Abstract
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal disorders causing patients to seek medical treatment. It is relatively resource intensive and the source of significant morbidity. Recent insights into the pathophysiology and treatment of IBS has given clinicians more options than ever to contend with this disorder. The purpose of our paper is to review older, “classic” treatments for IBS as well as newer agents and “alternative” therapies. We discuss the evidence base of these drugs and provide context to help develop appropriate treatment plans for IBS patients.
Keywords: Irritable bowel syndrome, Probiotics, Rifaximin, Lubiprostone, Linaclotide, Peppermint oil
Core tip: Gastroenterology practitioners have more agents than ever before to treat the symptoms associated with irritable bowel syndrome. Unfortunately, despite advances in our understanding of the pathophysiology of this disorder, targeted treatments do not yet exist. This review summarizes the recent evidence-based treatment of this disorder, including, older and newer agents.
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal disorders causing patients to seek medical treatment. It exerts significant economic burden and is responsible for considerable morbidity in Western countries[1]. Despite these costs and numerous investigations into the pathophysiology and treatment of this disorder, our understanding of IBS is still incomplete. Over the last ten years, increasing insight into the enteric nervous system and how its dysfunction may play a role in IBS pathology has emerged[2]. Additionally our increasing understanding of the gut microbiome and how its potential disruption may lead to IBS symptoms has also been highlighted[3]. However, with few exceptions, these insights have yet to lead to targeted treatment strategies for IBS. Currently, many clinicians use a treatment approach based on the predominant symptoms of the patient: constipation (IBS-C), diarrhea (IBS-D), or mixed symptoms (IBS-M) (Table 1)[4]. Several new drugs have recently been examined for IBS using this symptom-based approach. Two agents for IBS-C, lubiprostone and linaclotide have been approved by the United States Food and Drug Administration (FDA) for that specific indication[5]. To improve the evidence by which drugs for IBS are approved, the FDA has recently proposed standardized outcomes for approval studies as is discussed later in this paper. The purpose of this paper is to provide the clinician with a concise review of pharmacotherapy strategies for IBS. Consequently, it is divided into three sections: “classic” treatment options, “newer drugs,” such as lubiprostone and linaclotide, and “alternative” treatments such as probiotics and peppermint oil. In the last section we will also discuss emerging information on the so-called “pre-cebo” effect in IBS.
Table 1.
Irritable bowel syndrome subtypes
| Subtype | Definition |
| (symptoms classified using Bristol stool form scale) | |
| IBS with constipation (IBS-C) | > 25% of stools are hard or lumpy and < 25% of stools are loose/mushy or watery |
| IBS with diarrhea (IBS-D) | > 25% of stools are loose/mushy or watery stools and < 25% are hard or lumpy |
| Mixed IBS (IBS-M) | > 25% of stools are loose/mushy or watery stools and > 25% and hard or lumpy |
| Unsubtyped IBS | insufficient abnormality of stool consistency to meet criteria for IBS-C, D, or M (in the absence of antidiarrheals or laxatives) |
IBS: Irritable bowel syndrome.
CLASSIC TREATMENTS FOR IBS
Antidiarrheals
Loperamide is a synthetic opioid, which acts on intestinal muscles to prolong transit time and inhibit peristalsis. While loperamide has been studied in different subtypes of IBS, it may be particularly effective in IBS-D because of its ability to decrease fecal volume and transit time. A meta-analysis in 2000 found loperamide to be an effective agent in decreasing stool frequency and improving stool consistency, as well as demonstrating a modest improvement in global well being[6]. However, it does not appear that loperamide is effective in reducing abdominal pain in comparison to placebo. In fact, some studies show an increase in abdominal pain particularly when loperamide is used in IBS-C[7]. Other common antidiarrheal agents, such as diphenoxylate with atropine, have not been well studied in IBS and are likely to be less tolerated due to anticholinergic effects, such as sedation, dry mouth, constipation, and urinary retention.
Antidepressants
Antidepressants, such as the tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), and serotonin norepinephrine reuptake inhibitors, have been utilized in the treatment of various functional gastrointestinal disorders. Current treatment guidelines endorse the use of either TCAs or SSRIs for patients with IBS, although duloxetine has also been studied in small trials for this population[8,9]. These agents are believed to act via centrally-mediated antinociceptive pathways decreasing abdominal pain associated with IBS. In addition, these agents may affect the gastrointestinal tract by peripheral means particularly in gut transit times[8]. A recent Cochrane review pooled 15 TCA and SSRI trials[10]. The antidepressant class as a whole significantly decreased pain, IBS symptom scores, and overall global assessment. A subgroup analysis revealed only TCAs remained statistically significant for abdominal pain and improvement in symptom scores. However, this may be due to a smaller number of patients and trials studying the use of SSRIs to treat IBS. In addition, an earlier meta-analysis demonstrated a reduction in pain, bloating, and other symptoms, although it contained mostly TCA trials[11].
Despite the large differences in the amount of supporting data, many clinicians are reticent to prescribe TCAs instead of SSRIs given the poor tolerability of these agents. In fact, one trial utilizing desipramine found nearly one in five subjects of that treatment arm dropped out due to adverse reactions[12]. Secondary amine tricyclic antidepressants (e.g., nortriptyline) are typically better tolerated than tertiary amines (e.g., amitriptyline) because of decreased anticholinergic adverse effects. In addition, lower doses of TCAs as compared to doses used to treat depression seem adequate to provide IBS symptom relief. Despite a more favorable side effect profile, SSRI use is more controversial in IBS patients as the supporting evidence is not nearly as robust. Clinical guidelines do suggest hypothetically that SSRIs may be of more utility in IBS-C and TCAs may be of more benefit in IBS-D due to their respective effects on whole gut transit times[8]. Clinicians await head-to-head trials with these agents.
Antispasmodics
Medications that relax smooth muscle via anticholinergic mechanisms or calcium channel antagonism have been commonly utilized for the treatment of IBS. Among these are alverine, dicyclomine (with or without cimetropium), hyoscyamine, otilonium, pinaverium, scopolamine, and trimebutine. The availability of many of these medications varies from country to country. Generally, antispasmodics have been utilized for their effects on gastrointestinal motility in attempts to reduce abdominal pain associated with IBS. They have also been evaluated in combination with agents such as acetaminophen, simethacone, and benzodiazepines in attempts to improve gastrointestinal discomfort[13-15].
Unfortunately many of the studies evaluating antispasmodics are small, suffer from methodological issues, and often fail to evaluate individual symptoms or effect on IBS subtypes. Only a small number of trials include active comparators. A recent Cochrane review of 29 antispasmodic trials for IBS suggested that some, but not all antispasmodics may decrease abdominal pain[10]. Similarly some, but not all, antispasmodics improved IBS symptom scores and global assessment. A subgroup analysis showed benefit of the use of trimebutine, pinaverium, and combined dicyclomine/cimetropium in the treatment of IBS. Anticholinergic side effects of these agents often include dose-related vision disturbances, dry mouth, and dizziness. Moreover, antispasmodics can also cause constipation, thus they should be used cautiously in patients with IBS-C. Prescribers should consider the limitations of these medications when using them for IBS.
Bulking agents
Several bulking agents have been examined in the treatment of IBS. These include psyllium, calcium polycarbophil, bran, and ispaghula husk. These synthetic and naturally occurring fiber supplements are often used for their ability to increase stool frequency, quality, and transit time. Consequently, they are often attractive options in all subtypes of IBS, particularly IBS-C. Most of the trials involving these agents have been small and as a result, multiple meta-analyses have been undertaken. An early systematic review found that there may be a significant improvement in global IBS symptoms with soluble fibers (psyllium, calcium polycarbophil, ispaghula), but worsening symptoms with insoluble fiber (bran)[16]. However, this review suffered from significant heterogeneity. Furthermore, a recent Cochrane review of 12 randomized control trials showed that fiber supplements do not improve abdominal pain, IBS symptom scores, or global assessment. Other meta-analyses have had similar results[17].
Osmotic laxatives
Osmotic laxatives are often used in the treatment of IBS-C due their efficacy in chronic idiopathic constipation. These agents, including polyethylene glycol (PEG) 3350 and lactulose, work by increasing water in the intestinal lumen to decrease intestinal transit time. PEG 3350 (with or without electrolytes) has been utilized in only a few randomized control trials for the treatment of IBS[18,19]. It has been shown to be effective for relieving constipation associated with IBS, but no more effective than placebo for reducing abdominal pain, bloating, or other symptoms associated with IBS[18]. Lactulose has not been rigorously studied in IBS. In addition, lactulose may cause bloating resulting from fermentation in the intestinal lumen. Thus it should not be recommended for patients with IBS.
NEWER TREATMENTS FOR IBS
Lubiprostone
Lubiprostone is a gastrointestinal chloride-channel activator (specifically at the chloride channel 2 receptor) that enhances intestinal fluid secretion which leads to increased intestinal motility and facilitation of stool passage[20]. It was FDA approved in 2006 for the treatment of chronic idiopathic constipation (CIC) at a dose of 24 μg taken twice per day. Subsequently in 2008, its use was approved for IBS-C in women older than 18 years of age at a dose of 8 μg taken twice per day. This approval was based on the results of two 12 wk randomized phase III trials that were published in one manuscript in 2009[21]. The primary endpoint in this study was monthly responder status at three months. The definition of responder was developed between the study investigators and the FDA and thought to be more rigorous than previous trials of IBS treatments. In this study, a “monthly responder” was defined as subjects who reported moderate relief of IBS symptoms for four of four weeks or significant relief for more than two of 4 wk (Table 2). To be considered an “overall responder” (the primary efficacy endpoint), patients had to be a monthly responder for two of three months of the trial. Symptoms were recorded in a weekly electronic diary in which patients were asked “How would you rate your relief of IBS symptoms over the past week compared to how you felt before you entered the study?” Subjects’ responses were recorded on a seven-point scale that ranged from “significantly worse” to “significantly relieved”. Results of this study showed a statistically significant improvement in the primary efficacy endpoint (17.9% lubiprostone vs 10.1% placebo, P = 0.001; NNT = 13), as well as monthly response at months two and three (Table 2). Adverse events were frequent but similar between the lubiprostone and placebo groups, with gastrointestinal events occurring most frequently. There was no difference in serious adverse drug reactions (ADRs) or patients who discontinued treatment due to an adverse event (Table 3). ADRs to lubiprostone were reported at a lower rate in the IBS-C trials when compared to trials examining its other indications of opioid-induced constipation and CIC. This is likely due to lower systemic exposure (16 μg/d vs 48 μg/d) and the differences between disease states since placebo rates were higher in those trials.
Table 2.
Response rates for lubiprostone 12 wk phase III irritable bowel syndrome with constipation studies
| Lubiprostone | Placebo | P value | |
| Overall responder | 17.90% | 10.10% | 0.001 |
| Month 1 | 10.80% | 7.50% | 0.078 |
| Month 2 | 18.20% | 11.40% | 0.003 |
| Month 3 | 22.00% | 14.50% | 0.003 |
Table 3.
Adverse events for lubiprostone phase III irritable bowel syndrome with constipation studies
| Lubiprostone | Placebo | Lubiprostone | |
| 12 wk | 12 wk | 36 wk | |
| Serious | 1% | 1% | 1.90% |
| Treatment related | 22% | 21% | 25.40% |
| Nausea | 8% | 4% | 11% |
| Diarrhea | 6% | 4% | 11% |
| Abdominal distension | 2% | 2% | 3.70% |
| Discontinuation due to ADR | 5% | 7% | 4% |
ADR: Adverse drug reaction.
Patients who completed the 12 wk study were eligible for an additional open-label 36 wk extension study if they had been at least 70% compliant with the study medication[22]. The primary objective of this study was to assess long-term safety and tolerability. Treatment related ADRs were more frequent but similar to the 12 wk study with nausea and diarrhea reported most commonly (Table 3). The drug was tolerated well with only 4% of patients withdrawing due to adverse events. This rate was lower than the 12 wk study, however this likely reflects some selection bias since patients were not treatment naive (except those previously in the placebo arm).
Too few men with IBS-C were enrolled in the clinical trials with lubiprostone to draw any conclusions about its effectiveness in this population. Because the drug is associated with teratogenic effects in animals, the manufacturer recommends that women who could become pregnant have a negative pregnancy test before beginning therapy, as well as be able to comply with effective contraceptive measures during therapy. The drug is significantly more expensive than traditional laxatives, and should generally be reserved for patients who have failed other therapy for IBS-C.
Linaclotide
An agonist of guanylate cyclase, linaclotide is a unique agent which was recently approved by both American and European regulatory agencies for the treatment of IBS-C[23,24]. Stimulation of guanylate cyclase receptors leads to increased secretion of both guanylin and uroguanylin into the intestinal lumen where they act as a second messenger for both fluid and electrolyte release into the large bowel[25]. Linaclotide is minimally absorbed and has a strong affinity for the guanylate cyclase receptor. Preliminary clinical studies were conducted in the mid-2000s and found the drug to have significant effects on ascending colonic transit time and clinical symptoms related to stooling[26,27]. This led to phase III studies that were submitted for regulatory approval. One such study was performed by Rao and colleagues in a randomized, double-blinded fashion on 800 patients with IBS-C[28]. These patients were randomized in this 12-wk trial to linaclotide 266 mcg (n = 405) vs placebo (n = 395). As with most IBS studies, the majority of patients were white females who had met Rome II criteria for IBS-C. Exclusion criteria included cathartic colon, laxative or enema abuse, ischemic colitis, pelvic floor dysfunction, recent abdominal or pelvic surgery, or other conditions that would explain symptoms, such as inflammatory bowel disease. Of interest, this study was one of the first to use the United States FDA recommendations for trial design and outcomes in IBS studies[29]. Thus, one of the four primary outcomes in the trial was the combination of (1) an improvement of ≥ 30% from baseline in the average of the daily worst abdominal pain scores on standardized scales; and (2) an increase of ≥ 1 spontaneous bowel movements from baseline. Numerous secondary endpoints including patient assessed symptoms, such as abdominal discomfort, abdominal bloating, stool frequency and stool consistency were evaluated. In this study, the primary FDA endpoint was reached by 33.6% receiving linaclotide compared with 21.0% receiving placebo (OR = 1.9, 95%CI: 1.4-2.7, P < 0.0001; NNT = 8). All other primary and secondary efficacy endpoints were similar. Of interest was the group of patients who had improvement in abdominal pain of ≥ 30% (34.3% of linaclotide vs 27.1% placebo, OR = 1.4, 95%CI: 1.0-1.9, P = 0.0262; NNT = 14). This suggests that in addition to acting as a laxative, linaclotide has gut anti-nociceptive properties. The safety profile of linaclotide was favorable with diarrhea being the most common adverse effect reported (5.7% vs 0.3% in placebo-treated patients). Additionally, no serious or life-threatening adverse effects were reported in this study.
A similarly designed study was performed by Chey et al[30] to examine the long-term safety and efficacy of linaclotide in IBS-C. Subjects included 804 patients classified as having IBS-C by Rome II criteria and were randomized to either linaclotide 290 mcg or placebo once daily for 26 wk. Exclusion criteria and outcomes were virtually identical to the study discussed above. In this 26 wk study, linaclotide achieved the FDA outcome more frequently than placebo (33.7% vs 13.9% respectively, P < 0.0001; NNT = 6). As with the 12 wk study, all other primary and secondary efficacy endpoints showed similar benefits with linaclotide. Diarrhea was again the most common adverse effect reported, with 5.7% of patients dropping out of the study due to this effect. This study not only helped confirm linaclotide’s role in treating IBS-C, but it also showed durability of response, a notorious problem when addressing the evidence base of older treatments for this disorder. As mentioned above, these two studies were among the first to utilize the FDA recommended outcomes for IBS trials. It should be noted that other investigators have examined these outcomes and have suggested they may be conservative. Consequently, the true effect size of linaclotide in IBS-C may be greater than these studies suggest[31].
Most recently, a meta-analysis assessed all current randomized controlled trials of linaclotide for both chronic constipation as well as IBS-C[32]. For IBS-C, the investigators utilized the two studies listed above as well as a third trial for which the FDA primary outcome was compiled. When analyzing the data from these studies together, linaclotide was associated with a significant improvement in the FDA outcome [RR = 1.95 (95%CI: 1.3-2.9); NNT = 7 (95%CI: 5-11)]. The authors concluded that linaclotide was effective and had a robust effect size in treating IBS-C. Despite the growing evidence, the role of linaclotide for treating IBS-C in the United States is still uncertain. Given the published data, some experts have called for its placement as a first-line option for this disorder[33]. However, given its cost in the United States (roughly United States $900 monthly), and the reluctance of many third-party payers to cover it, its use will likely be reserved for those patients with IBS-C who have failed other treatments.
Rifaximin
As previously mentioned, a number of avenues concerning the pathogenesis of IBS have received considerable investigation in recent years. Among these lines of research is the relationship between host-gut microbiome. Disruption of this complex relationship, perhaps caused by small intestinal bacterial overgrowth (SIBO), may lead to symptoms attributed to IBS: constipation, abdominal pain and bloating, and change in bowel habit[34]. This may explain the subset of IBS patients who develop symptoms after a gut infection (so-called “post-infectious IBS”). After disruption of the normal gut microbiome and overgrowth of the small bowel by bacteria, the resulting inflammation may lead to chronic IBS-like symptoms[35]. For the practicing clinician, this does raise interesting questions, such as is SIBO a cause of IBS, particularly the diarrhea-predominant version of the disorder[36]? Or conversely, are some patients labeled as having IBS in reality suffering from SIBO? In either event, a therapeutic strategy aimed at treating SIBO in select patients with IBS-D may be rational.
Since traditional bacterial culture of the entire small bowel is impractical, experts have recommended using breath tests, such as the hydrogen or lactulose test to assess the possibility of SIBO[37]. Selective utilization of these tests, combined with assessment of patient symptoms may help to delineate IBS patients with a SIBO component to their disorder. A recent review of this subject provides an excellent overview for the clinician[37]. Once the determination that SIBO may be playing a factor in a patient’s IBS symptoms, should antimicrobials be used for treatment? And, if so, which agent would be preferred? The ideal agent would have little to no systemic absorption, would be active against common gut flora, and would have few adverse effects. Older agents traditionally used for bowel decontamination such as neomycin or metronidazole largely do not meet these criteria. Rifaximin is a drug chemically related to rifampin that has little to no systemic absorption and is well tolerated[38]. This agent has been used in patients with SIBO and has been examined in patients with IBS who do not have constipation. Currently, rifaximin is not FDA approved in the United States for IBS, however, several trials support its use for this indication.
An initial small randomized, controlled trial by Pimentel and co-workers in 87 patients with IBS suggested that a 10-d course of rifaximin 400 mg three times daily improved patient global scores of symptoms compared to placebo[39]. This improvement seemed to persist for the duration of the trial (10 wk) and led these investigators to confirm rifaximin’s utility in two larger studies named TARGET-1 and TARGET-2. The results of these trials were combined and published in 2011[40]. Both studies were identically designed and enrolled patients with IBS as assessed by the Rome II criteria. Key exclusion criteria included patients with a recent exposure to antibiotics, inflammatory bowel disease, diabetes, or use of other medications exclusively for IBS symptoms. Patients were randomized to rifaximin 550 mg twice daily for two wk or placebo and were followed for up to 10 wk after medication completion. The primary outcome was patients who reported qualitative relief of their global IBS symptoms. A key secondary endpoint was patient assessment of relief from abdominal bloating. A total of 1260 patients were enrolled in the two trials, making these studies among the largest in the IBS literature. In looking at the combined primary endpoint, 40.7% of rifaximin patients reported global improvement in symptoms compared to 31.7% of placebo patients (P < 0.001; NNT = 12) in the two studies combined. Numerous secondary endpoints, including abdominal bloating, were statistically better in the active treatment arm compared to placebo. This benefit was largely maintained throughout the study period, up to 10 wk after treatment ended. No significant adverse effects were reported in the rifaximin arm, and no cases of Clostridium difficile-associated diarrhea or ischemic colitis were seen. The authors concluded that a two-week course of rifaximin may provide lasting improvement of symptoms in patients with IBS without constipation.
One concern with the aforementioned study was the need to know durability of response to see if or when patients would need retreatment. The TARGET lead investigators performed a retrospective review of patients in their health-system who had received rifaximin for IBS[41]. Of the 71 patients evaluated, the majority did require retreatment for relapsing symptoms. However, patients who responded to one treatment generally also responded to subsequent ones. This is in accordance with a study in only SIBO patients that found a recurrence of symptoms in approximately half of patients nine months after rifaximin treatment[42]. Such patients may be required to receive multiple doses of an expensive antibiotic (roughly United States $700 per treatment course), raising the possibility of developing resistance[43].
Most recently, a meta-analysis was published examining the treatment effect of rifaximin in IBS patients[44]. The authors performed a systematic review that culminated in five articles subject to meta-analysis. The results of this analysis are consistent with individual trial data. Rifaximin was found to improve global IBS symptoms compared to placebo (OR = 1.57, 95%CI: 1.22-2.01; NNT = 11). Bloating symptoms also improved compared to placebo (OR = 1.55, 95%CI: 1.23-1.96; NNT = 11).
Given the price of rifaximin in the US, many patients or payers will be unwilling to assume the cost of the drug. Yet another cost consideration is whether all patients should undergo hydrogen or lactulose breath testing before rifaximin therapy. A recent study from Switzerland suggests that a high percentage of patients diagnosed with IBS will have positive breath testing, and when treated with rifaximin, will have a sustained response[45]. This suggests that, if available to the clinician, such testing should be performed to help guide therapy with rifaximin.
Other treatments, including prucalopride (a selective serotonin receptor agonist with prokinetic activity) may become viable options for IBS, but data to date are limited[46].
ALTERNATIVE TREATMENTS
Peppermint oil
Peppermint oil is an antispasmodic available over the counter in the United States that blocks calcium channels resulting in gastrointestinal smooth muscle relaxation. [8]According to the American College of Gastroenterology, peppermint oil may provide short-term relief of discomfort and abdominal pain in IBS and appears to be superior to placebo[17]. However, this conclusion is based on a small number of studies (Grade 2B), and there are no long-term studies to support chronic use. Additionally, there is large variation in the doses of oral peppermint oil (450-900 mg/d in 2-3 divided doses) and duration of therapy used in clinical trials (1-3 mo)[47-51]. The most common adverse effect reported with oral peppermint oil is gastroesophageal reflux. This is thought to be due in part to relaxation of the lower esophageal sphincter, and has led to the popularity of enteric-coated preparations that can bypass the upper gastrointestinal tract[52].
A 2008 meta-analysis including 4 trials (n = 392) provides support for the use of peppermint oil in IBS[17]. In this study, peppermint oil (n = 197) resulted in fewer patients reporting persistent symptoms compared to treatment with placebo (n = 195) for a duration of one to three months (26% vs 65% respectively, RR = 0.43, 95%CI: 0.32-0.59; NNT = 2.5). However, statistically significant heterogeneity was detected between studies (I2 = 31.1%, P = 0.23). Only one of the trials (n = 57) reported the type of IBS according to stool pattern, as two of the four trials included predate the use of these subgroups which were developed with the publication of the Rome II criteria in 1999. In this study, 25% of patients had predominant IBS-C and 75% had IBS-D[49]. Additionally, the treatment effect of peppermint oil was found to last for 4 wk after stopping therapy in over 50% of patients in this trial. Although other alternative therapies have been advocated to treat IBS, data on many of these treatments are limited. Oral capsaicin was examined in one small trial found a small improved in abdominal pain and bloating scores, but discontinuations due to initial intolerance was high[53].
Probiotics
Probiotics are dietary supplements that contain live or attenuated bacteria, or bacterial products, which when ingested, may have beneficial effects to a patient’s health by altering the gastrointestinal flora[54]. The precise mechanism of action of probiotics is not known. It is hypothesized that inflammation or disproportion of the gastrointestinal bacterial flora may play a part in the pathogenesis of IBS. The probiotic theory suggests that supplementation of the gastrointestinal flora with the right types and numbers of live microorganisms can improve the gut flora and promote health[55]. Additionally, there is evidence to suggest that certain strains of probiotics may stimulate an anti-inflammatory response or improve visceral hypersensitivity, which could theoretically lead to an improvement in symptoms of IBS[56]. Probiotics may comprise a formulation containing a single or mixed-culture of live microbes and are obtainable in diverse preparations, including fermented milk drinks, food products (snacks, chocolates, etc.), capsules, pills, and powders[57]. Side effects are generally minimal, although there are risks for patients who are immune compromised[58].
Several strains of probiotics have been studied, but the most commonly used organisms are the lactobacillae and bifidobacteria (Table 4). Several clinical trials have evaluated the effectiveness of a variety of probiotics in patients with IBS, and in general, probiotics can be used for patients with all types of IBS (IBS-D, IBS-C, and IBS-M). Nonetheless, the supportive evidence for treating IBS with probiotics is weak due to the heterogeneity of the studies and the varying probiotics evaluated[59]. Relating and summarizing these trials is difficult due to differences in study design, patient populations, dosing regimens, probiotic species utilized, and reported clinical end points. Regardless of these limitations, some recent systematic reviews and meta-analyses concluded that probiotics seem to be effective in patients with IBS[60-63]. A systematic review of data pooled from 10 randomized controlled trials (RCTs) involving 918 patients with IBS showed a significant benefit for probiotics vs placebo in reducing IBS symptoms and decreasing pain and flatulence [RR = 0.71, 95%CI: 0.57-0.88, I2 = 68%; NNT = 4 (95%CI: 3-12.5)][61]. An additional systematic review of 14 RCTs showed a moderate improvement in overall symptoms, abdominal pain, and flatulence in patients taking probiotics vs placebo (OR = 1.6; 95%CI: 1.2-2.2 for dichotomous data from seven trials and standardized mean difference = 0.23; 95%CI: 0.07-0.38 for continuous data from six trials)[60]. Several of the studies found improvement in primary end points compared with baseline, but only some were able to show significant improvement over placebo.
Table 4.
Probiotic strains
| Clinical condition | Effectiveness | Specific strain |
| IBS | B | Bifidobacterium infantis B5624 |
| IBS | B | VSL33 (composite containing multiple strains): |
| 3 strains of Bifidobacterium: | ||
| Bifidobacterium longum | ||
| Bifidobacterium finfantis | ||
| Bifidobacterium breve | ||
| 4 strains of Lactobacillus: | ||
| Lactobacillus acidophilus | ||
| Lactobacillus casei | ||
| Lactobacillus bulgaricus | ||
| Lactobacillus plantarum | ||
| 1 strain of Streptococcus salivarius, subspecies | ||
| Thermophilus | ||
| IBS | C | Bifidobacterium animalis |
| IBS | C | Lactobacillus plantarum 299V |
IBS: Irritable bowel syndrome.
Two types of probiotics were granted the highest rating for efficacy in the treatment of IBS (level “B”: based on positive, controlled studies and in spite of the presence of some negative studies) in the Recommendations for Probiotic Use from a Yale University Workshop[64]. The recommendation for Bifidobacterium infantis 35624 was concluded from two well-designed clinical trials[65,66] and has been labeled with the “B” rating since the 2008 update[67]. One particular mixture of probiotics, VSL#3 moved up from a “C” rating to a “B” rating based on the results from two trials[68,69]. A more recent study evaluated patients randomized to receive either placebo or Bifidobacterium bifidum MIMBb75[70]. This particular probiotic type reduced the global assessment of IBS symptoms (on a 7-point Likert scale) by 0.88 points vs 0.16 points (P < 0.0001) and had adequate symptom relief in 47% vs 11% (P < 0.0001, NNT = 3).
It is important to note that in the United States, regulatory authorities consider probiotics as dietary supplements that are not intended to diagnose, treat, cure, or mitigate the effects of diseases. It is advised that consumers should consult with a health care professional before consuming these products. Many of the available products have not been sufficiently tested for their effectiveness in IBS in satisfactorily designed clinical trials. Another critical factor is the issue of the type products being sold to the public and if their content have enough viable amounts of organisms to make a clinical difference[71]. Furthermore, a study by Mercer et al[72] evaluated how patients with IBS viewed probiotics. In this study, patients conveyed frustration that their more traditional IBS medications had worked at first, but became less effective over time. Patients in this study considered probiotics as an appealing potential therapeutic approach for those running out of pharmaceutical options.
Further research is needed to help identify the most effective probiotic species and strains, and the ideal regimen. However, with limited available treatments for IBS, the overall safety of probiotics lowers the bar for trying probiotic products in patients with IBS. Clinicians should not recommend probiotics as monotherapy in symptomatic patients with IBS, but rather in combination with current conventional treatments[57]. Based on the limited evidence for the use of probiotics in patients with IBS, the following organizations have developed guidelines to aid clinicians in their recommendations of products to patients. The National Institute for Health and Clinical Excellence in the United Kingdom has the following recommendation about the use of probiotics in IBS: “Probiotics do not appear to be harmful (unless they come from an unreliable source) and they might benefit people with IBS; they should be advised to take the product at the dose recommended by the manufacturer for at least four wk while monitoring the effect[73].” Additionally, recommendations from the American College of Gastroenterology Task Force on IBS resolved that Bifidobacteria and certain combinations of probiotics demonstrate some efficacy, and that in single-organism studies, lactobacilli do not appear effective for patients with IBS[8].
“Pre-cebo” effect
The placebo effect in clinical trials has long been known, and because of the vague nature of IBS symptoms and the use of primary outcomes that are often subjective in nature, high placebo response rates have been noted in IBS trials. However, Kim et al[74] have also described the potential for a “pre-cebo” effect in IBS, which impacts the treatment outcome even before the study begins. The pre-cebo effect describes the impact of consent language used in clinical trials on expectations of benefit from the study medication. This was studied in 59 patients with IBS-D who were randomized to one of 3 medication questionnaires (desipramine, alosetron, or rifaximin). Subjects were asked to rate the percent (0%-100%) improvement in symptoms that would be sufficient for the subject to feel adequate relief. Patients anticipating therapy for any of the three drugs had very high expectations of benefit (> 70%), and patients anticipating rifaxamin treatment had the highest expectation of improvement needed for satisfactory symptom relief (87.3%) compared to desipramine (73.4%, P < 0.001) and alosetron (76.8%, P = 0.049). This was thought to be due both to the wording used in the consent process, as well as any preconceived ideas about the study medication. The authors note that the high overall expectations may be a challenge to positive outcomes of therapy in any study, and this is particularly noteworthy in IBS because many trials depend on subjective measures of improvement that are patient driven.
CONCLUSION
Gastroenterology practitioners have more agents than ever before to treat the symptoms associated with IBS. Unfortunately, despite advances in our understanding of the pathophysiology of this disorder, targeted treatments do not yet exist. Based on the literature reviewed in this paper, the authors have constructed an algorithm to guide practicing clinicians who encounter this disorder (Figure 1). This algorithm is stratified to symptoms, economic costs, and level of evidence. Using this, or another systematic approach will enable practitioners who treat IBS to do so more efficiently, yet provide relief to a significant number of their patients with this disorder.
Figure 1.
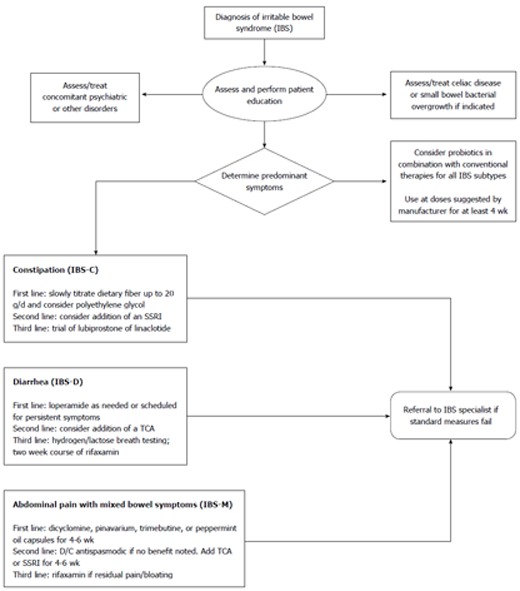
Treatment algorithm for irritable bowel syndrome. IBS: Irritable bowel syndrome; TCA: Tricyclic antidepressant; SSRI: Selective serotonin reuptake inhibitor; D/C: Discontinue.
Footnotes
P- Reviewers: Abraham P, Butterworth J, Bortolotti M, Stanghellini V, Wasim F S- Editor: Gou SX L- Editor: A E- Editor: Ma S
References
- 1.Mitra D, Davis KL, Baran RW. All-cause health care charges among managed care patients with constipation and comorbid irritable bowel syndrome. Postgrad Med. 2011;123:122–132. doi: 10.3810/pgm.2011.05.2290. [DOI] [PubMed] [Google Scholar]
- 2.Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. J Gastroenterol. 2012;47:1177–1185. doi: 10.1007/s00535-012-0627-7. [DOI] [PubMed] [Google Scholar]
- 3.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review: the efficacy of treatments for irritable bowel syndrome--a European perspective. Aliment Pharmacol Ther. 2006;24:183–205. doi: 10.1111/j.1365-2036.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- 5.Pohl D, Tutuian R, Fried M. Pharmacologic treatment of constipation: what is new? Curr Opin Pharmacol. 2008;8:724–728. doi: 10.1016/j.coph.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–147. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- 7.Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl. 1987;130:81–84. doi: 10.3109/00365528709091004. [DOI] [PubMed] [Google Scholar]
- 8.Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1–S35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 9.Brennan BP, Fogarty KV, Roberts JL, Reynolds KA, Pope HG, Hudson JI. Duloxetine in the treatment of irritable bowel syndrome: an open-label pilot study. Hum Psychopharmacol. 2009;24:423–428. doi: 10.1002/hup.1038. [DOI] [PubMed] [Google Scholar]
- 10.Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460. doi: 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 12.Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Emmott S, Proffitt V, Akman D, Frusciante K, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 13.Mueller-Lissner S, Tytgat GN, Paulo LG, Quigley EM, Bubeck J, Peil H, Schaefer E. Placebo- and paracetamol-controlled study on the efficacy and tolerability of hyoscine butylbromide in the treatment of patients with recurrent crampy abdominal pain. Aliment Pharmacol Ther. 2006;23:1741–1748. doi: 10.1111/j.1365-2036.2006.02818.x. [DOI] [PubMed] [Google Scholar]
- 14.Wittmann T, Paradowski L, Ducrotté P, Bueno L, Andro Delestrain MC. Clinical trial: the efficacy of alverine citrate/simeticone combination on abdominal pain/discomfort in irritable bowel syndrome--a randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2010;31:615–624. doi: 10.1111/j.1365-2036.2009.04216.x. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie JA, Truelove SC. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J. 1979;1:376–378. doi: 10.1136/bmj.1.6160.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:245–251. doi: 10.1111/j.0269-2813.2004.01862.x. [DOI] [PubMed] [Google Scholar]
- 17.Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, Moayyedi P. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman RW, Stanghellini V, Geraint M, Halphen M. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol. 2013;108:1508–1515. doi: 10.1038/ajg.2013.197. [DOI] [PubMed] [Google Scholar]
- 19.Awad RA, Camacho S. A randomized, double-blind, placebo-controlled trial of polyethylene glycol effects on fasting and postprandial rectal sensitivity and symptoms in hypersensitive constipation-predominant irritable bowel syndrome. Colorectal Dis. 2010;12:1131–1138. doi: 10.1111/j.1463-1318.2009.01990.x. [DOI] [PubMed] [Google Scholar]
- 20.Amitiza Product Information 2013. Bethesda, MD: Sucampo Pharmaceuticals, Inc., 2013. Accessed 9/1/13. Available from: http://general.takedapharm.com/content/file/PI.pdf?applicationCode=69CADEA4-354A-44BF-B5E7-017EBDF1333E&fileTypeCode=AMITIZAPI.
- 21.Drossman DA, Chey WD, Johanson JF, Fass R, Scott C, Panas R, Ueno R. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome--results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329–341. doi: 10.1111/j.1365-2036.2008.03881.x. [DOI] [PubMed] [Google Scholar]
- 22.Chey WD, Drossman DA, Johanson JF, Scott C, Panas RM, Ueno R. Safety and patient outcomes with lubiprostone for up to 52 weeks in patients with irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2012;35:587–599. doi: 10.1111/j.1365-2036.2011.04983.x. [DOI] [PubMed] [Google Scholar]
- 23.Linzess Product Information 2013. Cambridge, MA: Ironwood Pharmaceuticals, Inc., 2013. Accessed 7/8/13. Available from: http://www.frx.com/pi/linzess_pi.pdf.
- 24.European Medicines Agency. Constella Information Page 2013. Accessed 7/8/13. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002490/human_med_001597.jsp&mid=WC0b01ac058001d124.
- 25.Bryant AP, Busby RW, Bartolini WP, Cordero EA, Hannig G, Kessler MM, Pierce CM, Solinga RM, Tobin JV, Mahajan-Miklos S, et al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci. 2010;86:760–765. doi: 10.1016/j.lfs.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, Foxx-Orenstein A, Kurtz CB, Sharma V, Johnston JM, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–768. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 27.Johnston JM, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, O’Dea C, Baird M, Lembo AJ. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology. 2010;139:1877–1886.e2. doi: 10.1053/j.gastro.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 28.Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, Shi K, MacDougall JE, Shao JZ, Eng P, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714–1724; quiz p.1725. doi: 10.1038/ajg.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidance for industry: irritable bowel syndrome - clinical evaluation of drugs for treatment Food and Drug Administration 2012. Accessed 7/8/13. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM205269.pdf.
- 30.Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, MacDougall JE, Jia XD, Shao JZ, Fitch DA, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702–1712. doi: 10.1038/ajg.2012.254. [DOI] [PubMed] [Google Scholar]
- 31.Macdougall JE, Johnston JM, Lavins BJ, Nelson LM, Williams VS, Carson RT, Shiff SJ, Shi K, Kurtz CB, Baird MJ, et al. An evaluation of the FDA responder endpoint for IBS-C clinical trials: analysis of data from linaclotide Phase 3 clinical trials. Neurogastroenterol Motil. 2013;25:481–486. doi: 10.1111/nmo.12089. [DOI] [PubMed] [Google Scholar]
- 32.Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1084–1092.e3; quiz e68. doi: 10.1016/j.cgh.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 33.Brenner DM. Linaclotide for the treatment of irritable bowel syndrome with constipation: is it time to reshuffle the deck? Gastroenterology. 2013;145:476–478. doi: 10.1053/j.gastro.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19:62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 35.Pimentel M, Lezcano S. Irritable Bowel Syndrome: Bacterial Overgrowth--What’s Known and What to Do. Curr Treat Options Gastroenterol. 2007;10:328–337. doi: 10.1007/s11938-007-0076-1. [DOI] [PubMed] [Google Scholar]
- 36.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–530, e248. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312–317. doi: 10.5056/jnm.2011.17.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basseri RJ, Weitsman S, Barlow GM, Pimentel M. Antibiotics for the treatment of irritable bowel syndrome. Gastroenterol Hepatol (N Y) 2011;7:455–493. [PMC free article] [PubMed] [Google Scholar]
- 39.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 40.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 41.Pimentel M, Morales W, Chua K, Barlow G, Weitsman S, Kim G, Amichai MM, Pokkunuri V, Rook E, Mathur R, et al. Effects of rifaximin treatment and retreatment in nonconstipated IBS subjects. Dig Dis Sci. 2011;56:2067–2072. doi: 10.1007/s10620-011-1728-5. [DOI] [PubMed] [Google Scholar]
- 42.Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Novi M, Sottili S, Vitale G, Cesario V, Serricchio M, Cammarota G, et al. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am J Gastroenterol. 2008;103:2031–2035. doi: 10.1111/j.1572-0241.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- 43.Farrell DJ. Rifaximin in the treatment of irritable bowel syndrome: is there a high risk for development of antimicrobial resistance? J Clin Gastroenterol. 2013;47:205–211. doi: 10.1097/MCG.0b013e31827559a3. [DOI] [PubMed] [Google Scholar]
- 44.Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35; quiz 36. doi: 10.1038/ajg.2011.355. [DOI] [PubMed] [Google Scholar]
- 45.Meyrat P, Safroneeva E, Schoepfer AM. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment Pharmacol Ther. 2012;36:1084–1093. doi: 10.1111/apt.12087. [DOI] [PubMed] [Google Scholar]
- 46.Manabe N, Rao AS, Wong BS, Camilleri M. Emerging pharmacologic therapies for irritable bowel syndrome. Curr Gastroenterol Rep. 2010;12:408–416. doi: 10.1007/s11894-010-0124-1. [DOI] [PubMed] [Google Scholar]
- 47.Lech Y, Olesen KM, Hey H, Rask-Pedersen E, Vilien M, Ostergaard O. [Treatment of irritable bowel syndrome with peppermint oil. A double-blind study with a placebo] Ugeskr Laeger. 1988;150:2388–2389. [PubMed] [Google Scholar]
- 48.Liu JH, Chen GH, Yeh HZ, Huang CK, Poon SK. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol. 1997;32:765–768. doi: 10.1007/BF02936952. [DOI] [PubMed] [Google Scholar]
- 49.Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double blind placebo-controlled randomized trial. Dig Liver Dis. 2007;39:530–536. doi: 10.1016/j.dld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Capanni M, Surrenti E, Biagini M, Milani S, Surrenti C, Galli A. Efficacy of peppermint oil in the treatment of irritable bowel syndrome: a randomized, controlled trial. Gazz Med Ital. 2005;164:119–126. [Google Scholar]
- 51.Merat S, Khalili S, Mostajabi P, Ghorbani A, Ansari R, Malekzadeh R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig Dis Sci. 2010;55:1385–1390. doi: 10.1007/s10620-009-0854-9. [DOI] [PubMed] [Google Scholar]
- 52.Kligler B, Chaudhary S. Peppermint oil. Am Fam Physician. 2007;75:1027–1030. [PubMed] [Google Scholar]
- 53.Bortolotti M, Porta S. Effect of red pepper on symptoms of irritable bowel syndrome: preliminary study. Dig Dis Sci. 2011;56:3288–3295. doi: 10.1007/s10620-011-1740-9. [DOI] [PubMed] [Google Scholar]
- 54.Ford AC, Talley NJ. Irritable bowel syndrome. BMJ. 2012;345:e5836. doi: 10.1136/bmj.e5836. [DOI] [PubMed] [Google Scholar]
- 55.Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O’Sullivan GC, Kiely B, Collins JK, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ringel Y, Ringel-Kulka T. The rationale and clinical effectiveness of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2011;45 Suppl:S145–S148. doi: 10.1097/MCG.0b013e31822d32d3. [DOI] [PubMed] [Google Scholar]
- 58.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–1264; quiz 1446-1447. doi: 10.1093/ajcn/83.6.1256. [DOI] [PubMed] [Google Scholar]
- 59.Wilkins T, Pepitone C, Alex B, Schade RR. Diagnosis and management of IBS in adults. Am Fam Physician. 2012;86:419–426. [PubMed] [Google Scholar]
- 60.Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. doi: 10.1186/1471-230X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 62.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033–1049; quiz 1050. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 63.McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650–2661. doi: 10.3748/wjg.14.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Floch MH, Walker WA, Madsen K, Sanders ME, Macfarlane GT, Flint HJ, Dieleman LA, Ringel Y, Guandalini S, Kelly CP, et al. Recommendations for probiotic use-2011 update. J Clin Gastroenterol. 2011;45 Suppl:S168–S171. doi: 10.1097/MCG.0b013e318230928b. [DOI] [PubMed] [Google Scholar]
- 65.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 66.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 67.Floch MH, Walker WA, Guandalini S, Hibberd P, Gorbach S, Surawicz C, Sanders ME, Garcia-Tsao G, Quigley EM, Isolauri E, et al. Recommendations for probiotic use--2008. J Clin Gastroenterol. 2008;42 Suppl 2:S104–S108. doi: 10.1097/MCG.0b013e31816b903f. [DOI] [PubMed] [Google Scholar]
- 68.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 69.Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Sibal A, Romano C, Canani RB, Lionetti P, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 70.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 71.Whorwell PJ. Do probiotics improve symptoms in patients with irritable bowel syndrome? Ther Adv Gastroenterol. 2009;2(Suppl 1):S37–S44. doi: 10.1177/1756283X09335637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mercer M, Brinich MA, Geller G, Harrison K, Highland J, James K, Marshall P, McCormick JB, Tilburt J, Achkar JP, et al. How patients view probiotics: findings from a multicenter study of patients with inflammatory bowel disease and irritable bowel syndrome. J Clin Gastroenterol. 2012;46:138–144. doi: 10.1097/MCG.0b013e318225f545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.NICE (2008) Irritable Bowel Syndrome. Accessed 9/16/13. Available from: http://www.nice.org.uk/Guidance/CG61#summary.
- 74.Kim SE, Kubomoto S, Chua K, Amichai MM, Pimentel M. “Pre-cebo”: an unrecognized issue in the interpretation of adequate relief during irritable bowel syndrome drug trials. J Clin Gastroenterol. 2012;46:686–690. doi: 10.1097/MCG.0b013e31825828a7. [DOI] [PubMed] [Google Scholar]


