions of Mnn1 (Yip et al., 1994; Romero et al., 1999). The C. albicans MNN1 gene family contains 6 members, but only deletion of MNN14 attenuates virulence (Bates et al., 2013), suggesting a degree of functional redundancy between family members. In contrast to S. cerevisiae, the C. albicans N‐mannan contains β1,2‐mannose, which forms part of both the acid‐stable and acid‐labile mannan fractions (see below), which are attached through the actions of β1,2‐mannosyltransferases (BMTs). Bmt1 and Bmt3 are required for the addition of the first and second β1,2‐mannose units respectively (Mille et al., 2008). However, removal of β1,2‐mannose from the acid‐stable mannan fraction did not affect growth, morphology or compromise the cell wall integrity (Mille et al., 2008). Therefore, the functional significance of β1,2‐mannosylation remains to be clarified. However β‐mannan plays important roles in immune recognition (see later).
Phosphomannan
The β1,2‐mannose moiety, linked to the branched N‐glycan through a phosphodiester bond, is commonly known as phosphomannan (PM), or acid‐labile mannan. Loss of this mannan fraction is characterized by a reduced ability of C. albicans to bind the cationic dye Alcian Blue, due to the loss of negative charge in the cell wall, as a result in the reduction of phosphate content. In S. cerevisiae, the PM is attached to the outer N‐mannan chains via Mnn4 and Mnn6 (Karson and Ballou, 1978; Nakayama et al., 1998). ScMNN6 encodes the mannosylphosphate transferase (Odani et al., 1997), while ScMnn4 is a positive regulator of ScMnn6 (Odani et al., 1996). Deletion of the putative C. albicans MNN4 orthologue impairs Alcian Blue binding to the C. albicans cell wall, confirming that it also participates in the attachment of PM to the outer N‐mannan chains (Hobson et al., 2004), although it has not been confirmed if CaMnn4 is acting as the mannosylphosphate transferase, or a positive regulator of CaMnn6. However, the C. albicans mnn4Δ mutant does maintain β1,2‐mannose in the acid‐stable fraction (Hobson et al., 2004; Singleton et al., 2005). The PM glycoconjugate is extended by a family of BMTs, which attach a series of β1,2‐mannose residues to the initial α1,2‐mannose. Bmt2, Bmt3 and Bmt4 are required for the addition of the first, second and third β1,2‐mannose units of the acid‐labile mannan respectively (Mille et al., 2008). Deletion of the α1,2‐mannosyltransferases mnt3Δ, and mnt5Δ together also results in reduced Alcian Blue binding (Mora‐Montes et al., 2010), suggesting they are also involved in elaboration/attachment of the PM to the N‐mannan, although O‐mannan can also incorporate PM. Removal of the PM, by deletion of MNN4, increases the net hydrophobicity of the cell wall (Singleton et al., 2005), and increases the resistance of the N‐mannan to acetolysis (Hazen et al., 2007), which cleaves α1,6‐linkages. This increased resistance suggests that Mnn4, in addition to regulating the addition of PM to the α1,2‐mannan side‐chain, may also have a global affect on the synthesis of acid‐stable mannan. The PM is important for macrophage phagocytosis (McKenzie et al., 2010). In comparison, removal of O‐ or N‐mannan resulted in increased phagocytosis (McKenzie et al., 2010), and increased exposure of β‐glucan, which would increase recognition through the phagocytic receptor Dectin‐1 (see below).
Other enzymes
The majority of the mannosyltransferases are metalloenzymes which require a metal ion cofactor [predominately manganese (Mn2+)] for functionality (Bai et al., 2006). Therefore, ion transport within the ER and Golgi network is an important factor for mannan biosynthesis. Pmr1 is a P‐type ATPase required for transporting divalent cations (Ca2+/Mn2+) into the Golgi and maintaining manganese homeostasis. Disruption of PMR1 results in shortening of the branched N‐mannan and O‐mannan (Fig. 1), presumably due to the inhibition of several mannosyltransferases as a result of insufficient concentrations of cations within the Golgi (Bates et al., 2005). However, in comparison with the och1Δ mutant, the pmr1Δ has a thinner glucan‐chitin layer and longer, but less dense mannan fibrils.
Phospholipomannan
Phospholipomannan (PLM) is comprised of mannosylated sphingolipids, sharing a mannose moiety similar to that of PM, composed of β1,2‐mannose, covalently linked to the lipid domain by a phosphodiester bond with an α‐mannose unit. Deletion of MIT1 (Mannose Inositolphosphoceramide mannose Transferase) totally eliminated mannan from C. albicans PLM (Mille et al., 2004), suggesting that Mit1 is the sole transferase responsible for the addition of mannan to this lipid. The PLM is then elaborated with β‐mannose units, via the actions of Bmt5 and Bmt6 (Mille et al., 2012). Disruption of PLM significantly affected the C. albicans cell wall stress response due to calcium and SDS, but not Calcofluor White (Mille et al., 2004). Interestingly, blastospores shed PLM during early stages of macrophage phagocytosis, and the released PLM binds the surface of the macrophage (Jouault et al., 1998), where it participates in immune recognition of the fungal pathogen (see below).
In general, glycosylation mutants display similar phenotypes. For example, all glycosylation mutants studied, so far, show increased flocculation. For some of the mutants (och1Δ, mnt1Δ/mnt2Δ) this can be explained by a cell separation defect, at cytokinesis (Munro et al., 2005). However, this defect has not been observed for all the glycosylation mutants. One possible explanation is that the alterations to the glycosylation status of the cell wall affects the charge of the cell and hence the tendency to aggregate. It is also possible that the disruption of key regulatory cell wall processes affects the activity of glucanase and chitinase enzymes required for cell‐separation after cytokinesis. However, Gregori et al. recently showed that sub‐MIC concentrations of the β‐glucan synthase inhibitor caspofungin induce flocculation in an Efg1‐, Als1‐dependent manner, which could be inhibited by high concentrations of exogenous sugars (Gregori et al., 2011). Alternatively, overexpression of Als3 has been shown to induce flocculation. Expression of ALS proteins in the glycosylation mutants has not been studied, but evidence suggests that in addition to the glycome, the cell wall proteome is also altered in some mannosylation mutants, perhaps by inducing the unfolded protein response (Bates et al., 2005). Therefore, it is possible that manipulation of mannosylation alters many properties of the cell wall, which results in increased cell–cell adhesion, and could serve as an alternative mechanism for protection from the environment.
Effects of the environment on mannan composition
The fungal cell wall is dynamic, and its composition is mediated by components of the surrounding environment. For example, the presence of echinocandin antifungals results in increased chitin synthesis to compensate for the depletion of glucan, to maintain cell wall integrity (Walker et al., 2008). Recent investigations into the mannan composition have shown that the environment also modulates the structure of the protruding mannan fibrils. At the molecular level, NMR data suggest that the structural composition of the mannan is dependent on growth conditions (Kruppa et al., 2011; Lowman et al., 2011). Growth in alternative carbon sources reduced chitin and glucan levels and also diminished the mannan fibrillar layer (Ene et al., 2012). Moreover, damage to the mannosylation structures upregulates PMT1, PMT2 and PMT4 in an Msb2‐, Cek1‐, Ace2‐dependent manner (Cantero and Ernst, 2011). Therefore, different growth conditions are likely to activate cell wall signalling cascades to varying degrees, altering the expression of cell wall biosynthesis genes, and affecting the mannan composition. For a detailed review of cell wall signalling pathways we direct readers to the following recent review (Ernst and Pla, 2011).
Contribution of mannan to fungal immune recognition
Like many pathogens, C. albicans is detected and cleared predominantly through the actions of the innate immune system. Recognition of invading microbes is achieved by a variety of receptors on the surfaces of epithelia and myeloid cells. These include toll‐like receptors (TLRs), C‐type lectins (CTLs) and Nod‐like receptors (NDLs), which bind to specific epitopes on the pathogen surface (Medzhitov et al., 1997; Yang et al., 1998; Ariizumi et al., 2000). These so‐called pathogen recognition receptors (PRRs) and pathogen‐associated molecular patterns (PAMPs) now form the basis of our understanding of innate immune recognition. For example, TLR2, TLR4, dectin‐2, Mincle, DC‐SIGN and galectin‐3 have major roles in the recognition of fungal mannans (Fradin et al., 2000; Tada et al., 2002; Porcaro et al., 2003; Taylor et al., 2004; Rouabhia et al., 2005; McGreal et al., 2006), TLR9 recognizes fungal DNA (Miyazato et al., 2009), and dectin‐1 and complement receptor 3 (CR3) are the major PRRs involved in the detection of β‐glucans (Thornton et al., 1996; Brown and Gordon, 2001).
Participation of O‐mannan to immune recognition
O‐mannan is predominately recognized by TLR4 (Netea et al., 2006). Deletion of TLR4 results in reduced neutrophil infiltration and enhanced fungal burden in the peritoneal exudates, lymph nodes and spleen (Gasparoto et al., 2010). Co‐incubation of oral epithelial cells with purified C. albicans cell wall components confirmed that these PAMPs only induced expression of TLR4, but epithelial cytokine production was independent of TLR4 (Wagener et al., 2012). However, a recent study highlighted that TLR4 recognition, is largely dependent on the C. albicans strain under investigation (Netea et al., 2010). In this study, the susceptibility of TLR4−/− mice to C. albicans infection correlated with the dependence on TLR4 recognition, with disease progression unaltered in TLR4−/− mice when infected with a C. albicans strain known to be independent of TLR4 recognition (Netea et al., 2010). Therefore, data regarding TLR4 recognition should be interpreted with caution.
Participation of N‐mannan to immune recognition
N‐mannan is recognized by a multitude of receptors, which are expressed on different immune cells. The mannose receptor (MR) is an endocytic receptor thought to recognize terminal α1,2‐/α1,3‐mannose residues (Kéry et al., 1992; Netea et al., 2008). The MR is cleaved by a metalloproteinase producing a functional soluble (sMR) receptor (Martínez‐Pomares et al., 1998). The role of sMR in innate immunity has not been clarified, but the sMR may function to bind soluble mannan or degraded particles from phagocytosis events and present them to CR‐Fc+ cells surrounding the infection site (Martínez‐Pomares et al., 1998).
Due to the phagocytic nature of the MR, fungal cells with a low mannan content in their cell wall have reduced phagocytosis rates (Keppler‐Ross et al., 2010). Indeed, mutants with truncations in N‐ (mns1), phosphomannan (mnn4) and O‐linked mannan (mnt1/mnt2) exhibited delays in engulfment, but not in the rate of macrophage migration and chemotaxis towards Candida cells (Lewis et al., 2012). This is also true of neutrophils, where mannosylation mutants (for example, och1Δ, pmr1Δ and mnt1Δ/mnt2Δ) displayed a reduced phagocytosis index (Sheth et al., 2011). In neutrophils, at least, the decreased phagocytosis rate was found not to be due to lack of recognition, since neutrophils still had yeast bound to their surface. Instead, the reduced phagocytic index of the mannan‐deficient mutants seemed to be due to the failure of the neutrophils to engulf the mutants (Sheth et al., 2011). In contrast, alterations in other cell wall components, including glucan and chitin, did not markedly affect the efficiency of macrophages to phagocytose fungal cells (Keppler‐Ross et al., 2010). The MR is also responsible for the majority (70%) of dendritic cell (DC) recognition and internalization of C. albicans (Cambi et al., 2008). This recognition is mainly based on interactions with α1,2‐ or α1,3‐mannose, with the och1Δ and pmr1Δ mutants displaying reduce phagocytosis rates, while the mnt1Δ/mnt2Δ, mnn4Δ mutants, and the serotype B strains were still efficiently phagocytosed by DCs (Cambi et al., 2008).
Although the majority of C. albicans recognition by DCs occurs via the MR, DCs also express the C‐type lectin‐like receptor, DC‐SIGN. DC‐SIGN recognizes a variety of carbohydrate structures, including fructose and branched α‐mannan (Cambi et al., 2008), and can phagocytose Candida cells through the recognition of mannan (Cambi et al., 2003). The mouse orthologue of DC‐SIGN, SIGNR‐1, works in concert with Dectin‐1 to enhance the oxidative burst in macrophage cell lines (Takahara et al., 2011). Although DC‐SIGN and SIGNR‐1 are orthologues, they show distinct epitope specificity. For example, DC‐SIGN only recognizes α‐mannose residues with a free non‐reducing end (i.e. α‐mannose units at the end of the polymers), while SIGNR‐1 can also recognize α‐mannose units capped with additional α‐mannoses, or β‐mannose residues (Takahara et al., 2012).
In addition to the MR and DC‐SIGN, the C‐type lectin‐like receptor, dectin‐2 (Clec4n), has recently been identified as recognizing high mannose containing epitopes (> 7 terminal or branched α‐mannose residues) (McGreal et al., 2006), although the exact epitope (i.e. terminal, or branched α‐mannose units) recognized by dectin‐2 is unknown (Saijo et al., 2010). Deletion of dectin‐2 results in increased kidney fungal burdens and accelerated neutrophil infiltration, with Candida growth observed in the pelvis (Saijo et al., 2010), confirming that α‐mannan recognition via dectin‐2 is crucial for fungal detection and removal. Dectin‐2 recognition enhances secretion of IL‐1β, IL‐23 and IL‐6 and hence activates a protective Th17 response to the invading pathogen, as well as a less potent Th1 response (Saijo et al., 2010). In conjunction with this, C. albicans purified mannan is capable of inducing prostaglandin production from human PBMCs. β‐Glucan only enhanced prostaglandin levels in concert with TLR2 ligands (Smeekens et al., 2010). Furthermore, prostaglandin production is regulated via dectin‐2 and hence by mannan‐stimulation (Suram et al., 2010). Therefore, fungal mannan appears to play a critical role in inducing Th17 responses, presumably through the actions of CD14++/CD16− subsets of circulating monocytes which have elevated expression of the MR on their surface (Smeekens et al., 2011), to fungal pathogens.
The β‐mannan which caps the branches of N‐mannan is recognized by galectin‐3 (Fradin et al., 2000). Although galectin‐3 can bind to a variety of β1,2‐epitopes, only recognition of antigenic factor 5 (phosphate bound β1,2‐mannose units) or factor 6 (terminal α1,3‐mannose units) exert fungicidal effects on C. albicans. These affects are specific for Candida species that display β1,2‐linked mannose on their surface, as galectin‐3 does not bind fungal cells that lack this epitope (for example S. cerevisiae) (Kohatsu et al., 2006). Macrophages isolated from galectin‐3 deficient mice exhibited normal levels of uptake and phagocytosis of Candida (Jouault et al., 2006), suggesting that recognition of β1,2‐mannan is not important for fungal eradication. However, more recently Linden et al. have shown that Candida parapsilosis induces galectin‐3 secretion from neutrophils, and propose that soluble galectin‐3 functions as a pro‐inflammatory autocrine/paracrine signal to enhance neutrophil phagocytosis (Linden et al., 2013).
In addition to the receptors described above, the C‐type lectin‐like receptor, Mincle which is expressed on macrophages, has been proposed to recognize α‐mannose units, but not complete mannan polysaccharides (Yamasaki et al., 2009). However, some conflicts exist in the literature regarding the role of Mincle in fungal infections. Mincle−/− mice do not show increased susceptibility to systemic candidiasis, but they do display increased kidney burdens compared to control mice (Wells et al., 2008), suggesting that Mincle may play a role in fungal clearance. In agreement with this, TNFα secretion was reduced by 30% in Mincle−/− bone marrow‐derived macrophages after stimulation with C. albicans (Wells et al., 2008). In contrast, Mincle specifically recognizes Malassezia, and not C. albicans or Aspergillus species (Yamasaki et al., 2009). The differences observed in this study might, in part, be attributed to the different C. albicans strains used in each study, which potentially has been attributed to the ability of different organisms to express different α‐mannose epitopes.
Participation of phospholipomannan to immune recognition
Addition of purified PLM to macrophage‐like cells (J774) stimulates pro‐inflammatory cytokine secretion, suggesting that PLM contributes to innate immune recognition of C. albicans (Jouault et al., 1994; 1998). TLR knockout mice confirmed that PLM was recognized by TLR2, although bone marrow‐derived macrophages from TLR4−/− and TLR6−/− mice also showed reduced cytokine signalling in response to purified PLM, suggesting that these receptors may also function in the recognition of PLM (Jouault et al., 2003). However in keratinocytes, PLM induced pro‐inflammatory cytokine secretion (IL‐6 and IL‐8) was shown to be TLR2 dependent (Li et al., 2009). Therefore, the role of PLM in innate immune recognition may depend on the site of infection.
Mannan and fungal diagnostics
Early detection of invasive candidaemia (IC) is essential for a good prognosis, with mortality rates increasing from 15% (antifungal treatment initiated immediately after positive blood culture), to 40% when treatment is delayed by 72 h (Garey et al., 2006). Despite the new developments in disease diagnostics, Candida infections are still hard to diagnose, with many cases going unreported until autopsy. Diagnosis is now based on the non‐invasive detection of circulating polysaccharides from the fungal cell wall in blood samples. Two of the diagnostic tests focus on circulating mannan levels, while the other is directed against β‐glucan.
Mannan antigen detection
Mannan comprises up to 7% of the dry weight of C. albicans and is non‐covalently attached to the surface of the pathogen, and as a result is released into the circulation (Fukazawa, 1989). Therefore, patients with invasive candidaemia tend to have high circulating levels of mannan in their blood (mannanaemia). The first commercially available kit for the detection of mannan was Pastorex antigen agglutination kit, which gave varied results with a high percentage of false positives (Bailey et al., 1985; Lemieux et al., 1990). Currently, the conventional kit for testing sera for the presence of fungal mannan is the Platelia Candida antigen kit from Bio‐Rad, which is based on an enzyme‐linked immunosorbent assay (ELISA). The kit utilizes the rat monoclonal antibody EB‐CA1, which recognizes chains of α1,2‐mannose from the fungal cell wall in a size‐dependent manner, with five units being the minimum for efficient binding (Jacquinot et al., 1998). This assay assumes that mannan serum concentrations above 0.5 ng ml−1 are positive for candidaemia, and can lead to the identification of patients with candidaemia 7 weeks earlier than blood cultures (Nihtinen et al., 2011). The Platelia assay has a specificity of over 80% with a sensitivity of around 60% (Sendid et al., 1999; Alam et al., 2007; Mikulska et al., 2010; Mokaddas et al., 2011). However, increased sensitivity can be observed (70–100%) by decreasing the recommended cut‐off, but this increases false positives (Ellis et al., 2009; Mikulska et al., 2010). An alternative method is to use the assay in combination with another test like the anti‐mannan antibody detection kit (Arendrup et al., 2010; Mikulska et al., 2010). Initially there were concerns over the use of mannan as a diagnostic tool due to natural colonization of Candida. However, under these circumstances the mannan level remains within the cut‐off (i.e. below 0.5 ng ml−1), while they are greatly elevated in patients with invasive candidaemia (Mokaddas et al., 2010). Therefore, detection of mannan is a reliable diagnostic marker for invasive candidaemia. One factor that influences the accuracy of such diagnostics is the clearance of mannan from the circulation. Therefore, for high‐risk patients, such as those on immune suppressive therapy, or with neutropenia consistent monitoring of circulatory mannan levels may prove more beneficial than one‐off measurements.
Anti‐mannan antibody detection
As discussed in the previous section, mannan is immune‐stimulatory and as a consequence antibodies are generated against it, the presence of which can then be used as a diagnostic tool to identify patients with fungal infections. The detection of anti‐mannan antibodies is taken advantage of in the Platelia Candida Ab assay kit. This assay involves the use of Candida mannan coated plates, to which sera from the patient is applied. The presence of the antibodies is achieved through a sandwich ELISA. Several studies have reported that the average sensitivity of the kit to detect patients infected with Candida is 60% with a range between 44% and 100%. However, the anti‐mannan test is less specific than the Platelia Candida antigen kit, due to high circulation of mannan antibodies from uninfected, but heavily colonized individuals (Odds and Evans, 1980), and the reduced antibody response in immune suppressed patients (Jones, 1990). It was reported that use of the anti‐mannan antibody test in combination with the mannan antigen test increases the sensitivity to 80–90% (Mikulska et al., 2010). Greater accuracy can also be achieved through the combined testing for Candida mannan and β‐glucan, or Candida mannan, β‐glucan and Candida DNA (Alam et al., 2007). The use of these biological markers to detect IC in high‐risk patients has proven successful in the early detection of infection, producing positive results up to 7 days before a positive blood culture.
Other fungal species
Although much of the knowledge we have on the fungal cell wall has been based on studies from S. cerevisiae and C. albicans, which have similar cell wall structures, new insights are now coming from studies of other pathogenic fungi. These studies confirm that the structural organization of some elements of the fungal cell wall are well conserved, with most fungi having a common core comprised of chitin and β‐glucan in the inner wall layer and an outer layer of glycoproteins. The ratio of the components and the major carbohydrate components and the amount of glycoprotein in the wall vary significantly. For example, chitin forms only 2–5% of the dry weight of the C. albicans cell wall, while it accounts for over 10–20% of the dry weight of the walls of Aspergillus or Neurospora species. In Aspergillus species, the glucan layer is comprised of β1,3‐ and β1,4‐glucan, while C. albicans contains β1,3‐ and β1,6‐glucan (Fontaine et al., 2000). Some fungi have considerably less glycoproteins in their cell wall than C. albicans, and these proteins are glycosylated with polysaccharide structures other than mannan. In Aspergillus fumigatus, and Malassezia furfur the glycoproteins are glycosylated with polysaccharides composed of mannose and galactose monosaccharides, known as galactomannan (Latgé et al., 1994; Shibata et al., 2009), and circulating galactomannan levels are the most commonly used diagnostic marker for invasive aspergillosis (Rohrlich et al., 1996). In addition, long complex glycosylation structures such as the N‐mannan in C. albicans are not present in filamentous fungi, but instead N‐mannans are often shorter and terminate in galactofuranose (Leitão et al., 2003; Morelle et al., 2005). In some fungi, a polysaccharide capsule surrounds the cell wall. Cryptococcus neoformans and C. gattii are surrounded by a glucuronoxylomannan (GXM) and galactoxylomannan (GalXM) capsule, which forms a physical barrier protecting the fungus from the environment and host immune defences (O'Meara and Alspaugh, 2012). The capsule is also a major diagnostic marker, which can be visualized by India ink staining, or quantified through the detection of Cryptococcal antigen (CrAg) by latex aggregation, ELISA or lateral flow (Kozel and Bauman, 2012; O'Meara and Alspaugh, 2012).
Conclusions
The fungal cell wall is a dynamic structure important for maintaining cell shape, protection against environmental stress and immune recognition. The outer most layer of the fungal cell wall is comprised of glycosylated proteins, the carbohydrate structures of which serve as PAMPs that trigger immune recognition. A series of glycosylation mutants, which express altered mannan epitopes on the cell surface, have shed light on the role of different mannans in fungal immune recognition. Many of these mutants show similar phenotypic characteristics including increased flocculation, decreased growth rates, abnormal morphogenesis, temperature sensitivity, increased sensitivity to cell wall perturbing agents and a reduced ability to active host immune responses, all of which result in attenuated virulence. However, immune responses are dependent on the type of immune cell. For example, the mutants which are defective in mannan (och1Δ, mnt1Δ/mnt2Δ and mns1Δ) show a reduced ability to activate peripheral blood monocytes (Munro et al., 2005; Bates et al., 2006; Mora‐Montes et al., 2007), but are phagocytosed by macrophages at a higher rate than wild type (McKenzie et al., 2010), suggesting that recognition in monocytes is predominately driven by mannan through the TLR4 and the MR, while macrophage recognition is predominately mediated by β‐glucan, through dectin‐1. Moreover, during tissue invasion, where fungal β‐glucan exposure is increased, the immune stimulation becomes more dependent on β‐glucans (Wheeler et al., 2008). It is also important to consider that local host environmental signals can strongly influence cell wall structure and composition and so immune recognition of the wall is presented with a moving target (Kruppa et al., 2011; Lowman et al., 2011). During the infection process, C. albicans will be exposed to a plethora of signals including environments of different pH and CO2 levels, different carbon sources (Ene et al., 2012), etc., all of which may individually or simultaneously impact on the cell wall altering the way in which the immune system sees the fungus. The affect of host environmental cues on the fungal cell wall is currently an understudied area of fungal biology, but this area is important if we want to fully understand the extent of the interactions that occur between the host and pathogen during infection.
Acknowledgements
The authors declare no conflict of interest. Our work in the area is supported by grants from the Wellcome Trust (080088, 086827, 075470 and 099215; 097377), and by a FP7‐2007‐2013 grant agreement (HEALTH‐F2‐2010‐260338‐ALLFUN).
References
- Alam, F., Mustafa, A., and Khan, Z. (2007) Comparative evaluation of (1, 3)‐beta‐D‐glucan, mannan and anti‐mannan antibodies, and Candida species‐specific snPCR in patients with candidemia. BMC Infect Dis 7: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almirante, B., Rodríguez, D., Park, B.J., Cuenca‐Estrella, M., Planes, A.M., Almela, M., et al (2005) Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population‐based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 43: 1829–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup, M.C., Bergmann, O.J., Larsson, L., Nielsen, H.V., Jarløv, J.O., and Christensson, B. (2010) Detection of candidaemia in patients with and without underlying haematological disease. Clin Microbiol Infect 16: 855–862 [DOI] [PubMed] [Google Scholar]
- Ariizumi, K., Shen, G.‐L., Shikano, S., Xu, S., Ritter, R., Kumamoto, T., et al (2000) Identification of a novel, dendritic cell‐associated molecule, dectin‐1, by subtractive cDNA cloning. J Biol Chem 275: 20157–20167 [DOI] [PubMed] [Google Scholar]
- Bai, C., Xu, X.‐L., Chan, F.‐Y., Lee, R.T.H., and Wang, Y. (2006) MNN5 encodes an iron‐regulated alpha‐1,2‐mannosyltransferase important for protein glycosylation, cell wall integrity, morphogenesis, and virulence in Candida albicans. Eukaryot Cell 5: 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, J.W., Sada, E., Brass, C., and Bennett, J.E. (1985) Diagnosis of systemic candidiasis by latex agglutination for serum antigen. J Clin Microbiol 21: 749–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, S., MacCallum, D.M., Bertram, G., Munro, C.A., Hughes, H.B., Buurman, E.T., et al (2005) Candida albicans Pmr1p, a secretory pathway P‐type Ca2+/Mn2+‐ATPase, is required for glycosylation and virulence. J Biol Chem 280: 23408–23415 [DOI] [PubMed] [Google Scholar]
- Bates, S., Hughes, H.B., Munro, C.A., Thomas, W.P.H., MacCallum, D.M., Bertram, G., et al (2006) Outer chain N‐glycans are required for cell wall integrity and virulence of Candida albicans. J Biol Chem 281: 90–98 [DOI] [PubMed] [Google Scholar]
- Bates, S., Hall, R.A., Cheetham, J., Netea, M.G., MacCallum, D.M., Brown, A.J.P., et al (2013) Role of the Candida albicans MNN1 gene family in cell wall structure and virulence. BMC Res Notes 6: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, S.M., and Free, S.J. (2006) The structure and synthesis of the fungal cell wall. Bioessays 28: 799–808 [DOI] [PubMed] [Google Scholar]
- Brown, G.D., and Gordon, S. (2001) Immune recognition: a new receptor for [beta]‐glucans. Nature 413: 36–37 [DOI] [PubMed] [Google Scholar]
- Buurman, E.T., Westwater, C., Hube, B., Brown, A.J.P., Odds, F.C., and Gow, N.A.R. (1998) Molecular analysis of CaMnt1p, a mannosyl transferase important for adhesion and virulence of Candida albicans. Proc Natl Acad Sci USA 95: 7670–7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambi, A., Gijzen, K., de Vries, I.J.M., Torensma, R., Joosten, B., Adema, G.J., et al (2003) The C‐type lectin DC‐SIGN (CD209) is an antigen‐uptake receptor for Candida albicans on dendritic cells. Eur J Immunol 33: 532–538 [DOI] [PubMed] [Google Scholar]
- Cambi, A., Netea, M.G., Mora‐Montes, H.M., Gow, N.A.R., Hato, S.V., Lowman, D.W., et al (2008) Dendritic cell interaction with Candida albicans critically depends on N‐linked mannan. J Biol Chem 283: 20590–20599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero, P.D., and Ernst, J.F. (2011) Damage to the glycoshield activates PMT‐directed O‐mannosylation via the Msb2–Cek1 pathway in Candida albicans. Mol Microbiol 80: 715–725 [DOI] [PubMed] [Google Scholar]
- Corbucci, C., Cenci, E., Skrzypek, F., Gabrielli, E., Mosci, P., Ernst, J.F., et al (2007) Immune response to Candida albicans is preserved despite defect in O‐mannosylation of secretory proteins. Med Mycol 45: 709–719 [DOI] [PubMed] [Google Scholar]
- Díaz‐Jiménez, D.F., Mora‐Montes, H.M., Hernández‐Cervantes, A., Luna‐Arias, J.P., Gow, N.A.R., and Flores‐Carreón, A. (2012) Biochemical characterization of recombinant Candida albicans mannosyltransferases Mnt1, Mnt2 and Mnt5 reveals new functions in O‐ and N‐mannan biosynthesis. Biochem Biophys Res Commun 419: 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas, C.M., D'Ippolito, J.A., Shei, G.J., Meinz, M., Onishi, J., Marrinan, J.A., et al (1997) Identification of the FKS1 gene of Candida albicans as the essential target of 1,3‐beta‐D‐glucan synthase inhibitors. Antimicrob Agents Chemother 41: 2471–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünkler, A., Walther, A., Specht, C.A., and Wendland, J. (2005) Candida albicans CHT3 encodes the functional homolog of the Cts1 chitinase of Saccharomyces cerevisiae. Fungal Genet Biol 42: 935–947 [DOI] [PubMed] [Google Scholar]
- Ellis, M., Al‐Ramadi, B., Bernsen, R., Kristensen, J., Alizadeh, H., and Hedstrom, U. (2009) Prospective evaluation of mannan and anti‐mannan antibodies for diagnosis of invasive Candida infections in patients with neutropenic fever. J Med Microbiol 58: 606–615 [DOI] [PubMed] [Google Scholar]
- Ene, I.V., Adya, A.K., Wehmeier, S., Brand, A.C., MacCallum, D.M., Gow, N.A.R., and Brown, A.J.P. (2012) Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol 14: 1319–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, J.F., and Pla, J. (2011) Signaling the glycoshield: maintenance of the Candida albicans cell wall. Int J Med Microbiol 301: 378–383 [DOI] [PubMed] [Google Scholar]
- Fontaine, T., Simenel, C., Dubreucq, G., Adam, O., Delepierre, M., Lemoine, J., et al (2000) Molecular organization of the alkali‐insoluble fraction of Aspergillus fumigatus cell wall. J Biol Chem 275: 27594–27607 [DOI] [PubMed] [Google Scholar]
- Fradin, C., Poulain, D., and Jouault, T. (2000) beta ‐1,2‐linked oligomannosides from Candida albicans bind to a 32‐Kilodalton macrophage membrane protein homologous to the mammalian lectin Galectin‐3. Infect Immun 68: 4391–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa, Y. (1989) Antigenic structure of Candida albicans immunochemical basis of the serologic specificity of the mannans in yeasts. Immunol Ser 47: 37–62 [PubMed] [Google Scholar]
- Garey, K.W., Rege, M., Pai, M.P., Mingo, D.E., Suda, K.J., Turpin, R.S., and Bearden, D.T. (2006) Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi‐institutional study. Clin Infect Dis 43: 25–31 [DOI] [PubMed] [Google Scholar]
- Gasparoto, T.H., Tessarolli, V., Garlet, T.P., Torres, S.A., Garlet, G.P., da Silva, J.S., and Campanelli, A.P. (2010) Absence of functional TLR4 impairs response of macrophages after Candida albicans infection. Med Mycol 48: 1009–1017 [DOI] [PubMed] [Google Scholar]
- Gow, N.A., and Hube, B. (2012) Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol 15: 406–412 [DOI] [PubMed] [Google Scholar]
- Gregori, C., Glaser, W., Frohner, I.E., Reinoso‐Martín, C., Rupp, S., Schüller, C., and Kuchler, K. (2011) Efg1 controls caspofungin‐induced cell aggregation of Candida albicans through the adhesin Als1. Eukaryot Cell 10: 1694–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, R.A., Bates, S., Lenardon, M.D., MacCallum, D.M., Wagener, J., Lowman, D.W., et al (2013) The Mnn2 mannosyltransferase family modulates mannoprotein fibril length, immune recognition and virulence of Candida albicans. PLoS Pathog 9: e1003276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, H., and Yoda, K. (1997) Novel membrane protein complexes for protein glycosylation in the yeast Golgi apparatus. Biochem Biophys Res Commun 241: 682–686 [DOI] [PubMed] [Google Scholar]
- Hazen, K.C., Singleton, D.R., and Masuoka, J. (2007) Influence of outer region mannosylphosphorylation on N‐glycan formation by Candida albicans: normal acid‐stable N‐glycan formation requires acid‐labile mannosylphosphate addition. Glycobiology 17: 1052–1060 [DOI] [PubMed] [Google Scholar]
- Hobson, R.P., Munro, C.A., Bates, S., MacCallum, D.M., Cutler, J.E., Heinsbroek, S.E.M., et al (2004) Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J Biol Chem 279: 39628–39635 [DOI] [PubMed] [Google Scholar]
- Hoyer, L.L. (2001) The ALS gene family of Candida albicans. Trends Microbiol 9: 176–180 [DOI] [PubMed] [Google Scholar]
- Jacquinot, P.M., Plancke, Y., Sendid, B., Strecker, G., and Poulain, D. (1998) Nature of Candida albicans‐derived carbohydrate antigen recognized by a monoclonal antibody in patient sera and distribution over Candida species. FEMS Microbiol Lett 169: 131–138 [DOI] [PubMed] [Google Scholar]
- Jones, J.M. (1990) Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev 3: 32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouault, T., Bernigaud, A., Lepage, G., Trinel, P.A., and Poulain, D. (1994) The Candida albicans phospholipomannan induces in vitro production of tumour necrosis factor‐α from human and murine macrophages. Immunology 83: 268–273 [PMC free article] [PubMed] [Google Scholar]
- Jouault, T., Fradin, C., Trinel, P.‐A., Bernigaud, A., and Poulain, D. (1998) Early signal transduction induced by Candida albicans in macrophages through shedding of a glycolipid. J Infect Dis 178: 792–802 [DOI] [PubMed] [Google Scholar]
- Jouault, T., Ibata‐Ombetta, S., Takeuchi, O., Trinel, P.‐A., Sacchetti, P., Lefebvre, P., et al (2003) Candida albicans phospholipomannan is sensed through Toll‐like receptors. J Infect Dis 188: 165–172 [DOI] [PubMed] [Google Scholar]
- Jouault, T., El Abed‐El Behi, M., Martínez‐Esparza, M., Breuilh, L., Trinel, P., Chamaillard, M., et al (2006) Specific recognition of Candida albicans by macrophages requires galectin‐3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol 177: 4679–4687 [DOI] [PubMed] [Google Scholar]
- Jungmann, J., and Munro, S. (1998) Multi‐protein complexes in the cis Golgi of Saccharomyces cerevisiae with [alpha]‐1,6‐mannosyltransferase activity. EMBO J 17: 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson, E.M., and Ballou, C.E. (1978) Biosynthesis of yeast mannan. Properties of a mannosylphosphate transferase in Saccharomyces cerevisiae. J Biol Chem 253: 6484–6492 [PubMed] [Google Scholar]
- Kelleher, D.J., and Gillmore, R. (2006) An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology 16: 47–62 [DOI] [PubMed] [Google Scholar]
- Keppler‐Ross, S., Douglas, L., Konopka, J.B., and Dean, N. (2010) Recognition of yeast by murine macrophages requires mannan but not glucan. Eukaryot Cell 9: 1776–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kéry, V., Krepinský, J.J., Warren, C.D., Capek, P., and Stahl, P. (1992) Ligand recognition by purified human mannose receptor. Arch Biochem Biophys 298: 49–55 [DOI] [PubMed] [Google Scholar]
- Klevay, M.J., Ernst, E.J., Hollanbaugh, J.L., Miller, J.G., Pfaller, M.A., and Diekema, D.J. (2008) Therapy and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn Microbiol Infect Dis 60: 273–277 [DOI] [PubMed] [Google Scholar]
- Kohatsu, L., Hsu, D.K., Jegalian, A.G., Liu, F.‐T., and Baum, L.G. (2006) Galectin‐3 induces death of Candida species expressing specific beta1,2‐linked mannans. J Immunol 177: 4718–4726 [DOI] [PubMed] [Google Scholar]
- Kozel, T., and Bauman, S. (2012) CrAg lateral flow assay for cryptococcosis. Expert Opin Med Diagn 6: 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruppa, M., Greene, R.R., Noss, I., Lowman, D.W., and Williams, D.L. (2011) C. albicans increases cell wall mannoprotein, but not mannan, in response to blood, serum and cultivation at physiological temperature. Glycobiology 21: 1173–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé, J., Kobayashi, H., and Debeaupuis, J. (1994) Chemical and immunological characterization of the galactomannan secreted by Aspergillus fumigatus. Infect Immun 62: 5424–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé, J.‐P. (2007) The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol 66: 279–290 [DOI] [PubMed] [Google Scholar]
- Leitão, E.A., Bittencourt, V.C.B., Haido, R.M.T., Valente, A.P., Peter‐Katalinic, J., Letzel, M., et al (2003) β‐Galactofuranose‐containing O‐linked oligosaccharides present in the cell wall peptidogalactomannan of Aspergillus fumigatus contain immunodominant epitopes. Glycobiology 13: 681–692 [DOI] [PubMed] [Google Scholar]
- Lemieux, C., St‐Germain, G., Vincelette, J., Kaufman, L., and de Repentigny, L. (1990) Collaborative evaluation of antigen detection by a commercial latex agglutination test and enzyme immunoassay in the diagnosis of invasive candidiasis. J Clin Microbiol 28: 249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy, O., Gangneux, J.‐P., Montravers, P., Mira, J.‐P., Gouin, F., Sollet, J.‐P., et al (2009) Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 37: 1612–1618 [DOI] [PubMed] [Google Scholar]
- Lewis, L.E., Bain, J.M., Lowes, C., Gillespie, C., Rudkin, F.M., Gow, N.A.R., and Erwig, L.‐P. (2012) Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog 8: e1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M., Chen, Q., Shen, Y., and Liu, W. (2009) Candida albicans phospholipomannan triggers inflammatory responses of human keratinocytes through Toll‐like receptor 2. Exp Dermatol 18: 603–610 [DOI] [PubMed] [Google Scholar]
- Linden, J.R., Kunkel, D., Laforce‐Nesbitt, S.S., and Bliss, J.M. (2013) The role of galectin‐3 in phagocytosis of Candida albicans and Candida parapsilosis by human neutrophils. Cell Microbiol 15: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman, D.W., Ensley, H.E., Greene, R.R., Knagge, K.J., Williams, D.L., and Kruppa, M.D. (2011) Mannan structural complexity is decreased when Candida albicans is cultivated in blood or serum at physiological temperature. Carbohydr Res 346: 2752–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreal, E.P., Rosas, M., Brown, G.D., Zamze, S., Wong, S.Y.C., Gordon, S., et al (2006) The carbohydrate‐recognition domain of Dectin‐2 is a C‐type lectin with specificity for high mannose. Glycobiology 16: 422–430 [DOI] [PubMed] [Google Scholar]
- McKenzie, C.G.J., Koser, U., Lewis, L.E., Bain, J.M., Mora‐Montes, H.M., Barker, R.N., et al (2010) Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun 78: 1650–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Pomares, L., Mahoney, J.A., Káposzta, R., Linehan, S.A., Stahl, P.D., and Gordon, S. (1998) A functional soluble form of the murine mannose receptor is produced by macrophages in vitro and is present in mouse serum. J Biol Chem 273: 23376–23380 [DOI] [PubMed] [Google Scholar]
- Medzhitov, R., Preston‐Hurlburt, P., and Janeway, C.J. (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397 [DOI] [PubMed] [Google Scholar]
- Mikulska, M., Calandra, T., Sanguinetti, M., Poulain, D., Viscoli, C., and the Third European Conference on Infections in Leukemia Group (2010) The use of mannan antigen and anti‐mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit Care 14: R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mille, C., Janbon, G., Delplace, F., Ibata‐Ombetta, S., Gaillardin, C., Strecker, G., et al (2004) Inactivation of CaMIT1 inhibits Candida albicans phospholipomannan beta‐mannosylation, reduces virulence, and alters cell wall protein beta‐mannosylation. J Biol Chem 279: 47952–47960 [DOI] [PubMed] [Google Scholar]
- Mille, C., Bobrowicz, P., Trinel, P.A., Li, H., Maes, E., Guerardel, Y., et al (2008) Identification of a new family of genes involved in beta‐1,2‐mannosylation of glycans in Pichia pastoris and Candida albicans. J Biol Chem 283: 9724–9736 [DOI] [PubMed] [Google Scholar]
- Mille, C., Fradin, C., Delplace, F., Trinel, P.‐A., Masset, A., François, N., et al (2012) Members 5 and 6 of the Candida albicans BMT family encode enzymes acting specifically on β‐mannosylation of the phospholipomannan cell‐wall glycosphingolipid. Glycobiology 22: 1332–1342 [DOI] [PubMed] [Google Scholar]
- Miyazato, A., Nakamura, K., Yamamoto, N., Mora‐Montes, H.M., Tanaka, M., Abe, Y., et al (2009) Toll‐like receptor 9‐dependent activation of myeloid dendritic cells by deoxynucleic acids from Candida albicans. Infect Immun 77: 3056–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokaddas, E., Burhamah, M., Khan, Z., and Ahmad, S. (2010) Levels of (1‐3)‐beta‐D‐glucan, Candida mannan and Candida DNA in serum samples of pediatric cancer patients colonized with Candida species. BMC Infect Dis 10: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokaddas, E., Khan, Z.U., Ahmad, S., Nampoory, M.R.N., and Burhamah, M. (2011) Value of (1‐3)‐β‐D‐glucan, Candida mannan and Candida DNA detection in the diagnosis of candidaemia. Clin Microbiol Infect 17: 1549–1553 [DOI] [PubMed] [Google Scholar]
- Mora‐Montes, H.M., Bates, S., Netea, M.G., Diaz‐Jimenez, D.F., Lopez‐Romero, E., Zinker, S., et al (2007) Endoplasmic Reticulum alpha‐glycosidases of Candida albicans are required for N‐glycosylation, cell wall integrity, and normal host–fungus interaction. Eukaryot. Cell 6: 2184–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora‐Montes, H.M., Ponce‐Noyola, P., Villagómez‐Castro, J.C., Gow, N.A.R., Flores‐Carreón, A., and López‐Romero, E. (2009) Protein glucosylation in Candida. Future Microbiol 4: 1167–1183 [DOI] [PubMed] [Google Scholar]
- Mora‐Montes, H.M., Bates, S., Netea, M.G., Castillo, L., Brand, A., Buurman, E.T., et al (2010) A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host–fungus interactions. J Biol Chem 285: 12087–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelle, W., Bernard, M., Debeaupuis, J.‐P., Buitrago, M., Tabouret, M., and Latgé, J.‐P. (2005) Galactomannoproteins of Aspergillus fumigatus. Eukaryot Cell 4: 1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, C.A., Bates, S., Buurman, E.T., Hughes, H.B., MacCallum, D.M., Bertram, G., et al (2005) Mnt1p and Mnt2p of Candida albicans are partially redundant alpha‐1,2‐mannosyltransferases that participate in O‐linked mannosylation and are required for adhesion and virulence. J Biol Chem 280: 1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murciano, C., Moyes, D.L., Runglall, M., Islam, A., Mille, C., Fradin, C., et al (2011) Candida albicans cell wall glycosylation may be indirectly required for activation of epithelial cell proinflammatory responses. Infect Immun 79: 4902–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, K., Feng, Y., Tanaka, A., and Jigami, Y. (1998) The involvement of mnn4 and mnn6 mutations in mannosylphosphorylation of O‐linked oligosaccharide in yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1425: 255–262 [DOI] [PubMed] [Google Scholar]
- Netea, M.G., Gow, N.A.R., Munro, C.A., Bates, S., Collins, C., Ferwerda, G., et al (2006) Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll‐like receptors. J Clin Invest 116: 1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea, M.G., Brown, G.D., Kullberg, B.J., and Gow, N.A.R. (2008) An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6: 67–78 [DOI] [PubMed] [Google Scholar]
- Netea, M.G., Gow, N.A.R., Joosten, L.A.B., Verschueren, I., van der Meer, J.W.M., and Kullberg, B.J. (2010) Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med Mycol 48: 897–903 [DOI] [PubMed] [Google Scholar]
- Nihtinen, A., Anttila, V.J., Richardson, M., Ruutu, T., Juvonen, E., Meri, T., and Volin, L. (2011) Factors influencing the performance level of Candida mannan antigen testing in allogeneic stem cell transplant recipients not receiving fluconazole prophylaxis. Transpl Infect Dis 13: 266–272 [DOI] [PubMed] [Google Scholar]
- Nobile, C.J., Nett, J.E., Andes, D.R., and Mitchell, A.P. (2006) Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell 5: 1604–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara, T.R., and Alspaugh, J.A. (2012) The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev 25: 387–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odani, T., Shimma, Y., Tanaka, A., and Jigami, Y. (1996) Cloning and analysis of the MNN4 gene required for phosphorylation of N‐linked oligosaccharides in Saccharomyces cerevisiae. Glycobiology 6: 805–810 [DOI] [PubMed] [Google Scholar]
- Odani, T., Shimma, Y., Wang, X.H., and Jigami, Y. (1997) Mannosylphosphate transfer to cell wall mannan is regulated by the transcriptional level of the MNN4 gene in Saccharomyces cerevisiae. FEBS Lett 420: 186–190 [DOI] [PubMed] [Google Scholar]
- Odds, F.C., and Evans, E.G. (1980) Distribution of pathogenic yeasts and humoral antibodies to candida among hospital inpatients. J Clin Pathol 33: 750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltroche‐Llacsahuanga, H., Goyard, S., d′Enfert, C., Prill, S.K.H., and Ernst, J.F. (2006) Protein O‐mannosyltransferase isoforms regulate biofilm formation in Candida albicans. Antimicrob Agents Chemother 50: 3488–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcaro, I., Vidal, M., Jouvert, S., Stahl, P.D., and Giaimis, J. (2003) Mannose receptor contribution to Candida albicans phagocytosis by murine E‐clone J774 macrophages. J Leukoc Biol 74: 206–215 [DOI] [PubMed] [Google Scholar]
- Prill, S.K.H., Klinkert, B., Timpel, C., Gale, C.A., Schröppel, K., and Ernst, J.F. (2005) PMT family of Candida albicans: five protein mannosyltransferase isoforms affect growth, morphogenesis and antifungal resistance. Mol Microbiol 55: 546–560 [DOI] [PubMed] [Google Scholar]
- Rohrlich, P., Sarfati, J., Mariani, P., Duval, M., Carol, A., Saint‐Martin, C., et al (1996) Prospective sandwich enzyme‐linked immunosorbent assay for serum galactomannan: early predictive value and clinical use in invasive aspergillosis. Pediatr Infect Dis J 15: 232–237 [DOI] [PubMed] [Google Scholar]
- Romero, P.A., Lussier, M., Veronneau, S., Sdicu, A.‐M., Herscovics, A., and Bussey, H. (1999) Mnt2p and Mnt3p of Saccharomyces cerevisiae are members of the Mnn1p family of α‐1,3‐mannosyltransferases responsible for adding the terminal mannose residues of O‐linked oligosaccharides. Glycobiology 9: 1045–1051 [DOI] [PubMed] [Google Scholar]
- Rouabhia, M., Schaller, M., Corbucci, C., Vecchiarelli, A., Prill, S.K.‐H., Giasson, L., and Ernst, J.F. (2005) Virulence of the fungal pathogen Candida albicans requires the five isoforms of protein mannosyltransferases. Infect Immun 73: 4571–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo, S., Ikeda, S., Yamabe, K., Kakuta, S., Ishigame, H., Akitsu, A., et al (2010) Dectin‐2 recognition of [alpha]‐mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32: 681–691 [DOI] [PubMed] [Google Scholar]
- Sendid, B., Tabouret, M., Poirot, J.L., Mathieu, D., Fruit, J., and Poulain, D. (1999) New enzyme immunoassays for sensitive detection of circulating Candida albicans mannan and antimannan antibodies: useful combined test for diagnosis of systemic candidiasis. J Clin Microbiol 37: 1510–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth, C.C., Hall, R., Lewis, L., Brown, A.J.P., Odds, F.C., Erwig, L.P., and Gow, N.A.R. (2011) Glycosylation status of the C. albicans cell wall affects the efficiency of neutrophil phagocytosis and killing but not cytokine signaling. Med Mycol 49: 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, N., Saitoh, T., Tadokoro, Y., and Okawa, Y. (2009) The cell wall galactomannan antigen from Malassezia furfur and Malassezia pachydermatis contains beta‐1,6‐linked linear galactofuranosyl residues and its detection has diagnostic potential. Microbiology 155: 3420–3429 [DOI] [PubMed] [Google Scholar]
- Singleton, D.R., Masuoka, J., and Hazen, K.C. (2005) Surface hydrophobicity changes of two Candida albicans serotype B mnn4Δ mutants. Eukaryot Cell 4: 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens, S.P., van de Veerdonk, F.L., van der Meer, J.W.M., Kullberg, B.J., Joosten, L.A.B., and Netea, M.G. (2010) The Candida Th17 response is dependent on mannan‐ and beta‐glucan‐induced prostaglandin E2. Int Immunol 22: 889–895 [DOI] [PubMed] [Google Scholar]
- Smeekens, S.P., van de Veerdonk, F.L., Joosten, L.A.B., Jacobs, L., Jansen, T., Williams, D.L., et al (2011) The classical CD14++ CD16− monocytes, but not the patrolling CD14+ CD16+ monocytes, promote Th17 responses to Candida albicans. Eur J Immunol 41: 2915–2924 [DOI] [PubMed] [Google Scholar]
- Sobel, J.D. (2007) Vulvovaginal candidosis. Lancet 369: 1961–1971 [DOI] [PubMed] [Google Scholar]
- Southard, S.B., Specht, C.A., Mishra, C., Chen‐Weiner, J., and Robbins, P.W. (1999) Molecular analysis of the Candida albicans homolog of Saccharomyces cerevisiae MNN9, required for glycosylation of cell wall mannoproteins. J Bacteriol 181: 7439–7448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suram, S., Gangelhoff, T.A., Taylor, P.R., Rosas, M., Brown, G.D., Bonventre, J.V., et al (2010) Pathways regulating cytosolic phospholipase A2 activation and eicosanoid production in macrophages by Candida albicans. J Biol Chem 285: 30676–30685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada, H., Nemoto, E., Shimauchi, H., Watanabe, T., Mikami, T., Matsumoto, T., et al (2002) Saccharomyces cerevisiae and Candida albicans‐derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14‐ and Toll‐like receptor 4‐dependent manner. Microbiol Immunol 46: 503–512 [DOI] [PubMed] [Google Scholar]
- Takahara, K., Tokieda, S., Nagaoka, K., Takeda, T., Kimura, Y., and Inaba, K. (2011) C‐type lectin SIGNR1 enhances cellular oxidative burst response against C. albicans in cooperation with Dectin‐1. Eur J Immunol 41: 1435–1444 [DOI] [PubMed] [Google Scholar]
- Takahara, K., Arita, T., Tokieda, S., Shibata, N., Okawa, Y., Tateno, H., et al (2012) Difference in fine specificity to polysaccharides of C. albicans mannoprotein between mouse SIGNR1 and human DC‐SIGN. Infect Immun 80: 1699–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, P.R., Brown, G.D., Herre, J., Williams, D.L., Willment, J.A., and Gordon, S. (2004) The role of SIGNR1 and the beta‐glucan receptor (Dectin‐1) in the nonopsonic recognition of yeast by specific macrophages. J Immunol 172: 1157–1162 [DOI] [PubMed] [Google Scholar]
- Thornton, B.P., Vĕtvicka, V., Pitman, M., Goldman, R.C., and Ross, G.D. (1996) Analysis of the sugar specificity and molecular location of the beta‐glucan‐binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol 156: 1235–1246 [PubMed] [Google Scholar]
- Timpel, C., Strahl‐Bolsinger, S., Ziegelbauer, K., and Ernst, J.F. (1998) Multiple functions of Pmt1p‐mediated protein O‐mannosylation in the fungal pathogen Candida albicans. J Biol Chem 273: 20837–20846 [DOI] [PubMed] [Google Scholar]
- Timpel, C., Zink, S., Strahl‐Bolsinger, S., Schroppel, K., and Ernst, J. (2000) Morphogenesis, adhesive properties, and antifungal resistance depend on the Pmt6 protein mannosyltransferase in the fungal pathogen Candida albicans. J Bacteriol 182: 3063–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener, J., Weindl, G., de Groot, P.W.J., de Boer, A.D., Kaesler, S., Thavaraj, S., et al (2012) Glycosylation of Candida albicans cell wall proteins is critical for induction of innate immune responses and apoptosis of epithelial cells. PLoS ONE 7: e50518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, L.A., Munro, C.A., Bruijn, I., Lenardon, M.D., McKinnon, A., and Gow, N.A. (2008) Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog 4: e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, C.A., Salvage‐Jones, J.A., Li, X., Hitchens, K., Butcher, S., Murray, R.Z., et al (2008) The macrophage‐inducible C‐type lectin, Mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180: 7404–7413 [DOI] [PubMed] [Google Scholar]
- Wheeler, R.T., Kombe, D., Agarwala, S.D., and Fink, G.R. (2008) Dynamic, morphotype‐specific Candida albicans beta‐glucan exposure during infection and drug treatment. PLoS Pathog 4: e1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, S., Matsumoto, M., Takeuchi, O., Matsuzawa, T., Ishikawa, E., Sakuma, M., et al (2009) C‐type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA 106: 1897–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R.‐B., Mark, M.R., Gray, A., Huang, A., Xie, M.H., Zhang, M., et al (1998) Toll‐like receptor‐2 mediates lipopolysaccharide‐induced cellular signalling. Nature 395: 284–288 [DOI] [PubMed] [Google Scholar]
- Yip, C.L., Welch, S.K., Klebl, F., Gilbert, T., Seidel, P., Grant, F.J., et al (1994) Cloning and analysis of the Saccharomyces cerevisiae MNN9 and MNN1 genes required for complex glycosylation of secreted proteins. Proc Natl Acad Sci USA 91: 2723–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Daniels, K.J., Oh, S.‐H., Green, C.B., Yeater, K.M., Soll, D.R., and Hoyer, L.L. (2006) Candida albicans Als3p is required for wild‐type biofilm formation on silicone elastomer surfaces. Microbiology 152: 2287–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]


