Abstract
Obesity and its related metabolic disorders, including insulin resistance and chronic inflammation, increase the risk of colorectal cancer (CRC). This observation suggests that the metabolic abnormalities associated with obesity can be effective targets for preventing the development of CRC in obese individuals. In recent years, many studies using obese and diabetic animal models have been conducted to investigate the chemoprevention of CRC using pharmaceutical or nutritional interventions. Pitavastatin, a medicine used to treat hyperlipidemia, prevents the development of obesity-related colorectal carcinogenesis by attenuating chronic inflammation. Anti-hypertensive medicines, such as captopril and telmisartan, also suppress the formation of colonic preneoplastic lesions in obese and diabetic mice. In addition, several phytochemicals, including green tea catechins, have been reported to improve metabolic disorders and prevent the development of various cancers, including CRC. Moreover, the administration of branched-chain amino acids, which improves protein malnutrition and prevents the progression of hepatic failure, is effective for suppressing obesity-related colon carcinogenesis, which is thought to be associated with improvements in insulin resistance. In the present article, we summarize the detailed relationship between metabolic abnormalities and the development of CRC. This review also outlines recent evidence, in particular drawing from basic and clinical examinations using either pharmaceutical or nutritional intervention that suggests that targeting metabolic alterations may be an effective strategy for preventing the development of CRC in obese individuals.
Keywords: Colorectal cancer, Obesity, Green tea catechin, Branched-chain amino acid, Chemoprevention
Core tip: Obesity and its related metabolic disorders increase the risk of colorectal cancer (CRC). Many studies using obese animal models have been conducted to investigate the chemoprevention of CRC using pharmaceutical or nutritional interventions. Lipid-lowering and anti-hypertensive medicines suppress the development of colonic preneoplastic lesions in obese mice. Green tea catechins improve metabolic disorders and prevent the development of CRC. The administration of branched-chain amino acids may be effective for suppressing obesity-related CRC. This review summarizes recent evidence that suggests that targeting metabolic alterations may be an effective strategy for preventing the development of CRC in obese individuals.
INTRODUCTION
Obesity is recognized to be a serious health problem that is becoming more prevalent worldwide[1]. It frequently causes a number of medical problems, including type 2 diabetes mellitus, cardiovascular diseases, hypertension, and dyslipidemia[2]. In addition, recent epidemiological and experimental evidence indicates that obesity and its related metabolic abnormalities, especially diabetes mellitus, are associated with the development of certain types of epithelial malignancies, including colorectal cancer (CRC)[2-6]. Renehan et al[7] revealed in a large-scale meta-analysis that the magnitude of the risk of CRC is greater in obese males than in non-obese males.
Several pathophysiological mechanisms that correlate obesity with colorectal carcinogenesis have been demonstrated, including the occurrence of insulin resistance and adipocytokine imbalances, alterations in the insulin-like growth factor-1 (IGF-1)/IGF-1 receptor (IGF-1R) axis, chronic inflammation, and the induction of oxidative stress[2-6,8]. These findings also suggest that targeting obesity-associated pathophysiological disorders using nutritional or pharmaceutical interventions is a promising strategy for suppressing obesity-related colorectal carcinogenesis. For example, pitavastatin, a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, suppresses the development of colonic preneoplastic lesions by attenuating chronic inflammation in obese and diabetic mice[9]. Anti-hypertensive medicines, such as captopril and telmisartan, also prevent obesity-related colorectal carcinogenesis, and this suppressive effect appears to be associated with the reduction of oxidative stress and chronic inflammation[10].
Recently, green tea catechins (GTCs) have received significant attention due to their beneficial impact on health, as they are reported to improve metabolic abnormalities and prevent cancer[11-15]. Another phytochemical, curcumin, a component of turmeric, also demonstrates suppressive effects against colorectal carcinogenesis in obese mice[16]. Supplementation with branched-chain amino acids (BCAA: leucine, isoleucine, and valine), which can inhibit the progression of hepatic failure in patients with chronic liver disease[17-19], suppresses obesity-related colorectal carcinogenesis by improving insulin resistance in obese and diabetic mice[20].
The present review summarizes multiple mechanisms by which obesity and its related metabolic alterations influence the development of CRC, particularly focusing on the emergence of insulin resistance and the subsequent inflammatory cascade. This article also aims to review the possibility that nutritional or pharmaceutical approaches targeting pathophysiological conditions caused by obesity is effective in preventing obesity-related colorectal carcinogenesis.
POTENTIAL PATHOPHYSIOLOGICAL MECHANISMS CORRELATING OBESITY TO THE DEVELOPMENT OF CRC
Among the various obesity-related metabolic disorders, insulin resistance and hyperinsulinemia are considered to be pivotal risk factors for the development of CRC[21]. Insulin itself and the insulin-regulated signal transduction network play important roles in oncogenesis[22-24]. Insulin stimulates the growth of CRC cells[25] and promotes colorectal tumor growth in animal models[26]. In addition, IGF-1, an important endocrine and paracrine regulator of tissue growth and metabolism, is biologically activated by insulin resistance[27,28]. A number of studies have shown that the IGF-1/IGF-1R axis plays a significant role in the carcinogenesis of various cancers, including CRC[22-24]. Insulin resistance alters the IGF/IGF-1R axis, which contributes to the development of CRC[29,30]. The binding of insulin and IGF-1 to their respective receptors on cancer and/or precancerous cells activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is responsible for cellular processes, such as growth, proliferation, and survival[22,23]. Moreover, insulin resistance and an increased fat mass create an oxidative stress environment in tissues and increase the expression of various pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which stimulate tumor growth and progression[31-35]. Increased oxidative stress promotes DNA damage and activates the PI3K/Akt signaling pathway, both of which play essential roles in cancer development[36,37]. Hence, insulin resistance and subsequent inflammatory cascades involving increased oxidative stress are thought to be significant factors in the development of obesity-related CRC.
The overproduction of fat storage causes an imbalance in adipocytokines, increasing the levels of leptin and decreasing the levels of adiponectin in the serum. This imbalance also contributes to obesity-associated carcinogenesis[38,39]. Leptin induces the production of pro-inflammatory cytokines TNF-α and IL-6[40,41], which may lead to tumor growth and progression as stated above. With respect to CRC, leptin is reported to stimulate CRC cell growth[42]. In addition, a positive association between the circulating leptin levels and the development of CRC has been indicated in an epidemiological study[43]. These findings suggest that obesity-induced abnormalities cooperatively increase the risk of cancer, including CRC, in obese individuals (Figure 1).
Figure 1.
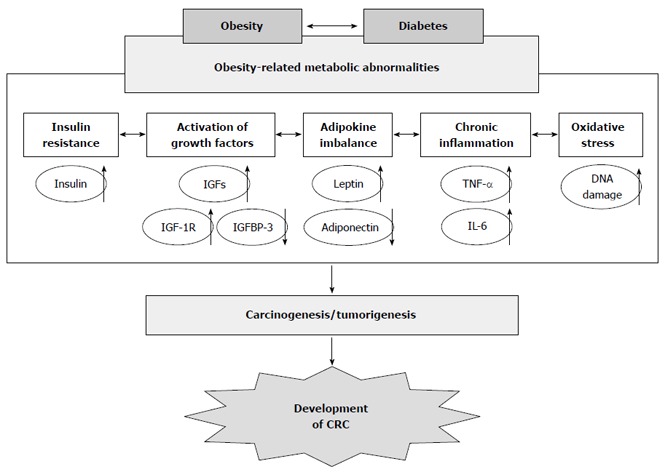
Proposed mechanisms linking obesity and its related metabolic abnormalities to the development of colorectal cancer. CRC: Colorectal cancer; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6; IGF-1: Insulin-like growth factor-1; IGFBP-3: IGF-binding protein-3.
CHEMOPREVENTIVE EFFECTS OF GREEN TEA CATECHINS ON THE DEVELOPMENT OF METABOLIC ABNORMALITIES AND CRC
In recent years, GTCs have received considerable attention due to their salutary influence on health. Several studies have indicated that GTCs possess various useful properties, such as anti-obesity effects[11]. A recent meta-analysis of clinical trials demonstrated that GTCs appear to reduce body weight and fat[44]. Rains et al[45] reported several potential mechanisms whereby GTCs may affect body weight: GTCs may increase energy expenditures, promote lipid oxidation, and decrease nutrient absorption. Laboratory, epidemiological, and human interventional investigations have also shown the effects of GTCs in ameliorating metabolic syndrome[46,47]. In rodent models of obesity and diabetes, the administration of green tea or its constituents results in the significant reduction of body weight and improvements in hyperglycemia, hyperinsulinemia, hyperleptinemia, hepatic steatosis, and liver dysfunction[48-50]. Treatment with GTCs also decreases the plasma levels of insulin, TNF-α, and IL-6 in a rat model of insulin resistance[51]. These observations suggest that long-term supplementation of GTCs may be useful for preventing the progression of obesity-related metabolic abnormalities. In addition to their anti-obesity effects, GTCs are known to possess anti-cancer and cancer prevention properties[12-15]. Positive evidence for the chemopreventive effects of tea preparations against premalignant lesions has been provided in human intervention studies. Li et al[52] reported the preventive effects of tea on human oral precancerous mucosal lesion leukoplakia. Clinical efficacy of green tea extract in patients with human papilloma virus infected cervical lesions was demonstrated by Ahn et al[53]. The inhibitory effect of GTCs on the development of high-grade prostate intraepithelial neoplasia was also reported[54,55]. Moreover, a pilot study showed that the administration of GTCs successfully prevents the development of colorectal adenomas, which are considered to be precancerous lesions of CRC[56].
Several properties of GTCs are responsible for their anti-cancer and cancer prevention effects, including their antioxidant and anti-inflammatory actions[15,57]. A number of reported studies have indicated that GTCs, especially the major biologically active component epigallocatechin gallate (EGCG), inhibit proliferation and induce apoptosis in cancer cells by modulating the activities of diverse receptor tyrosine kinases (RTKs) and their downstream signaling pathways, such as the Ras/extracellular signal-regulated kinase and PI3K/Akt signaling pathways[12-14,58-61]. EGCG suppresses cell growth by inhibiting the activation of IGF-1R, a member of the RTK family, in human CRC cell lines. This inhibition is associated with a decrease in the expression of IGF-1/2 and an increase in the expression of IGF-binding protein-3 (IGFBP-3), which negatively controls the function of the IGF/IGF-1R axis[62,63]. Taken together, these reports indicate that the IGF/IGF-1R axis, which plays a critical role in both the development of cancer and occurrence of obesity-induced pathological events[22,23], is a critical target of GTCs.
PREVENTION OF OBESITY-RELATED CRC VIA A NUTRACEUTICAL APPROACH
Recent investigations have shown that an increased amount of body fat and high body mass index are associated with an increased risk of colorectal malignancy[2,5-7]. While the prolonged high consumption of red and processed meat may also increase the risk of CRC[64], there is persuasive evidence that positive dietary habits, especially a high level of consumption of fruits and vegetables, can reduce the risk of this malignancy[65]. C57BLKS/J- +Leprdb/+Leprdb (db/db) mice, which are genetically altered and those leptin receptors are mutated, express phenotypes of obesity and having type 2 diabetes mellitus in addition to hyperlipidemia, hyperinsulinemia, and hyperleptinemia[66]. A preclinical animal model using db/db mice was established by Hirose et al[67] in which the intraperitoneal administration of colonic carcinogen azoxymethane (AOM) is thought to be markedly useful for determining the underlying mechanisms of how specific agents prevent the development of obesity-related CRC. Furthermore, db/db mice are susceptible to AOM, as AOM-induced colonic precancerous lesions, aberrant crypt foci (ACF) and β-catenin accumulated crypts (BCAC) develop to an obviously greater extent in these mice than in control mice[67].
Diets supplemented with certain types of flavonoids, including citrus compounds, suppress the development of premalignant lesions of CRC in db/db mice[68,69]. We also used this experimental rodent model to examine the obesity-related cancer chemopreventive effects of curcumin, a yellow pigment found in the rhizome of the spice turmeric, which is known to possess both anti-inflammatory and cancer prevention properties[70-72]. A report by Kubota et al[16] revealed that supplementation with curcumin effectively prevents the development of colonic preneoplastic lesions in db/db mice treated with AOM injections in association with the inhibition of the NF-κB activity and the TNF-α, IL-6, and cyclooxygenase-2 (COX-2) expression in the colonic mucosa and improvements in adipocytokine imbalances.
In the same manner, we employed a rodent model to investigate in detail the effects of EGCG and BCAA on the prevention of obesity-related colorectal carcinogenesis. The mucosa in the db/db mice colon expresses increased levels of IGF-1R, the phosphorylated form of IGF-1R (p-IGF-1R), β-catenin, and COX-2[73]. We observed that drinking water containing EGCG caused a significant reduction in the number of ACF and BCAC, which accumulate IGF-1R proteins, and this decreasing effect was associated with the inhibition of the expression of IGF-1R, p-IGF-1R, the phosphorylated form of glycogen synthase kinase-3β (GSK-3β), β-catenin, COX-2, and cyclin D1 on the colonic mucosa[73]. EGCG also decreased the serum levels of IGF-1, insulin, triglycerides, total cholesterol, and leptin, while increasing the serum level of IGFBP-3[73]. In accordance with the results of this study, another study showed that supplementation with BCAA markedly decreased the number of ACF and BCAC compared with that observed in the control diet-fed groups by inhibiting the phosphorylation of IGF-1R, GSK-3β, and Akt in the colonic mucosa[20]. In that study, the serum levels of insulin, IGF-1, IGF-2, triglycerides, total cholesterol, and leptin were also decreased in the BCAA-treated mice[20].
Taken together, these findings suggest that both EGCG and BCAA successfully suppress the development of preneoplastic lesions of obesity-related CRC via diverse mechanisms, including the suppression of the IGF/IGF-1R axis and improvements in hyperlipidemia, hyperinsulinemia, and hyperleptinemia. Therefore, nutraceutical approaches, for example, the administration of EGCG or BCAA, may be useful for use in the chemoprevention of colorectal tumorigenesis in obese individuals (Figure 2).
Figure 2.
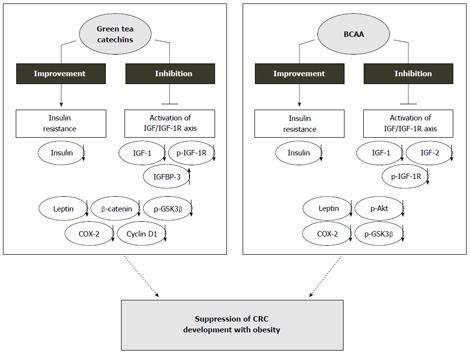
Mechanisms of action of green tea catechins and branched-chain amino acids in the inhibition of obesity-related colorectal carcinogenesis. IGF-1: Insulin-like growth factor-1; BCAA: Branched-chain amino acids; COX-2: Cyclooxygenase-2; IGF-1R: Insulin-like growth factors-1 receptor; GSK-3β: Glycogen synthase kinase-3β.
PREVENTION OF OBESITY-RELATED CRC VIA A PHARMACEUTICAL APPROACH
Obesity often leads to various medical problems, including hypertension and dyslipidemia[2]. Hyperactivity of the renin-angiotensin system (RAS) has been shown to be involved in the etiology of high blood pressure, obesity, and metabolic syndrome[74]. There is convincing evidence that the RAS is frequently upregulated in human malignancies due to systemic oxidative stress and hypoxia, which trigger a state of chronic inflammation[75]. We investigated the effects of an angiotensin-converting enzyme inhibitor, captopril, and an angiotensin-II type 1 receptor blocker, telmisartan, both of which can inhibit the RAS, on the development of colonic preneoplastic lesions in an obesity-related CRC model[10]. The administration of either captopril or telmisartan significantly reduced the number of ACF and BCAC and decreased the expression of TNF-α in the colonic mucosa. Oxidative stress throughout the body is also decreased by the administration of either captopril or telmisartan[10].
Statins, HMG-CoA reductase inhibitors, are widely used for the treatment of dyslipidemia. In addition to their lipid-lowering effects, statins have been shown to possess anti-cancer properties[76,77]. Statins induce apoptosis in human CRC cells and attenuate inflammation-related colon carcinogenesis in mice[78,79]. Moreover, epidemiological studies have indicated the chemopreventive effects of statins on various types of cancer, including CRC[76,77,80]. We conducted a study using a lipophilic statin, pitavastatin, to examine the cancer prevention effects of such drugs on obesity-related colon carcinogenesis in db/db obese mice treated with AOM injection[9]. Pitavastatin treatment achieved a marked reduction in the number of BCAC by inhibiting proliferation and surrounding inflammation, in which the expression levels of TNF-α, IL-6, and COX-2 in the colonic mucosa were decreased. In addition, pitavastatin also decreased the serum levels of total cholesterol, TNF-α, IL-6, and leptin, while increasing the serum level of adiponectin[9].
These observations suggest that both anti-hypertensive and lipid-lowering agents suppress obesity-related colorectal carcinogenesis. The potential mechanisms involve improving dyslipidemia and hyperleptinemia and attenuating chronic inflammation in the colonic mucosa by decreasing the expression of pro-inflammatory cytokines. Therefore, the pharmaceutical approach described above appears to be a feasible strategy for the chemoprevention of obesity-related CRC, as these medicines exert an original pharmacological effect against the development of obesity-related metabolic disorders in addition to their cancer prevention effects.
CONCLUSION
Obesity and its related metabolic disorders, which are associated with an increased risk of several life-threatening diseases, including cancer, are critical health problems that must be addressed. Among human cancers, CRC is one of the most representative malignancies influenced by obesity. In this review, we reported the potential efficacy of nutraceutical and pharmaceutical approaches for targeting obesity-related metabolic alterations. Restoring such abnormalities to a regular state is a promising strategy for preventing the development of obesity-related CRC. Tea catechins, especially GTCs and its active constituent EGCG, can be considered feasible agents for preventing carcinogenesis, as several human interventional trials have demonstrated the efficacy of GTCs as chemopreventive agents without serious adverse effects[54,56,61]. BCAA and certain types of statins and anti-hypertensive medicines are also thought to be potential agents because they are widely used in clinical practice and their safety has been adequately proven. Moreover, a randomized controlled trial demonstrated that BCAA supplementation can prevent liver cancer in obese individuals[19,81]. Therefore, further advanced translational research should be conducted to examine whether active interventions using these agents can prevent the development and recurrence of CRC in patients with obesity.
Footnotes
P- Reviewers: de Bree E, Elisaf MS S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 3.Aleksandrova K, Nimptsch K, Pischon T. Obesity and colorectal cancer. Front Biosci (Elite Ed) 2013;5:61–77. doi: 10.2741/e596. [DOI] [PubMed] [Google Scholar]
- 4.Anderson AS, Caswell S. Obesity management--an opportunity for cancer prevention. Surgeon. 2009;7:282–285. doi: 10.1016/s1479-666x(09)80005-x. [DOI] [PubMed] [Google Scholar]
- 5.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–291. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Nino ME. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol. 2013;2013:697521. doi: 10.1155/2013/697521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasuda Y, Shimizu M, Shirakami Y, Sakai H, Kubota M, Hata K, Hirose Y, Tsurumi H, Tanaka T, Moriwaki H. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci. 2010;101:1701–1707. doi: 10.1111/j.1349-7006.2010.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubota M, Shimizu M, Sakai H, Yasuda Y, Ohno T, Kochi T, Tsurumi H, Tanaka T, Moriwaki H. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem Biophys Res Commun. 2011;410:108–113. doi: 10.1016/j.bbrc.2011.05.115. [DOI] [PubMed] [Google Scholar]
- 11.Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res. 2006;50:188–210. doi: 10.1002/mnfr.200500109. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol Nutr Food Res. 2011;55:832–843. doi: 10.1002/mnfr.201000622. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu M, Shirakami Y, Moriwaki H. Targeting receptor tyrosine kinases for chemoprevention by green tea catechin, EGCG. Int J Mol Sci. 2008;9:1034–1049. doi: 10.3390/ijms9061034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu M, Weinstein IB. Modulation of signal transduction by tea catechins and related phytochemicals. Mutat Res. 2005;591:147–160. doi: 10.1016/j.mrfmmm.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubota M, Shimizu M, Sakai H, Yasuda Y, Terakura D, Baba A, Ohno T, Tsurumi H, Tanaka T, Moriwaki H. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr Cancer. 2012;64:72–79. doi: 10.1080/01635581.2012.630554. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi T, Nagao Y, Matsuoka H, Ide T, Sata M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int J Mol Med. 2008;22:105–112. [PubMed] [Google Scholar]
- 18.Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 19.Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu M, Shirakami Y, Iwasa J, Shiraki M, Yasuda Y, Hata K, Hirose Y, Tsurumi H, Tanaka T, Moriwaki H. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin Cancer Res. 2009;15:3068–3075. doi: 10.1158/1078-0432.CCR-08-2093. [DOI] [PubMed] [Google Scholar]
- 21.Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia. 2003;46:595–607. doi: 10.1007/s00125-003-1109-5. [DOI] [PubMed] [Google Scholar]
- 22.Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7:11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- 23.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 25.Björk J, Nilsson J, Hultcrantz R, Johansson C. Growth-regulatory effects of sensory neuropeptides, epidermal growth factor, insulin, and somatostatin on the non-transformed intestinal epithelial cell line IEC-6 and the colon cancer cell line HT 29. Scand J Gastroenterol. 1993;28:879–884. doi: 10.3109/00365529309103129. [DOI] [PubMed] [Google Scholar]
- 26.Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev. 1996;5:1013–1015. [PubMed] [Google Scholar]
- 27.Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem Pharmacol. 2004;68:1003–1015. doi: 10.1016/j.bcp.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 29.Durai R, Yang W, Gupta S, Seifalian AM, Winslet MC. The role of the insulin-like growth factor system in colorectal cancer: review of current knowledge. Int J Colorectal Dis. 2005;20:203–220. doi: 10.1007/s00384-004-0675-4. [DOI] [PubMed] [Google Scholar]
- 30.Singh P, Rubin N. Insulinlike growth factors and binding proteins in colon cancer. Gastroenterology. 1993;105:1218–1237. doi: 10.1016/0016-5085(93)90971-e. [DOI] [PubMed] [Google Scholar]
- 31.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 32.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 33.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 34.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Flores MB, Rocha GZ, Damas-Souza DM, Osório-Costa F, Dias MM, Ropelle ER, Camargo JA, de Carvalho RB, Carvalho HF, Saad MJ, et al. Obesity-induced increase in tumor necrosis factor-α leads to development of colon cancer in mice. Gastroenterology. 2012;143:741–753.e1-4. doi: 10.1053/j.gastro.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 36.Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal. 2006;8:1765–1774. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- 37.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 38.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 39.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 40.Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27:1507–1515. doi: 10.1093/carcin/bgl018. [DOI] [PubMed] [Google Scholar]
- 41.Molina A, Vendrell J, Gutiérrez C, Simón I, Masdevall C, Soler J, Gómez JM. Insulin resistance, leptin and TNF-alpha system in morbidly obese women after gastric bypass. Obes Surg. 2003;13:615–621. doi: 10.1381/096089203322190844. [DOI] [PubMed] [Google Scholar]
- 42.Amemori S, Ootani A, Aoki S, Fujise T, Shimoda R, Kakimoto T, Shiraishi R, Sakata Y, Tsunada S, Iwakiri R, et al. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G923–G929. doi: 10.1152/ajpgi.00145.2006. [DOI] [PubMed] [Google Scholar]
- 43.Stattin P, Lukanova A, Biessy C, Söderberg S, Palmqvist R, Kaaks R, Olsson T, Jellum E. Obesity and colon cancer: does leptin provide a link? Int J Cancer. 2004;109:149–152. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- 44.Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes (Lond) 2009;33:956–961. doi: 10.1038/ijo.2009.135. [DOI] [PubMed] [Google Scholar]
- 45.Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011;22:1–7. doi: 10.1016/j.jnutbio.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Grove KA, Lambert JD. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J Nutr. 2010;140:446–453. doi: 10.3945/jn.109.115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thielecke F, Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome - a review. Phytochemistry. 2009;70:11–24. doi: 10.1016/j.phytochem.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord. 2002;26:1459–1464. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- 50.Ramadan G, El-Beih NM, Abd El-Ghffar EA. Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. Br J Nutr. 2009;102:1611–1619. doi: 10.1017/S000711450999208X. [DOI] [PubMed] [Google Scholar]
- 51.Qin B, Polansky MM, Harry D, Anderson RA. Green tea polyphenols improve cardiac muscle mRNA and protein levels of signal pathways related to insulin and lipid metabolism and inflammation in insulin-resistant rats. Mol Nutr Food Res. 2010;54 Suppl 1:S14–S23. doi: 10.1002/mnfr.200900306. [DOI] [PubMed] [Google Scholar]
- 52.Li N, Sun Z, Han C, Chen J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc Soc Exp Biol Med. 1999;220:218–224. doi: 10.1046/j.1525-1373.1999.d01-37.x. [DOI] [PubMed] [Google Scholar]
- 53.Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, Bae SM, Lee IP. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev. 2003;12:383–390. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 55.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. Eur Urol. 2008;54:472–473. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, Suganuma M, Fujiki H, Moriwaki H. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020–3025. doi: 10.1158/1055-9965.EPI-08-0528. [DOI] [PubMed] [Google Scholar]
- 57.Shirakami Y, Shimizu M, Tsurumi H, Hara Y, Tanaka T, Moriwaki H. EGCG and Polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DDS. Mol Med Rep. 2008;1:355–361. [PubMed] [Google Scholar]
- 58.Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L, Weinstein IB. The inhibitory effect of (-)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–6501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 59.Adachi S, Nagao T, To S, Joe AK, Shimizu M, Matsushima-Nishiwaki R, Kozawa O, Moriwaki H, Maxfield FR, Weinstein IB. (-)-Epigallocatechin gallate causes internalization of the epidermal growth factor receptor in human colon cancer cells. Carcinogenesis. 2008;29:1986–1993. doi: 10.1093/carcin/bgn128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shirakami Y, Shimizu M, Adachi S, Sakai H, Nakagawa T, Yasuda Y, Tsurumi H, Hara Y, Moriwaki H. (-)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009;100:1957–1962. doi: 10.1111/j.1349-7006.2009.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shirakami Y, Shimizu M, Moriwaki H. Cancer chemoprevention with green tea catechins: from bench to bed. Curr Drug Targets. 2012;13:1842–1857. doi: 10.2174/138945012804545506. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem Biophys Res Commun. 2005;334:947–953. doi: 10.1016/j.bbrc.2005.06.182. [DOI] [PubMed] [Google Scholar]
- 63.Shimizu M, Shirakami Y, Sakai H, Tatebe H, Nakagawa T, Hara Y, Weinstein IB, Moriwaki H. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008;262:10–18. doi: 10.1016/j.canlet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 64.Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, Rodriguez C, Sinha R, Calle EE. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–182. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 65.Forte A, De Sanctis R, Leonetti G, Manfredelli S, Urbano V, Bezzi M. Dietary chemoprevention of colorectal cancer. Ann Ital Chir. 2008;79:261–267. [PubMed] [Google Scholar]
- 66.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 67.Hirose Y, Hata K, Kuno T, Yoshida K, Sakata K, Yamada Y, Tanaka T, Reddy BS, Mori H. Enhancement of development of azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Carcinogenesis. 2004;25:821–825. doi: 10.1093/carcin/bgh059. [DOI] [PubMed] [Google Scholar]
- 68.Hayashi K, Suzuki R, Miyamoto S, Shin-Ichiroh Y, Kohno H, Sugie S, Takashima S, Tanaka T. Citrus auraptene suppresses azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db mice. Nutr Cancer. 2007;58:75–84. doi: 10.1080/01635580701308216. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki R, Kohno H, Yasui Y, Hata K, Sugie S, Miyamoto S, Sugawara K, Sumida T, Hirose Y, Tanaka T. Diet supplemented with citrus unshiu segment membrane suppresses chemically induced colonic preneoplastic lesions and fatty liver in male db/db mice. Int J Cancer. 2007;120:252–258. doi: 10.1002/ijc.22240. [DOI] [PubMed] [Google Scholar]
- 70.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 71.Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–118. doi: 10.1016/s0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 72.Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu M, Shirakami Y, Sakai H, Adachi S, Hata K, Hirose Y, Tsurumi H, Tanaka T, Moriwaki H. (-)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev Res (Phila) 2008;1:298–304. doi: 10.1158/1940-6207.CAPR-08-0045. [DOI] [PubMed] [Google Scholar]
- 74.de Kloet AD, Krause EG, Woods SC. The renin angiotensin system and the metabolic syndrome. Physiol Behav. 2010;100:525–534. doi: 10.1016/j.physbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith GR, Missailidis S. Cancer, inflammation and the AT1 and AT2 receptors. J Inflamm (Lond) 2004;1:3. doi: 10.1186/1476-9255-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 77.Gauthaman K, Fong CY, Bongso A. Statins, stem cells, and cancer. J Cell Biochem. 2009;106:975–983. doi: 10.1002/jcb.22092. [DOI] [PubMed] [Google Scholar]
- 78.Cho SJ, Kim JS, Kim JM, Lee JY, Jung HC, Song IS. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. Int J Cancer. 2008;123:951–957. doi: 10.1002/ijc.23593. [DOI] [PubMed] [Google Scholar]
- 79.Yasui Y, Suzuki R, Miyamoto S, Tsukamoto T, Sugie S, Kohno H, Tanaka T. A lipophilic statin, pitavastatin, suppresses inflammation-associated mouse colon carcinogenesis. Int J Cancer. 2007;121:2331–2339. doi: 10.1002/ijc.22976. [DOI] [PubMed] [Google Scholar]
- 80.Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 81.Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204–214. doi: 10.1016/j.hepres.2006.04.007. [DOI] [PubMed] [Google Scholar]


