Abstract
Aim
To compare absolute values of regional cerebral tissue oxygenation (cStO2) during haemodynamic transition after birth and repeatability during steady state for two commercial near‐infrared spectroscopy (NIRS) devices.
Methods
In a prospective observational study, the INVOS 5100C and FORE‐SIGHT were compared on 12 term newborns delivered by elective caesarean section. During the 10 min following umbilical cord clamping, cStO2 was measured simultaneously with the neonatal sensors from each device. Repeated measurements were taken the following day.
Results
Three and 8 min after clamping, the mean cStO2 value increased from 53.4% (CI 36.8–69.9%) to 86.0% (CI 80.2–91.7%) for INVOS and from 61.6% (CI 55.4–67.8%) to 82.2% (CI 77.7–86.7%) for FORE‐SIGHT. The Bland–Altman plot revealed decreasing difference (INVOS minus FORE‐SIGHT) (D) in absolute values (A) with increasing cStO2 (D = 0.5A – 38.19 p = <0.001). The mean steady‐state value on day two was 78.4% (CI 74.6–82.2%) and 86.2% (CI 85.0–87.4%) for INVOS and FORE‐SIGHT, respectively. The within‐subject standard deviation during steady‐state repeated measurements was 4.8% ± 0.86 for INVOS and 2.8% ± 0.5 for FORE‐SIGHT.
Conclusion
The INVOS and FORE‐SIGHT cStO2 estimates showed oxygenation‐level‐dependent difference during birth transition. The better repeatability of FORE‐SIGHT could be due to the lower response to change in saturation.
Keywords: Cerebral oxygenation, Near‐infrared spectroscopy, Newborn, Transition
Abbreviations
- cStO2
Regional cerebral tissue oxygenation
- NIRS
Near‐infrared spectroscopy
- SpO2
Arterial oxygen saturation
Key notes.
This study compared two commercial near‐infrared spectroscopy (NIRS) devices, investigating cerebral oxygenation in 12 term newborn infants born by elective caesarean section.
As already well known, arterial saturation and cerebral oxygenation increased rapidly from three to 10 min after cord clamping.
We demonstrated a systematic oxygen‐level‐dependent difference between the two commercial cerebral oximeters, approved for clinical use in newborn infants, which have not been shown before.
Introduction
With near‐infrared spectroscopy (NIRS), it is possible to monitor oxygenation in the brain tissue continuously and noninvasively 1. This might be of clinical value as it enables assessment of end‐organ oxygen balance. Neonates are often born in a state of hemodynamic instability 5, so trend monitoring is not sufficient as the ‘baseline’ state is uncertain. Specific knowledge of the different NIRS devices in neonates, with regard to absolute values, dynamic range, repeatability and cStO2 values, is necessary for clinical use.
The present study compares two commercial devices, the FORE‐SIGHT cerebral oximeter (CASMED, Branford, CT, USA) and the INVOS 5100C (COVIDIEN, Mansfield, MA, USA) by measuring cerebral oxygenation on healthy infants simultaneously during birth transition. These are then complemented by repeated steady‐state measurements when the infant is stable the following day. We used the natural phenomenon of transition for estimation of the dynamic properties of the devices, as it has been demonstrated that arterial and cerebral oxygenation is low in the first minutes after birth and gradually increases to a steady state after 7–8 min 6, as a consequence of establishment of ventilation and changes in cardiac function and cerebral haemodynamics 5.
Methods
The project was approved by the Danish Regional Committee on Biomedical Research Ethics (journal no. H‐3‐2012‐016). To have 80% power to detect a mean difference of 5% in cStO2 with a standard deviation of 5% and a significance level of 0.05, 12 infants were needed. We included term newborns with a gestational age >37 weeks, who were expected to be delivered by an uncomplicated elective caesarean section. Children who had an Apgar score <8 or who needed respiratory support or supplementary oxygen during the transition were excluded. Infants with genetically defined syndromes, congenital malformations or too much hair in the fronto‐parietal region of the forehead were also excluded. Written consent was obtained from the parents before the elective caesarean section. We used the INVOS 5100C with the OxyAlert neonatal sensors and the FORE‐SIGHT cerebral oximeters with the Small Dual sensors. Arterial oxygen saturation (SpO2) and pulse were measured continuously with the Masimo SET pulse oximeter (Masimo Coorporation, Irvine, CA, USA) on the right hand wrist. The instruments were all connected to an IntelliVue MP70 monitor (Philips, Eindhoven, the Netherlands) and stored in 1 Hz in IxTrend 2.0 (Ixcellence GmbH, Wildaum, Germany). The Apgar scores were registered by the midwife independently of the NIRS or pulseoximetry data. The same investigator (TWH) carried out all the NIRS measurements.
Devices
The INVOS 5100C with the OxyAlert™ neonatal sensor is approved for use in patients <5 kg. It uses light‐emitting diodes (wavelength 730 and 810 nm) and source–detector distances of 30 and 40 mm. The maximum reading is 95% and the minimum reading is 15%.
The FORE‐SIGHT™ cerebral oximeter with the neonatal Small Dual sensor approved for use in patients <8 kg using a 4‐wavelength laser source (wavelength 690, 780, 805 and 850) with one detector at a source–detector distance of 25 mm. The maximum reading is 100%, and the minimum reading is 0%.
Procedure during transition
Immediately after birth, the infants were laid in a prone position on the mother's chest, which is the routine procedure in the obstetric ward, to mimic the initial contact after a normal vaginal delivery. The head and hand of the newborn were cleaned with towels to reduce vernix and amniotic fluid, which could influence sensor signal quality. The infant was then wrapped in warm towels. Immediately after the head was dried, the two NIRS sensors were positioned in the fronto‐parietal region on each side of the newborn's head with a distance of at least 6 cm from the light source of one sensor to the detector of the other sensor to minimise signal interference. The light sources of the sensors were always closest to the midline. The position of the sensors was held in position throughout the 10 min but was alternated between the right and left hemisphere among patients. The timer was started at the point of umbilical cord clamping. The sensors were positioned as soon as possible and held in position by hand for 10 min from cord clamping. The pulse oximeter (Sp02) was positioned on the right arm or wrist (at the preductal level), as soon as possible. A standard physical examination was carried out after the measurements were finished.
Repeatability measurements
Repeated NIRS measurements were carried out more than 24 h after delivery. First, the two NIRS sensors were positioned on the right and left fronto‐parietal region within the recommended distance of more than six centimetres, to assess any possible interference between the two sensors. One sensor was removed and replaced with the other sensor in the same location to assess whether the cStO2 from that sensor changed. Then, six measurements of 30 sec each were carried out on the fronto‐parietal region with each device, to examine repeatability and a possible difference between the left and right hemispheres. The sensors were totally removed from the infant's head between measurements.
Data analysis and statistics
Absolute values for cerebral oxygenation during the steady state and the differences between the hemispheres were compared by paired t‐test. The mean absolute values for arterial saturation, INVOS and FORE‐SIGHT at 3 and 8 min were calculated as the average of 30 sec of recording. The steady‐state repeated measurements within‐subject standard deviation (Sw) was estimated from the square root of the residual mean square in a one‐way ANOVA with infant as factor. A Bland–Altman plot 11 with 95% limits of agreements (±1.96 standard deviation) was used to visualise the agreement between the cStO2 values of the FORE‐SIGHT and INVOS during transition. The statistical significance of the oxygenation‐level dependence of the bias was tested by ANCOVA with average as the outcome variable and subject as the predictor variable. Signal interference was tested by paired t‐test. A signal‐to‐noise ratio was calculated as the ratio between the slope during transition and the within‐subject standard deviation for the repeated measurements. All graphs were constructed using the OmniGraphSketcher version 1.2.4 (The Omni Group, Seattle, WA, USA), and statistical tests were carried out in SPSS statistics version 20 (IBM, Armonk, NY, USA). A p < 0.05 was considered significant.
Results
Between October and December 2012, 14 neonates were initially included in the study with parental consent and 12 infants were actually studied (Table 1). Two infants were excluded, as one was taken for resuscitation immediately after birth and one was born before the scheduled caesarean section. No infants were excluded due to too much hair in the sensor area. All mothers received spinal anaesthesia prior to caesarean section. One infant was excluded from the transitional data analysis due to exceptionally unstable signals of both the pulse oximeter and the two cerebral oximeters, with no correlation between the three devices throughout the study period. After birth, the cerebral cStO2 of the INVOS and FORE‐SIGHT increased progressively from 53.4% (CI 36.8–69.9%) to 86.0% (CI 80.2–91.7%) for INVOS and from 61.6% (CI 55.4–67.8%) to 82.2% (CI 77.7–86.7%) for FORE‐SIGHT, at 3 and 8 min, respectively (Table 2). The min–max absolute value range recorded was 15%–95% for INVOS and 26%–93% for FORE‐SIGHT. The Bland–Altman plot (Figure 3) revealed an oxygenation‐level‐dependent bias with limits of agreement of ± 15.4% demonstrating a decreasing difference (INVOS minus FORE‐SIGHT) (D) in absolute values (A) with increasing cStO2 (D = 0.5A – 38.19 p = <0.001) having INVOS reading a lower value at low levels of oxygenation. There was no statistically significant difference between cStO2 from the left and right hemispheres either after cord clamping or on the following day. The INVOS measurement increased by a 1.3 percentage point when the FORE‐SIGHT was switched on at the same time (p = 0.049), whereas the FORE‐SIGHT reading was independent of the INVOS being on or off. On day two, the mean values were 78.4% (CI 74.6–82.2%) and 86.2% (CI 85.0–87.4%) for INVOS and FORE‐SIGHT, respectively (p < 0.0001). The repeatabilities were 4.8% ± 0.86 and 2.8% ± 0.5 with a signal‐to‐noise ratio of 8.6 and 8.8 for INVOS and FORE‐SIGHT, respectively.
Table 1. Patient characteristics of the cohort.
| Patients (N = 12) | Mean (range) |
|---|---|
| GA, weeks | 38.9 (38.1–39.6) |
| Weight, g | 3417 (2550–4010) |
| Head circumference, cm | 35.6 (34–38) |
| APGAR 1 min | 10 (9–10) |
| APGAR 5 min | 10 (9–10) |
| pH umbilical artery | 7.25 (7.06–7.33) |
Table 2. Arterial and cerebral saturation after umbilical clamping (mean, 95% CI).
| Time | SpO2 | INVOS cStO2 | FORE‐SIGHT cStO2 |
|---|---|---|---|
| 3 min (N = 10) | 65.0 (52.9–77.9) | 53.4 (36.8–69.9) | 61.6 (55.4–67.8) |
| 8 min (N = 11) | 88.4 (83.6–93.1) | 86.0 (80.2–91.7) | 82.2 (77.7–86.7) |
Figure 3.
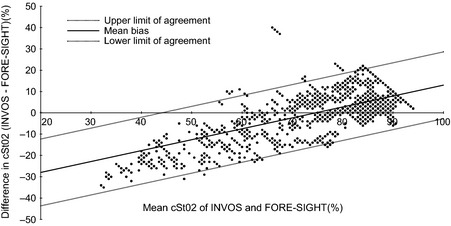
Bland–Altman plot (mean, limits of agreement).
Discussion
We compared absolute values of arterial and cerebral oxygenation during haemodynamic transition after birth and repeatability during steady state for two commercial NIRS devices, the INVOS 5100C and the FORE‐SIGHT. As expected and already well known, SpO2 and cStO2 increased in the minutes following umbilical cord clamping (Figure 1). However, the increase in cerebral oxygenation as estimated by the FORE‐SIGHT was significantly less compared with the INVOS (Figure 2a, b).
Figure 1.
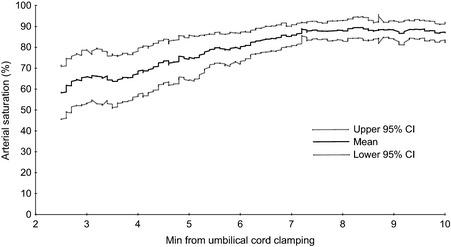
Arterial saturation using pulse oximeter (mean, 95% CI).
Figure 2.

a. Cerebral oxygenation for INVOS (mean, 95% CI). b. Cerebral oxygenation for FORE‐SIGHT (mean, 95% CI).
The strength of our study is that it allows device comparison during low oxygen levels together with assessment of repeatability of measurements. This has to our knowledge not been done before. Limitations include possible sensor cross‐talk, which is an inherent issue when doing measurement with several sensors at a time. However, great care was taken to ensure proper distance between sensors, and our analysis of the magnitude of this problem suggests that it is minor. Furthermore, cerebral oxygenation has no gold standard, which prohibits any conclusions of the appropriateness of values from each device to be made.
Transition
This study agrees with previous research when it comes to the general trend of cerebral oxygenation in the first 10 min after umbilical cord clamping. Using the INVOS 5100C with the neonatal sensor, Urlesberger et al. 8 examined term infants born by vaginal and caesarean section. Mean values were about 50% at 3 min and 80% at 8 min (estimations from graphs), similar to our findings. In ours, as well as in other studies, the infants were hyperoxygenated at eight to 10 min after birth possibly followed by a subsequent decrease to their ‘actual’ steady state, as suggested by Noori and co‐workers 5. This trend was similar to our INVOS cStO2 data, having a value of 86.0% at 8 min during the plateau after caesarean section and a value of 78.2% the following day during the testing of the reproducibility of measurement. Almaazmi et al. recently observed a median cStO2 of 52% at 3 min of age and 73% at 10 min of age using the FORE‐SIGHT cerebral oximeter with the small sensor during transition in 46 healthy term newborns 13. This finding is about ten percentage points less than our measurements. However, delivery of the body was defined as birth and start time in the study by Almaazmi et al., not as umbilical cord clamping as in our and other studies. This could make a difference when comparing cStO2 of various studies at specific times.
Three of 11 infants in our study had a cStO2 value of 95% for >30 sec in the last two minutes when using the INVOS neonatal sensor. The difference from the values obtained by the FORE‐SIGHT neonatal sensor was not much different in this area as seen in the Bland–Altman plot (Figure 3). Considering that cStO2 reflects the oxygen saturation in veins (70% to 80%), capillaries (5%) and arteries (15% to 20%) 8, the values of 95% are implausible. Furthermore, a mean SpO2 of 88.4% and cStO2 estimates of 86.0% and 82.2% with INVOS and FORE‐SIGHT devices, respectively, at 8 min suggest an unreasonably low oxygen extraction, indicating that at least the INVOS, but probably both devices, overestimates the cStO2 at higher levels of oxygenation. Urlesberger 7, Pocivalnik 15 and Kratky 6 use ‘Quality Criteria’ excluding all NIRS values equal or above the simultaneous SpO2. Although it seems reasonable to exclude these as artefacts, it becomes difficult to do so in a device test setting when testing the sensors of two different instruments, especially when using the imperfect pulse oximeter as part of the exclusion criteria. Therefore, we did not apply exclusion criteria for high cStO2 values as information about all values is equally important when comparing devices.
Repeatability
We found a lower within‐subject standard deviation (Sw) of 2.8% for the FORE‐SIGHT than for the INVOS (4.8%) when replacing the sensor repeatedly the day after birth. However, the signal‐to‐noise ratio was similar for the two devices at 8.6 for INVOS and 8.8 for FORE‐SIGHT. INVOS detected a larger change in rStO2 values from 3 to 8 min (dynamic range) at the cost of noise, whereas FORE‐SIGHT demonstrated less noise but also showed a narrower oxygenation range during transition. Dullenkopf and colleagues found 95% limits of agreement of ±17.7% for the NIRO 300 during sensor exchanges, corresponding to a within‐subject standard deviation (Sw) of 6.4% 16. Sorensen 17 and Hyttel‐Sorensen 18, in separate studies, found an Sw of 5.2% and 5.4% for the NIRO 300 and the INVOS 5100 with the adult Somasensor, respectively.
The difference between devices and sensors
The INVOS adult and paediatric sensors are identical in optical geometry and diode wavelengths, but apparently differ in analysis algorithm and perhaps also in calibration techniques. Values from the neonatal head differ by approximately 10%, as found by Dullenkoft et al. 1, whereas when comparing the neonatal sensor and the paediatric sensor, no significant difference seems to be found 20. Dix et al. 21 recently found a similar difference between the cStO2 of the adult sensor compared to both the paediatric and neonatal sensor of 10–14% for the three most used devices, NIRO, INVOS and FORE‐SIGHT. A mean difference of 11.3% ± 5.4 and 13.8% ± 7.9 was also found by Dullenkopf et al. 1 when the paediatric sensor of INVOS 5100 was compared to their own adult Somasensor and the NIRO 300, respectively. Pocivalnik and colleagues 15 examined eight term and 29 preterm and also found a 10% difference between the NIRO 300 (73.1% ± 6.9) and the INVOS 5100 with the neonatal sensor (84.1% ± 6.4). The reason for this difference in the absolute values of the adult and paediatric/neonatal sensors is not known, but could be a simple matter of different calibrations.
In none of the mentioned studies reported in the literature were attempts made to examine whether the differences were oxygenation‐level dependent. We demonstrated that this is the case for the INVOS and the FORE‐SIGHT instrument, both equipped with their ‘neonatal’ sensors. Thus, the difference was more than 20% in the low area of oxygenation (below 50% in cStO2) between the two devices. This difference could come from the difference in sensor geometry, the different algorithms and/or the differences in calibration procedures. Whereas the INVOS uses spatially resolved spectroscopy (with source–detector distances of 30 and 40 mm), the FORE‐SIGHT with its Small Dual sensor uses ‘wavelength’‐resolved spectroscopy, with a single detector and a source–detector distance of 25 mm. Thus, the penetration depth of the light path is slightly deeper with the INVOS device, while the INVOS claims to be able to subtract the more superficial tissue layers from the signal with the use of two light detectors. However, the actual sources of device differences remain to be clearly elucidated and would probably require access to the raw optical data and exact algorithms and calibrations.
One of the purposes of NIRS is to obtain a direct and non‐invasive assessment of cerebral oxygenation, so that events such as hypoxia, ischaemia or hyperoxia in the preterm can be identified, evaluated and perhaps intervened upon in future. For this to happen, NIRS cStO2 measurements need to be reliable and reproducible. This is not yet the case as seen from our and other investigators' results 16. When the absolute values on commercial devices differ significantly, it is challenging to define normal ranges and clinical limits of concern, when a low cStO2 of 40% in one device might be 60% in another. More importantly, randomised clinical trials are needed to demonstrate whether NIRS can reduce the risk of clinically relevant endpoints, such as death or neurodevelopmental handicap 22. With this study set‐up, it was feasible to measure the oxygenation‐level‐dependent difference between two devices together with assessing repeatability. It would be interesting to do a similar set‐up comparing other devices in future.
Conclusion
We demonstrated an oxygen‐level‐dependent difference between two commercial cerebral oximeters approved for clinical use in newborn infants. In particular, their readings differed as much as 20% at the lowest range of saturations. Although one of the devices appeared to have better repeatability during repositioning, this advantage was proportional to a lower response to change in saturation.
Funding Source
The Augustinus Foundation funded the research year for TWH.
Competing Interests
We declare that we have no conflicts of interest.
Acknowledgements
We express our gratitude to the families who gave us permission to study their newborn infants, and the midwives, obstetricians and other staff who helped us make this trial possible.
References
- 1.Dullenkopf A. Measurement of cerebral oxygenation state in anaesthetized children using the INVOS 5100 cerebral oximeter. Peadiatr Anaest 2003; 3: 1–8 [DOI] [PubMed] [Google Scholar]
- 2.Greisen G. Is near‐infrared spectroscopy living up to its promises? Semin Fetal Neonatal Med 2006; 11: 498–502 [DOI] [PubMed] [Google Scholar]
- 3.Yoshitani K, Kawaguchi M, Tatsumi K, Kitaguchi K, Furuya H. A comparison of the INVOS 4100 and the NIRO 300 near‐infrared spectrophotometers. Anesth Analg 2002; 94: 586–90 [DOI] [PubMed] [Google Scholar]
- 4.Komiyama T, Quaresima V, Shigematsu H, Ferrari M. Comparison of two spatially resolved near‐infrared photometers in the detection of tissue oxygen saturation: poor reliability at very low oxygen saturation. Clin Sci 2001; 101: 715–8 [PubMed] [Google Scholar]
- 5.Noori S, Wlodaver A, Gottipati V, McCoy M, Schultz D, Escobedo M. Transitional changes in cardiac and cerebral hemodynamics in term neonates at birth. J Pediatr 2012; 160: 943–8 [DOI] [PubMed] [Google Scholar]
- 6.Kratky E, Pichler G, Rehak T, Avian A, Pocivalnik M, Müller W, et al. Regional cerebral oxygen saturation in newborn infants in the first 15 min of life after vaginal delivery. Physiol Meas 2012; 33: 95–102 [DOI] [PubMed] [Google Scholar]
- 7.Urlesberger B, Grossauer K, Pocivalnik M, Avian A, Müller W, Pichler G. Regional oxygen saturation of the brain and peripheral tissue during birth transition of term infants. J Pediatr 2010; 157: 740–4 [DOI] [PubMed] [Google Scholar]
- 8.Urlesberger B, Kratky E, Rehak T, Pocivalnik M, Avian A, Czihak J, et al. Regional oxygen saturation of the brain during birth transition of term infants: comparison between elective cesarean and vaginal deliveries. J Pediatr 2011; 159: 404–8 [DOI] [PubMed] [Google Scholar]
- 9.Fauchère J‐C, Schulz G, Haensse D, Keller E, Ersch J, Bucher HU, et al. Near‐infrared spectroscopy measurements of cerebral oxygenation in newborns during immediate postnatal adaptation. J Pediatr 2010; 156: 372–6 [DOI] [PubMed] [Google Scholar]
- 10.Isobe K, Kusaka T, Fujikawa Y, Okubo K, Nagano K, Yasuda S, et al. Measurement of cerebral oxygenation in neonates after vaginal delivery and cesarean section using full‐spectrum near infrared spectroscopy. Comp Biochem Physiol Part A Mol Integr Physiol 2002; 132: 133–8 [DOI] [PubMed] [Google Scholar]
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–10 [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8: 135–60 [DOI] [PubMed] [Google Scholar]
- 13.Almaazmi M, Schmid MB, Havers S, Reister F, Lindner W, Fuchs H, et al. Cerebral near‐infrared spectroscopy during transition of healthy term newborns. Neonatology 2013; 103: 246–51 [DOI] [PubMed] [Google Scholar]
- 14.Fuchs H, Lindner W, Buschko A, Almazam M, Hummler HD, Schmid MB. Brain oxygenation monitoring during neonatal resuscitation of very low birth weight infants. J Perinatology 2012; 32; 356–62 [DOI] [PubMed] [Google Scholar]
- 15.Pocivalnik M, Pichler G, Zotter H, Tax N, Müller W, Urlesberger B. Regional tissue oxygen saturation: comparability and reproducibility of different devices. J Biomed Opt 2011; 16: 057004. [DOI] [PubMed] [Google Scholar]
- 16.Dullenkopf A, Kolarova A, Schulz G, Frey B, Baenziger O, Weiss M. Reproducibility of cerebral oxygenation measurement in neonates and infants in the clinical setting using the NIRO 300 oximeter. Pediatr Crit Care Med 2005; 6: 344–7 [DOI] [PubMed] [Google Scholar]
- 17.Sorensen LC, Greisen G. Precision of measurement of cerebral tissue oxygenation index using near‐infrared spectroscopy in preterm neonates. J Biomed Opt 2006; 11 [DOI] [PubMed] [Google Scholar]
- 18.Hyttel‐Sorensen S, Sorensen LC, Riera J, Greisen G. Tissue oximetry: a comparison of mean values of regional tissue saturation, reproducibility and dynamic range of four NIRS‐instruments on the human forearm. Biomed Opt Express 2011; 2: 3047–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyttel‐Sorensen S, Hessel TW, Greisen G. Peripheral tissue oximetry: comparing three commercial near infrared spectroscopy oximeters on the human forearm. J Clin Monit Comput 2013; 28: 149–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris N, Pichler G, Pocivalnik M, Brandner A, Müller W, Urlesberger B. Cerebral regional oxygen saturation (crSO2): are different sensors comparable? Pediatr Anesth 2012; 12. doi: 10.1111/j.1460‐9592.2012.03895.x [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Dix LM, van Bel F, Baerts W, Lemmers PMA. Comparing near‐infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr Res 2013; 74: 557–63 [DOI] [PubMed] [Google Scholar]
- 22.Hyttel‐Sorensen S, Austin T, van Bel F, Benders M, Claris O, Dempsey EM, et al. Clinical use of cerebral oximetry in extremely preterm infants is feasible. Dan Med J 2013; 60 [PubMed] [Google Scholar]
- 23.Greisen G, Leung T, Wolf M. Has the time come to use near‐infrared spectroscopy as a routine clinical tool in preterm infants undergoing intensive care? Phil Trans R Soc A 2011; 369: 4440–51 [DOI] [PMC free article] [PubMed] [Google Scholar]


