Abstract
Laryngopharyngeal reflux (LPR) occurs when gastric contents pass the upper esophageal sphincter, causing symptoms such as hoarseness, sore throat, coughing, excess throat mucus, and globus. The pattern of reflux is different in LPR and gastroesophageal reflux. LPR usually occurs during the daytime in the upright position whereas gastroesophageal reflux disease more often occurs in the supine position at night-time or during sleep. Ambulatory 24-h double pH-probe monitoring is the gold standard diagnostic tool for LPR. Acid suppression with proton pump inhibitor on a long-term basis is the mainstay of treatment. Helicobacter pylori (H. pylori) is found in many sites including laryngeal mucosa and interarytenoid region. In this paper, we aim to present the relationship between LPR and H. pylori and review the current literature.
Keywords: Laryngophrayngeal reflux, Helicobacter pylori, Gastroesophageal reflux disease, Proton pump inhibitors
Core tip: This paper reviews the literature regarding the relationship between laryngophrayngeal reflux (LPR) and Helicobacter pylori. The otolaryngology perspective of LPR and the importance of endoscopic examination are emphasized.
INTRODUCTION
Gastroesophageal reflux (GER) can be a normal physiological phenomenon that occurs in most people, especially after meals. GER disease (GERD) develops when the reflux causes symptoms like heartburn and acid regurgitation. Laryngopharyngeal reflux (LPR) happens when gastric contents pass the upper esophageal sphincter, causing symptoms such as hoarseness, sore throat, coughing, excess throat mucus, and globus. The pattern of reflux is different in LPR and GER. LPR usually occurs during the daytime in the upright position, whereas GERD takes place more often in the supine position at night-time or during sleep[1]. Interestingly, the patients are different in terms of body type as well. There are reports suggesting a relationship between GERD and obesity[2,3]. In contrast, in a group of patients with laryngeal and pharyngeal symptoms, those with abnormal pharyngeal reflux events did not have a higher mean body mass index (BMI) than those with normal BMI[4]. A significantly higher percentage of esophageal reflux events was seen in obese versus non-obese participants. The authors concluded that abnormal esophageal reflux (GERD) is associated with increasing BMI and obesity, although this was not true for patients with pharyngeal reflux.
Helicobacter pylori (H. pylori) was originally identified by Marshall and Warren[5]. It was called a Campylobacter-like organism at first. It is a Gram-negative bacterium with a spiral shape and four to six flagella. It is obligate microaerophilic, and urease, catalase and oxidase positive. Although it is susceptible to acid, it is protected from the harmful effects of acid by both its motility and its ability to convert urea to ammonium by urease and form a basic milieu around itself. Although it occurs less commonly in developed countries and in children, and is more common in developing countries and adults, its prevalence varies between different regions and socioeconomic strata of the same country. Probable routes of contamination are fecal-oral, oral-oral, gastro-oral (reflux and vomiting), and iatrogenic (e.g., insufficiently disinfected endoscopes)[6]. The relationship of H. pylori with gastritis, peptic ulcer, gastric carcinoma, and mucosa-associated lymphoid tissue lymphoma has been demonstrated in many studies[7]. Different noninvasive [urea breath test (UBT), serological tests, and stool tests] and invasive [histological and microbiological examination of biopsy materials, rapid urease test, and polymerase chain reaction (PCR)] tests with varying specificity and sensitivity may be performed for the diagnosis for H. pylori in tissue[8]. H. pylori is localized primarily in the gastric mucosa. It is reported that the microorganism may exist in paranasal sinuses, tonsils, adenoids, and even middle ear mucosa[9-12]. It may also exist in atypical locations like dental plaque and saliva[13,14]. In many other studies H. pylori could not be found in tonsils, adenoids, dental plaque, saliva, or the oral cavity, which may mean that these tissues are only temporary colonization sites[15-18].
It has been estimated that half of otolaryngology patients with laryngeal and voice disorders have LPR[19]. LPR is considered one of the most important and common factors causing inflammation in the upper airways. The tissue damage is demonstrated in both animals and humans. It may be caused by direct exposure to acid, pepsin and bile, and by vagally mediated reflexes[20,21]. Besides acid and pepsin, the presence of H. pylori may be related to the symptoms and findings of LPR.
The variance between esophageal symptoms and upper aerodigestive tract disease may reflect the relative susceptibility of the epithelium of the larynx and pharynx to reflux-related injury. LPR may also occur in healthy individuals without symptoms or laryngeal pathology. LPR-related laryngeal disease and findings tend to resolve over a longer time and more often need higher levels of medication and therapy. LPR has an impact on various laryngeal pathologies including stenosis, malignancy, benign lesions, dysphagia, and functional disorders.
DIAGNOSIS
Laryngeal and voice disorders may present with diverse clinical manifestations. Many voice clinicians recommend that LPR be routinely assessed in patients with laryngeal and voice disorders; however, even among otolaryngologists who have a relatively high index of suspicion for LPR, it appears that this disorder is still often underdiagnosed and undertreated[22]. The symptoms, manifestations, patterns, and mechanisms of LPR and GERD are different. Patients with LPR usually deny symptoms of heartburn and/or regurgitation[21]. Less than half of otorhinolaryngology patients with LPR documented by pH monitoring complain of heartburn or regurgitation[23].
As with the majority of diseases, the diagnosis of LPR begins with history taking. It is then confirmed by laryngoscopy and subsequently validated by response to a trial of proton pump inhibitor (PPI) therapy. Although some institutions do perform routine pH testing, for the majority of cases this testing is reserved for refractory or complicated cases. The most common symptoms associated with LPR are cough, throat clearing, sore throat, globus, excess throat mucus, choking, and asthma. However, these entities have a multifactorial etiology and may be caused by recent sinusitis or other respiratory infections, smoking, voice abuse, and allergy, or these symptoms may be lacking. Therefore, accurate diagnosis based on history is a challenge. Belafsky et al[24] developed the Reflux Symptom Index (RSI), a self-administered nine-item questionnaire to help categorize the severity of LPR (Table 1). An RSI > 13 is considered abnormal. Symptoms of GERD, which include heartburn, chest pain, indigestion or acid regurgitation, are important, but it should be noted that more than half of the patients with LPR do not have these classic GERD symptoms[25]. Laryngeal examination is the second step in the diagnostic evaluation. The findings of LPR on laryngeal examination vary considerably. According to Belafsky et al[25-27], laryngeal edema is the hallmark finding of LPR. However, most otolaryngologists rely solely on the findings of erythema or posterior laryngitis (PL) (Figure 1A). Unfortunately, those findings are not present in many LPR patients. In our experience, edema (Figure 1B) is the principal, and most common, finding of LPR along with PL. PL is characterized by edema or hypertrophy, and sometimes erythema and hyperemia on the posterior wall of the glottis. Inflammation may reach the medial surface of the arytenoid cartilages and aryepiglottic folds. Furthermore, diffuse vocal fold edema and infraglottic edema reaching from the anterior commissure to the posterior wall may create an illusion of sulcus vocalis[27]. The nature of endolaryngeal mucus if it is thick and tenacious also points to PL (Figure 1C). LPR patients may present with one or all of these findings[21]. The difficulty in making an LPR diagnosis is that the findings are sometimes quite subtle; signs of inflammation and irritation are absent, and patients may have a normal-looking larynx (Figure 1D). Therefore, a high index of suspicion is needed. Reflux finding score (RFS) may be useful in categorizing the severity of the mucosal injury on laryngoscopy[26] (Table 2). Laryngoscopy should be done by both flexible and rigid endoscopes. There are also controversial studies on this matter. Branski et al[28] have reported on a series of patients in whom reflux findings were scrutinized by five observers reviewing videotaped examinations. The conclusions of the paper were that significant variability exists in describing reflux-related findings. In another important study by Hicks et al[29], 100 normal subjects underwent laryngoscopy; their examinations were then reviewed by otolaryngologists and speech-language pathologists to estimate the presence of reflux-related lesions in these healthy volunteers. The key finding in this study was that nearly 80% of the normal volunteers had an interarytenoid bar or posterior commissure hypertrophy. Unfortunately, this finding has been taken by many to be an indication that laryngoscopy is not useful in the clinical evaluation of reflux disease. However, it is important to realize that the physician’s own perception during a laryngoscopy strongly affects the diagnosis. That is, mild laryngeal edema might be the sole finding of LPR.
Table 1.
Reflux Symptom Index
| Within the past month, how did the following problems affect you? | 0 = no problem | 5 = severe problem |
| Hoarseness or a problem with your voice | 0 1 2 | 3 4 5 |
| Clearing your throat | 0 1 2 | 3 4 5 |
| Excess throat mucus or postnasal drip | 0 1 2 | 3 4 5 |
| Difficulty swallowing food, liquids, or pills | 0 1 2 | 3 4 5 |
| Coughing after you ate or after lying down | 0 1 2 | 3 4 5 |
| Breathing difficulties or choking episodes | 0 1 2 | 3 4 5 |
| Troublesome or annoying cough | 0 1 2 | 3 4 5 |
| Sensations of something sticking in your throat or a lump in your throat | 0 1 2 | 3 4 5 |
| Heartburn, chest pain, indigestion, or stomach acid coming up | 0 1 2 | 3 4 5 |
A total score of 13 is thought to be clinically significant.
Figure 1.
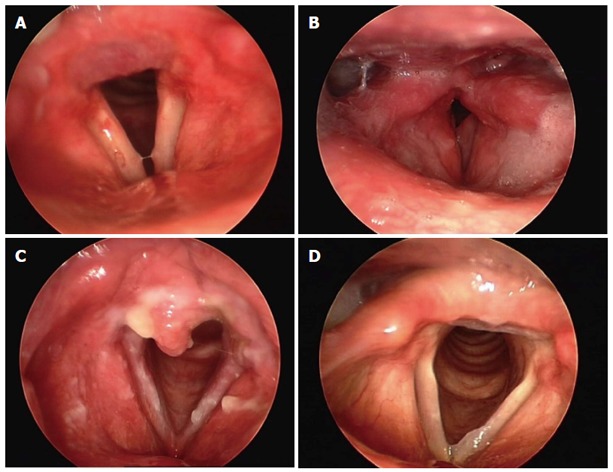
Diagnosis. A: Posterior laryngitis and erythema of the arytenoids; B: Laryngeal edema secondary to laryngopharyngeal reflux (LPR); C: Excessive posterior laryngitis. Note the thick mucus over the interarytenoid area and vocal cords; D: Normal larynx with only a mild posterior laryngitis treated by proton pump inhibitors with documented LPR by ambulatory double probe pH measurement.
Table 2.
Reflux finding score
| Subglottic edema | 2 = present |
| 0 = absent | |
| Ventricular obliteration | 2 = partial |
| 4 = complete | |
| Erythema/hyperemia | 2 = arytenoids only |
| 4 = diffuse | |
| Vocal cord edema | 1 = mild |
| 2 = moderate | |
| 3 = severe | |
| 4 = polypoid | |
| Diffuse laryngeal edema | 1 = mild |
| 2 = moderate | |
| 3 = severe | |
| 4 = obstructing | |
| Posterior commissure hypertrophy | 1 = mild |
| 2 = moderate | |
| 3 = severe | |
| 4 = obstructing | |
| Granuloma/granulation | 0 = absent |
| 2 = present | |
| Thick endolaryngeal mucus/other | 0 = absent |
| 2 = present | |
| Total |
A total score of 7 is thought to be clinically significant.
Traditional diagnostic tests for GERD lack both sensitivity and specificity for LPR. As mentioned before, these two patient groups differ in terms of symptoms and diagnosis. Barium esophagography, radionuclide scanning, the Bernstein acid-perfusion test, and esophagoscopy with biopsy are all often negative in LPR patients[21]. This is probably because most LPR patients do not develop esophagitis, because esophageal mucosa is more resistant to acid- and pepsin-related injury than the laryngeal and pharyngeal mucosa is[21,23]. Therefore, evaluating a patient depending on GERD protocols may lead the otolaryngologist to misdiagnosis. At the present time, ambulatory 24-h double pH probe (simultaneous esophageal and pharyngeal) monitoring has become the diagnostic gold standard for LPR[30-32]. The upper probe must be placed in a consistent zone at or above (2 cm) the functional upper esophageal sphincter. This allows the lower probe to be placed about 5 cm above the lower esophageal sphincter. However, it is expensive and is not widely available. Nevertheless, pH monitoring effectively documents LPR with a high degree of specificity and sensitivity. Esophageal manometry is also important for accurate placement of the pH electrodes and particularly useful in patients with chronic cough.
Endoscopic examination of the esophagus (transnasal esophagoscopy; TNE) is performed in the clinical setting with or without sedation. It is generally used to check GERD-related complications and exclude other diseases. TNE allows otolaryngologists to screen the esophagus. In a large series, Postma et al[33] reported that 50% of the patients had positive findings on TNE, including 17% esophagitis, 8% hiatal hernia, 5% Barrett’s metaplasia, 5% Candida esophagitis, and 4% stricture. Esophagoscopy alone does not diagnose LPR, and only a small percentage of LPR patients have abnormal esophagoscopy.
H. pylori infects the stomach, usually during childhood. The most common cause of peptic ulcers, H. pylori infection, is present in about half the world’s population. There are several different methods to test for H. pylori infection.
Breath test (carbon isotope-urea breath test or UBT)
Up to 2 wk before the test, the patient must stop taking any antibiotics, bismuth-containing medications such as Pepto-Bismol, and PPIs. The patient swallows a special substance containing urea (a waste product the body produces as it breaks down protein) that has been made harmlessly radioactive. If H. pylori is present, the bacteria convert the urea into CO2, which is detected and recorded in the exhaled breath after 10 min. This test can identify almost all people who have H. pylori and confirm that the infection has been fully treated.
Blood tests
Blood tests are used to measure H. pylori IgG, and H. pylori CagA IgG antibodies. This test is not as accurate as the other tests. These blood tests can be used to diagnose whether an H. pylori infection is present. However, the test cannot determine whether you have an infection at the time of the test or how long you have had it because the test remains positive for several years, even if the infection is cured. As a result, it cannot be used to see if the infection has been eradicated.
Stool test
A test to detect the genetic traces of H. pylori in the feces appears to be as accurate as the breath test for initially detecting the bacteria, and for detecting recurrences after antibiotic therapy. This test can also be used to diagnose H. pylori infection and confirm that it has been eradicated.
Biopsy
The most accurate way to identify the presence of H. pylori is by taking a tissue biopsy. H. pylori DNA was screened using a nested PCR amplification method for a portion of the 23S rRNA gene. Tissue samples can also be cultured on homogenized brain-heart infusion agar. Suspected colonies are tested and catalase-, oxidase- and urease-positive, curved Gram-negative rods are defined as H. pylori.
Another important diagnostic tool is an empiric trial of PPI therapy over a prolonged period, which has been proposed as a valid diagnostic test for LPR. The typical regime is twice daily PPI therapy for 1-6 mo. This recommendation is based on the fact that we have not identified the specific symptom combination, or combination of symptoms and laryngeal signs, pathognomonic to LPR. Besides, ambulatory 24-h double-probe pH measurement is not available in all clinics. The principal disadvantage of PPI therapy is its high cost, patient unwillingness, and placebo effect. Nevertheless it is a useful diagnostic tool in many cases.
TREATMENT
Treatment for LPR includes lifestyle modifications, acid-suppressive medication, and surgical therapy. Lifestyle modifications include elevation of the head of the bed, decreased intake of fat, citrus, tomato, chocolate, caffeine, and alcohol, cessation of smoking, and avoiding recumbency and further eating 3 h before bedtime. These measures are helpful if there is associated abnormal esophageal acid exposure[1]. If only LPR is present, these measures may be less meaningful because pharyngeal reflux occurs most often in the upright position during the daytime. Although Hanson et al[34] have described a 50% response rate to these measures alone in patients with chronic laryngitis, there are minimal supportive data on the efficacy of these measures in LPR. Medical acid suppression is the most important and common method of treatment. The treatment of LPR has dramatically changed since the introduction of PPIs, which are the most widely used drugs for the treatment of reflux. They maintain a potent and consistent effect on gastric acid secretion with few adverse effects. Comparisons between the five available compounds (omeprazole, rabeprazole, lansoprazole, esomeprazole, and pantoprazole) shows that they have a similar antisecretory potency on a milligram basis. Treatment recommendation at present is twice daily dosing of PPIs for at least 3-4 mo. Most authors suggest a longer duration of at least 6 mo up to 1 year[22,25]. Symptoms frequently improve before the laryngoscopic findings resolve[25]. Although PPIs effectively reduce the acid secretion, reflux still continues, meaning that the larynx and pharynx are still exposed to pepsin and bile.
Surgical therapy or antireflux surgery has been shown to be effective for patients with aggressive or life-threatening LPR[35]. The main procedure for antireflux surgery is Nissen fundoplication. The fundus of the stomach is wrapped around the lower esophageal sphincter to provide an antireflux barrier. Patients who have good control of GER and LPR symptoms with acid suppression may not need surgical intervention. However, in patients who do not respond to medical therapy, the symptoms can be attributed to pepsin and bile reflux. This patient group are considered to the best candidates for Nissen fundoplication[36]. However, it is not a widely accepted treatment choice.
No single drug cures H. pylori infection. Treatment involves taking several medications for 14 d. The recommended first-line therapy is PPI-clarithromycin-amoxicillin or metronidazole. The consensus is that 14 rather than 7 d treatment has a slight advantage in terms of treatment success. With regard to second-line therapies, bismuth-based quadruple therapies remain the best option. If unavailable, PPI-amoxicillin or tetracycline and metronidazole are recommended. There are increasing numbers of patients with H. pylori infection that is resistant to antibiotics, so it is important to take all the medications prescribed and to have a test that confirms that the infection has been cleared. Antimicrobial susceptibility testing is required in the resistant cases or treatment failures[37].
DISCUSSION
Regarding the relationship between LPR and H. pylori, the literature is limited. Rouev et al[38] compared 46 patients with GERD and LPR symptoms and found that there was an increasing tendency in GERD patients that develop LPR symptoms. They found 11 patients with H. pylori infection but the treatment did not affect the overall outcome. In one of the first studies investigating the relationship between H. pylori positivity and LPR, Oridate et al[39] compared H. pylori antibody positivity, laryngopharyngeal reflux symptoms, objective laryngopharyngeal findings, and rate of response to acid-suppressive therapy in 42 patients who were diagnosed with GERD. They found that the laryngopharyngeal, but not esophageal, symptom relief induced by acid suppression was significantly lower among H. pylori antibody-negative than antibody-positive cases. This was a surprising finding. Kountouras et al[40] have suggested that the increasing incidence of GERD complications after H. pylori eradication may be explained not just by the diminishing prevalence of H. pylori infection, but rather by healing of H. pylori-associated peptic ulcer disease, which coexists with GERD. The appearance of GERD depends on the esophageal acid exposure, and its symptomatology is related to acid hypersecretion; a condition that predisposes to peptic ulcer disease. Given that the vast majority of peptic ulcer cases are caused by H. pylori infection, the bacterium could therefore also promote GERD development by inducing esophageal acidity, but this does not necessarily promote LPR. Cekin et al[41] found no association between H. pylori and LPR status. In addition, they analyzed two subgroups based on whether their lesions were benign or malignant/premalignant and found a significant relationship between LPR positivity and the presence of malignant/premalignant laryngeal lesions. Again, they found no association between H. pylori status and either of the two subgroup categories.
LPR in the pediatric population is believed to contribute to failure to thrive, laryngomalacia, recurrent respiratory papillomatosis, chronic cough, hoarseness, esophagitis, and aspiration, among other pathologies. Thus, LPR should be considered as a chronic disease with a variety of presentations. High clinical suspicion along with consultation with an otolaryngologist, who can evaluate for laryngeal findings, is necessary to diagnose LPR accurately[42]. The majority of infected persons acquire the bacteria during early childhood and one of the risk factors may be immunological. These factors are possibly the cause of divergent manifestations of H. pylori infection in children compared with adults.
Tezer et al[43] have concluded that the expression of H. pylori positivity and degree of GERD correlated with LPR in 45 patients. H. pylori positivity and degree of GERD were more adverse in patients with an RFS of ≥ 7. However, their findings relied only on RFS; ambulatory 24-h double pH probe monitoring was not used. Toros et al[44] have investigated 45 patients. Although the percentage of H. pylori positivity was high, there was no significant relationship between the symptoms and H. pylori positivity. All patients underwent medical therapy mostly for gastroenterological indications rather than laryngopharyngeal symptoms. In a recent article by Youssef and Ahmed[45], H. pylori treatment and LPR symptom resolution was investigated. H. pylori stool antigen (HPSA) test was positive in 57% of the study group. Patients with negative HPSA were treated with esomeprazole as a single modality with a reported improvement score of 96.6%. Patients with positive HPSA test results were divided into two groups: one received only esomeprazole, with reported improvement in 40%, whereas the second group was treated with esomeprazole, plus amoxicillin sodium and clarithromycin (triple therapy) and reported a 90% incidence of symptom improvement. The incidence of H. pylori infection in patients with LPR was 57%. They concluded that H. pylori infection should be considered when treatment is prescribed to patients with LPR because the standard therapy for GERD might be insufficient. Also, the use of triple therapy for LPR with H. pylori infection might result in a higher cure rate. However, in a study by Ercan et al[46], 32 LPR patients were investigated regarding the presence of H. pylori and sex, age, degree of gastritis and esophagitis, and also the number of reflux episodes, fractional acid exposure times regarding proximal probe readings. They found that there was no relationship between the presence of H. pylori and LPR. Islam et al[47] took biopsies from the vocal fold and interarytenoid region of 50 patients. H. pylori was not found in the histological specimens of the vocal fold and interarytenoid region. The presence of H. pylori in the gastric mucosa, determined by UBT and H. pylori antibodies, does not have an effect on the RFS and RSI. In their prospective study, Siupsinskiene et al[48] found H. pylori in the biopsy material from the larynx in more than one-third of the patients, equally suffering from benign laryngeal disease and laryngeal cancer, but significantly more often than in the control group. Patients with chronic laryngitis and laryngeal cancer showed the highest rate of H. pylori infection in the larynx. However, the relationship was not clearly identified and they concluded that further studies are needed to confirm the importance of H. pylori infection for the development of different laryngeal diseases, as well as the effect of H. pylori eradication on the course of laryngeal diseases.
CONCLUSION
Detailed history taking and laryngoscopic examination constitute the basis for diagnosis of LPR. Most LPR patients have only mild symptoms. Unlike GERD patients, they seldom have heartburn or regurgitation. Laryngoscopic examination most commonly demonstrates findings in the posterior glottis and vocal folds. Laryngeal edema is an important indicator for LPR that is most often neglected. Ambulatory 24-h double pH-probe monitoring is the gold standard diagnostic tool for LPR. Acid suppression with PPI on a long-term basis is the mainstay of treatment; a trial of PPIs may also be useful as a diagnostic maneuver but it should be at least 4 mo. Laryngeal acid and pepsin sensitivity is greater in oropharyngeal mucosa than esophageal mucosa and this constitutes the main difference of LPR and GERD pathophysiology. H. pylori is found in many sites, including laryngeal mucosa and interarytenoid region; however, the importance of this colonization and its effects on disease progress and treatment outcome is yet to be identified with prospective clinical studies.
Footnotes
P- Reviewers: Murakami K, Naito Y S- Editor: Gou SX L- Editor: Kerr C E- Editor: Wang CH
References
- 1.Ylitalo R. Reflux and Its Impact on Laryngology. Textbook of Laryngology. In: Merati AL, Bielamowicz SA, editors. Hong Kong: Plural Publishing; 2006. pp. 203–303. [Google Scholar]
- 2.Smith KJ, O’Brien SM, Smithers BM, Gotley DC, Webb PM, Green AC, Whiteman DC. Interactions among smoking, obesity, and symptoms of acid reflux in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:2481–2486. doi: 10.1158/1055-9965.EPI-05-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein DJ, El-Serag HB, Kuczynski J, Kramer JR, Sampliner RE. The association of body mass index with Barrett’s oesophagus. Aliment Pharmacol Ther. 2005;22:1005–1010. doi: 10.1111/j.1365-2036.2005.02674.x. [DOI] [PubMed] [Google Scholar]
- 4.Halum SL, Postma GN, Johnston C, Belafsky PC, Koufman JA. Patients with isolated laryngopharyngeal reflux are not obese. Laryngoscope. 2005;115:1042–1045. doi: 10.1097/01.MLG.0000162656.05715.57. [DOI] [PubMed] [Google Scholar]
- 5.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell H, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2002;7 Suppl 1:8–16. doi: 10.1046/j.1523-5378.7.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 7.Eslick GD. Helicobacter pylori infection causes gastric cancer? A review of the epidemiological, meta-analytic, and experimental evidence. World J Gastroenterol. 2006;12:2991–2999. doi: 10.3748/wjg.v12.i19.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280–322. doi: 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morinaka S, Ichimiya M, Nakamura H. Detection of Helicobacter pylori in nasal and maxillary sinus specimens from patients with chronic sinusitis. Laryngoscope. 2003;113:1557–1563. doi: 10.1097/00005537-200309000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Cirak MY, Ozdek A, Yilmaz D, Bayiz U, Samim E, Turet S. Detection of Helicobacter pylori and its CagA gene in tonsil and adenoid tissues by PCR. Arch Otolaryngol Head Neck Surg. 2003;129:1225–1229. doi: 10.1001/archotol.129.11.1225. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz T, Ceylan M, Akyön Y, Ozçakýr O, Gürsel B. Helicobacter pylori: a possible association with otitis media with effusion. Otolaryngol Head Neck Surg. 2006;134:772–777. doi: 10.1016/j.otohns.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Ozmen S, Yücel OT, Sinici I, Ozmen OA, Süslü AE, Oğretmenoğlu O, Onerci M. Nasal pepsin assay and pH monitoring in chronic rhinosinusitis. Laryngoscope. 2008;118:890–894. doi: 10.1097/MLG.0b013e318165e324. [DOI] [PubMed] [Google Scholar]
- 13.Cammarota G, Tursi A, Montalto M, Papa A, Veneto G, Bernardi S, Boari A, Colizzi V, Fedeli G, Gasbarrini G. Role of dental plaque in the transmission of Helicobacter pylori infection. J Clin Gastroenterol. 1996;22:174–177. doi: 10.1097/00004836-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Musich PR, Ha T, Ferguson DA, Patel NR, Chi DS, Thomas E. High prevalence of Helicobacter pylori in saliva demonstrated by a novel PCR assay. J Clin Pathol. 1995;48:662–666. doi: 10.1136/jcp.48.7.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernander S, Dalén J, Gästrin B, Hedenborg L, Lamke LO, Ohrn R. Absence of Helicobacter pylori in dental plaques in Helicobacter pylori positive dyspeptic patients. Eur J Clin Microbiol Infect Dis. 1993;12:282–285. doi: 10.1007/BF01967259. [DOI] [PubMed] [Google Scholar]
- 16.Di Bonaventura G, Catamo G, Neri M, Neri G, Piccolomini R. Absence of Helicobacter pylori in tonsillar swabs from dyspeptic patients. New Microbiol. 2000;23:445–448. [PubMed] [Google Scholar]
- 17.Skinner LJ, Winter DC, Curran AJ, Barnes C, Kennedy S, Maguire AJ, Charles DA, Timon CI, Burns HP. Helicobacter pylori and tonsillectomy. Clin Otolaryngol Allied Sci. 2001;26:505–509. doi: 10.1046/j.1365-2273.2001.00513.x. [DOI] [PubMed] [Google Scholar]
- 18.Bitar MA, Soweid A, Mahfouz R, Zaatari G, Fuleihan N. Is Helicobacter pylori really present in the adenoids of children? Eur Arch Otorhinolaryngol. 2005;262:987–992. doi: 10.1007/s00405-005-0926-1. [DOI] [PubMed] [Google Scholar]
- 19.Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg. 2000;123:385–388. doi: 10.1067/mhn.2000.109935. [DOI] [PubMed] [Google Scholar]
- 20.Shaker R, Dodds WJ, Ren J, Hogan WJ, Arndorfer RC. Esophagoglottal closure reflex: a mechanism of airway protection. Gastroenterology. 1992;102:857–861. doi: 10.1016/0016-5085(92)90169-y. [DOI] [PubMed] [Google Scholar]
- 21.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1–78. doi: 10.1002/lary.1991.101.s53.1. [DOI] [PubMed] [Google Scholar]
- 22.Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32–35. doi: 10.1067/mhn.2002.125760. [DOI] [PubMed] [Google Scholar]
- 23.Koufman JA, Belafsky PC, Bach KK, Daniel E, Postma GN. Prevalence of esophagitis in patients with pH-documented laryngopharyngeal reflux. Laryngoscope. 2002;112:1606–1609. doi: 10.1097/00005537-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI) J Voice. 2002;16:274–277. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 25.Belafsky PC, Postma GN, Koufman JA. Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope. 2001;111:979–981. doi: 10.1097/00005537-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS) Laryngoscope. 2001;111:1313–1317. doi: 10.1097/00005537-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Belafsky PC, Postma GN, Koufman JA. The association between laryngeal pseudosulcus and laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2002;126:649–652. doi: 10.1067/mhn.2002.125603. [DOI] [PubMed] [Google Scholar]
- 28.Branski RC, Bhattacharyya N, Shapiro J. The reliability of the assessment of endoscopic laryngeal findings associated with laryngopharyngeal reflux disease. Laryngoscope. 2002;112:1019–1024. doi: 10.1097/00005537-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Hicks DM, Ours TM, Abelson TI, Vaezi MF, Richter JE. The prevalence of hypopharynx findings associated with gastroesophageal reflux in normal volunteers. J Voice. 2002;16:564–579. doi: 10.1016/s0892-1997(02)00132-7. [DOI] [PubMed] [Google Scholar]
- 30.Grøntved AM, West F. pH monitoring in patients with benign voice disorders. Acta Otolaryngol Suppl. 2000;543:229–231. doi: 10.1080/000164800454468. [DOI] [PubMed] [Google Scholar]
- 31.Postma GN. Ambulatory pH monitoring methodology. Ann Otol Rhinol Laryngol Suppl. 2000;184:10–14. doi: 10.1177/0003489400109s1003. [DOI] [PubMed] [Google Scholar]
- 32.Wiener GJ, Koufman JA, Wu WC, Cooper JB, Richter JE, Castell DO. Chronic hoarseness secondary to gastroesophageal reflux disease: documentation with 24-h ambulatory pH monitoring. Am J Gastroenterol. 1989;84:1503–1508. [PubMed] [Google Scholar]
- 33.Postma GN, Cohen JT, Belafsky PC, Halum SL, Gupta SK, Bach KK, Koufman JA. Transnasal esophagoscopy: revisited (over 700 consecutive cases) Laryngoscope. 2005;115:321–323. doi: 10.1097/01.mlg.0000154741.25443.fe. [DOI] [PubMed] [Google Scholar]
- 34.Hanson DG, Kamel PL, Kahrilas PJ. Outcomes of antireflux therapy for the treatment of chronic laryngitis. Ann Otol Rhinol Laryngol. 1995;104:550–555. doi: 10.1177/000348949510400709. [DOI] [PubMed] [Google Scholar]
- 35.Westcott CJ, Hopkins MB, Bach K, Postma GN, Belafsky PC, Koufman JA. Fundoplication for laryngopharyngeal reflux disease. J Am Coll Surg. 2004;199:23–30. doi: 10.1016/j.jamcollsurg.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Lindstrom DR, Wallace J, Loehrl TA, Merati AL, Toohill RJ. Nissen fundoplication surgery for extraesophageal manifestations of gastroesophageal reflux (EER) Laryngoscope. 2002;112:1762–1765. doi: 10.1097/00005537-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Selgrad M, Malfertheiner P. Treatment of Helicobacter pylori. Curr Opin Gastroenterol. 2011;27:565–570. doi: 10.1097/MOG.0b013e32834bb818. [DOI] [PubMed] [Google Scholar]
- 38.Rouev P, Chakarski I, Doskov D, Dimov G, Staykova E. Laryngopharyngeal symptoms and gastroesophageal reflux disease. J Voice. 2005;19:476–480. doi: 10.1016/j.jvoice.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Oridate N, Takeda H, Yamamoto J, Asaka M, Mesuda Y, Nishizawa N, Mori M, Furuta Y, Fukuda S. Helicobacter pylori seropositivity predicts outcomes of acid suppression therapy for laryngopharyngeal reflux symptoms. Laryngoscope. 2006;116:547–553. doi: 10.1097/01.MLG.0000201907.24514.6A. [DOI] [PubMed] [Google Scholar]
- 40.Kountouras J, Zavos C, Chatzopoulos D. The role of gastric Helicobacter pylori infection in laryngopharyngeal reflux disease. Otolaryngol Head Neck Surg. 2007;136:334; author reply 334–335. doi: 10.1016/j.otohns.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Cekin E, Ozyurt M, Erkul E, Ergunay K, Cincik H, Kapucu B, Gungor A. The association between Helicobacter pylori and laryngopharyngeal reflux in laryngeal pathologies. Ear Nose Throat J. 2012;91:E6–E9. doi: 10.1177/014556131209100314. [DOI] [PubMed] [Google Scholar]
- 42.Venkatesan NN, Pine HS, Underbrink M. Laryngopharyngeal reflux disease in children. Pediatr Clin North Am. 2013;60:865–878. doi: 10.1016/j.pcl.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tezer MS, Kockar MC, Koçkar O, Celik A. Laryngopharyngeal reflux finding scores correlate with gastroesophageal reflux disease and Helicobacter pylori expression. Acta Otolaryngol. 2006;126:958–961. doi: 10.1080/00016480500529314. [DOI] [PubMed] [Google Scholar]
- 44.Toros SZ, Toros AB, Yüksel OD, Ozel L, Akkaynak C, Naiboglu B. Association of laryngopharyngeal manifestations and gastroesophageal reflux. Eur Arch Otorhinolaryngol. 2009;266:403–409. doi: 10.1007/s00405-008-0761-2. [DOI] [PubMed] [Google Scholar]
- 45.Youssef TF, Ahmed MR. Treatment of clinically diagnosed laryngopharyngeal reflux disease. Arch Otolaryngol Head Neck Surg. 2010;136:1089–1092. doi: 10.1001/archoto.2010.165. [DOI] [PubMed] [Google Scholar]
- 46.Ercan I, Cakir BO, Uzel TS, Sakiz D, Karaca C, Turgut S. The role of gastric Helicobacter pylori infection in laryngopharyngeal reflux disease. Otolaryngol Head Neck Surg. 2006;135:52–55. doi: 10.1016/j.otohns.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Islam A, Oguz H, Yucel M, Koca G, Gonultas MA, Arslan N, Demirci M. Does Helicobacter pylori exist in vocal fold pathologies and in the interarytenoid region? Dysphagia. 2013;28:382–387. doi: 10.1007/s00455-012-9444-7. [DOI] [PubMed] [Google Scholar]
- 48.Siupsinskiene N, Jurgutaviciute V, Katutiene I, Janciauskas D, Vaitkus S, Adamonis K. Helicobacter pylori infection in laryngeal diseases. Eur Arch Otorhinolaryngol. 2013;270:2283–2288. doi: 10.1007/s00405-013-2475-3. [DOI] [PubMed] [Google Scholar]


