Abstract
AIM: To determine the baseline hepatitis B surface antigen (HBsAg) levels during the different phases of chronic hepatitis B (CHB) patients in China.
METHODS: Six hundred and twenty-three hepatitis B virus or un-infected patients not receiving antiviral therapy were analyzed in a cross-sectional study. The CHB patients were classified into five phases: immune-tolerant (IT, n = 108), immune-clearance (IC, n = 161), hepatitis B e antigen negative hepatitis (ENH, n = 149), low-replicative (LR, n = 135), and liver cirrhosis (LC, n = 70). HBsAg was quantified (Abbott ARCHITECT assay) and correlated with hepatitis B virus (HBV) DNA, and serum alanine aminotransferase/aspartate aminotransferase (ALT/AST) in each phase of CHB was also determined.
RESULTS: Median HBsAg titers were different in each phase of CHB (P < 0.001): IT (4.85 log10 IU/mL), IC (4.36 log10 IU/mL), ENH (2.95 log10 IU/mL), LR (3.18 log10 IU/mL) and LC (2.69 log10 IU/mL). HBsAg titers were highest in the IT phase and lowest in the LC phase. Serum HBsAg titers showed a strong correlation with HBV viral load in the IC phase (r = 0.683, P < 0.001). No correlation between serum HBsAg level and ALT/AST was observed.
CONCLUSION: The mean baseline HBsAg levels differ significantly during the five phases of CHB, providing evidence on the natural history of HBV infection. HBsAg quantification may predict the effects of immune-modulator or oral nucleos(t)ide analogue therapy.
Keywords: Hepatitis B surface antigen quantification, Chronic hepatitis B, Natural history, Perspective
Core tip: The quantification of serum hepatitis B surface antigen (HBsAg) has been recently advocated as a favorable marker of disease activity in chronic hepatitis B (CHB). Knowledge of HBsAg in the natural history of chronic hepatitis B is important for the management of the disease, but there is a lack of corresponding data on the base level of HBsAg in the natural history of CHB in China. Hence, the aim of this cross-sectional study was to evaluate the levels of HBsAg in consecutive phases of the natural history of hepatitis B virus-infection without the influence of antiviral treatment before, including the patients’ progression to liver cirrhosis.
INTRODUCTION
The liver and peripheral blood of patients with chronic hepatitis B (CHB) contains large amounts of viral proteins, especially hepatitis B virus (HBV) surface antigens[1]. Detection of hepatitis B surface antigen (HBsAg) in serum is a fundamental diagnostic marker of HBV infection[2]. During the natural history of HBV infection and during antiviral therapy, the level of HBsAg in CHB changes over time, and the loss of HBsAg and the development of anti-HBs antibodies (HBsAg-seroconversion) are the ultimate goals of anti-HBV therapy, as they are believed to represent successful immunological control of active HBV replication[3-6].
The quantification of serum HBsAg has recently been advocated as a reliable marker of disease activity in CHB[7-11]. Knowledge of HBsAg in the natural history of CHB is important for disease management. The natural history of CHB has five phases: immune-tolerant (IT), immune-clearance (IC), non/low-replicative (LR), hepatitis B e antigen negative hepatitis (ENH) and liver cirrhosis (LC). These phases may not occur in all patients or sequentially[3]. As the natural course of HBV infection proceeds, liver histology may progressively worsen and some patients with CHB develop liver failure or hepatocellular carcinoma (HCC)[12,13].
Despite the usefulness of HBsAg quantification as a predictor for CHB, there is a lack of corresponding data on the baseline level of HBsAg during the natural history of CHB in China. Hence, the aim of this cross-sectional study was to evaluate the levels of HBsAg in the consecutive phases of HBV infection in patients not receiving antiviral therapy, and the patients’ progress to liver cirrhosis.
MATERIALS AND METHODS
Patients
Six hundred and twenty-three patients with HBV infection, not receiving antiviral therapy, from the First Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China) were included in this cross-sectional study. Two hundred and seventy-nine patients were hepatitis B e antigen (HBeAg)(+) and 344 were HBeAg(-). There were 456 males and 167 females with a median age of 34 years (range: 18-65 years).
None of the patients were co-infected with hepatitis A virus, hepatitis C virus, hepatitis delta virus, hepatitis E virus or human immunodeficiency virus. Markers such as ceruloplasmin, anti-nuclear antibodies and anti-mitochondrial antibodies for possible co-existing autoimmune disorders and metabolic liver disease were negative. Patients with HCC and end-stage liver diseases were excluded. All useful indicators including patient age, biochemical parameters, and serum HBsAg and HBeAg levels were determined. HBV DNA levels in these patients were obtained when the serum samples were collected.
The patients were categorized into the five phases of CHB: immune-tolerant (IT, n = 108), immune-clearance (IC, n = 161), HBeAg negative hepatitis (ENH, n = 149), low-replicative (LR, n = 135), and liver cirrhosis (LC, n = 70), which were diagnosed based on clinical manifestations or pathology. The classification of these patients was based on the 2012 European Association for the Study of the Liver (EASL), Clinical Practice Guidelines-management of chronic hepatitis B in Asia-Pacific and the 2010 Chinese Clinical Practice Guidelines of chronic hepatitis B[3,14,15] (Table 1).
Table 1.
Definitions of phases of persistent hepatitis B virus infection
| Phase | HBV-DNA (IU/mL) | HBeAg status | ALT (U/L) | Liver imaging |
| IT | > 107 | + | ≤ ULN | No cirrhosis |
| IC | > 2000 | + | ≥ 2ULN | No cirrhosis |
| ENH | > 2000 | - | ≥ 2ULN | No cirrhosis |
| LR | < 2000 | - | ≤ ULN | No cirrhosis |
| LC | Any | +/- | Any | Cirrhosis |
IT: Immune tolerance phase; IC: Immune clearance phase; ENH: HBeAg(-) hepatitis; LR: Low-replicative; LC: Liver cirrhosis induced by hepatitis B virus; HBeAg: Hepatitis B e antigen; HBV: Hepatitis B virus; ULN: Upper limit of normal; “+”: Positive; “-”: Negative.
The study protocol, which conformed to the guidelines of the Declaration of Helsinki, was approved by the Ethics Review Committee of the First Affiliated Hospital of Zhejiang University School of Medicine.
Serum HBsAg quantification
Serum HBsAg levels were quantified using the Abbott ARCHITECT assay (Abbott Diagnostics, Germany) following the manufacturers’ instructions. The dynamic range was 0.05-250 IU/mL. Samples were diluted 1:500 or 1:1000 using ARCHITECT HBsAg Manual Diluent (Abbott Diagnostics) if > 250 IU/mL.
HBV DNA measurement
Serum HBV DNA was quantified by the Determination Kit for hepatitis B virus DNA (Life River, China) with a detection range of 5 × 102 IU/mL-108 IU/mL for samples with HBV DNA > 108 IU/mL, and the HBV DNA assay was repeated after a dilution of 1:1000.
HBV genotyping
The HBV genotypes were determined using sequence detection via PCR. All products were directly sequenced with a HBV Genotype Real Time PCR Kit (ZJ Bio-Tech, Shanghai, China) and run on a MegaBACE 500 according to the manufacturer’s instructions. HBV genome sequence analysis software was used to analyze the results.
Statistical analysis
Variables were compared between groups, using the Mann-Whitney U and Fisher’s exact tests for univariate comparisons, and the Kruskall-Wallis test and ANOVA for multivariate comparisons. Pearson’s correlation coefficient (r) was used to describe the correlation between two variables. Statistical analysis was performed using SPSS 16.0 software.
RESULTS
All patients with HBV infection were enrolled in the study from January 2012 to January 2013 and were classified into the five phases of CHB: IT (n = 108), IC (n = 161), ENH (n = 149), LR (n = 135) and LC (n = 70). More male patients (73.2%) than female patients were enrolled into this cohort study. HBeAg(+) patients were significantly younger than HBeAg(-) patients (P < 0.001), and the patients in the IT phase were younger than those in the IC phase (P = 0.035), while the patients in the LC phase were older than those in the LR and ENH phases (P < 0.001). There were no significant differences between the LR and ENH phase with regard to patient age (P = 0.584). A detailed description of the patients is shown in Table 2.
Table 2.
Baseline population characteristics
| Immune tolerant | Immune clearance | HBeAg negative hepatitis | Low replicative | Liver cirrhosis | P value | |
| HBeAg status | Positive | Positive | Negative | Negative | Positive/negative | |
| Sex M/F | 60/48 | 127/34 | 124/25 | 95/40 | 50/20 | < 0.001 |
| Age (yr) | 26 (18-44) | 29 (18-45) | 41 (22-60) | 39 (22-65) | 49 (26-65) | < 0.001 |
| ALT (U/L) | 25 (9-40) | 188 (80-1322) | 40 (80-3755) | 23 (17-39) | 45 (9-341) | < 0.001 |
| AST (U/L) | 20 (6-36) | 100 (7-1063) | 228 (20-2136) | 14 (9-38) | 49 (10-302) | < 0.001 |
| HBsAg (log10 IU/mL) | 4.85 (3.97-5.15) | 4.36 (3.08-5.25) | 2.95 (0.82-4.89) | 3.18 (2.53-3.60) | 2.69 (2.20-4.04) | < 0.001 |
| HBV DNA (log10 IU/mL) | 8.50 (7.12-9.49) | 7.93 (3.93-9.23) | 5.73 (3.3-9.28) | 2.64 (2.18-3.30) | 4.68 (3.28-8.75) | < 0.001 |
| Genotype | ||||||
| B/C | 60/48 | 83/78 | 86/62 | 74/61 | 37/33 | 0.006 |
HBsAg: Hepatitis B s antigen; HBeAg: Hepatitis B e antigen; HBV: Hepatitis B virus; ALT: Alanine transaminase; AST: Aspartate aminotransferase.
Distribution of serum HBsAg levels
Serum HBsAg levels were widely distributed, and there were significant differences in serum HBsAg levels between the HBV infected patients in the different phases. The highest HBsAg level among the five phases was found in the IT group (median 4.85 log10 IU/mL), followed by the IC group with a median HBsAg level of 4.36 log10 IU/mL, a moderate HBsAg level was found in the ENH (2.95 log10 IU/mL) and LR groups (3.18 log10 IU/mL), and the lowest level was observed in the LC group (2.69 log10 IU/mL) (Table 2 and Figure 1A). The median HBsAg level in HBeAg(+) patients was significantly higher than that in HBeAg(-) patients.
Figure 1.
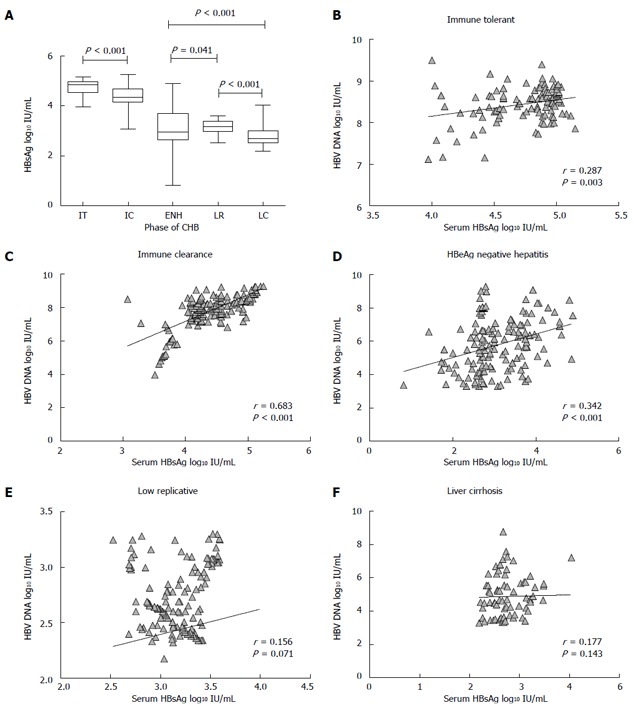
Distribution of hepatitis B surface antigen titers during natural history of chronic hepatitis B and correlation between serum hepatitis B surface antigen titers and hepatitis B virus DNA in different phases of chronic hepatitis B. A: Median values with 95% confidence interval; B-D: The ratio of serum hepatitis B surface antigen (HBsAg) to hepatitis B virus (HBV) DNA levels in the five phases of chronic hepatitis B (CHB). Dots represent individual values, bars 95%CI of median, and numbers are median values.
Correlation between HBsAg titers and HBV DNA
The correlations between serum HBsAg titers and serum HBV DNA in each phase of CHB are shown in Figure 1B-F. There was a strong correlation in the IC phase (r = 0.683, P < 0.0001), a modest correlation in the ENH (r = 0.342, P = 0.001) and IT (r = 0.287, P = 0.003) phases, but a poor correlation between serum HBsAg titers and serum HBV DNA in the LR (r = 0.156, P = 0.071) and LC (r = 0.177, P = 0.143) phases.
The ratio of HBsAg (log10 IU/mL) to HBV DNA in each phase of CHB was also determined (Figure 2). The HBsAg/HBV DNA ratio was significantly higher in the LR phase compared to the IT, IC, ENH and LC phases (1.17 vs 0.56, 0.56, 0.54 and 0.57, respectively, P < 0.001).
Figure 2.
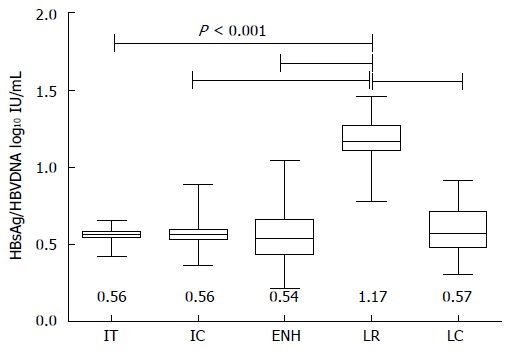
Ratio of hepatitis B surface antigen and hepatitis B virus DNA in each phase of chronic hepatitis B. Median values with 95% confidence interval (of median) are shown. IT: Immune-tolerant; IC: Immune-clearance; ENH: Hepatitis B e antigen negative hepatitis; LR: Low-replicative; LC: Liver cirrhosis; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus.
All patients were genotype B or C. In both viral genotypes, median HBsAg titers were also different among the five phases of CHB, however, there was no difference in the median HBsAg titers between genotype B and C in all five phases.
Correlation between HBsAg titers and clinical parameters
Serum HBsAg was not associated with ALT and AST levels in any of the phases of persistent CHB (Table 3). No significant correlations between serum HBsAg levels and gender, INR, serum bilirubin, albumin, sodium or cholinesterase levels were observed. Quantification of serum HBsAg was not associated with age (Table 3).
Table 3.
Correlation between hepatitis B s antigen and laboratory parameters in different phases
| IT (n = 108) |
IC (n = 161) |
ENH (n = 149) |
LR (n = 135) |
LC (n = 70) |
||||||
| r | P value | r | P value | r | P value | r | P value | r | P value | |
| Age | 0.153 | 0.144 | 0.025 | 0.757 | -0.053 | 0.519 | -0.102 | 0.319 | 0.254 | 0.134 |
| HBV-DNA (log10 IU/mL) | 0.287 | 0.003 | 0.683 | < 0.001 | 0.342 | < 0.001 | 0.156 | 0.071 | 0.177 | 0.143 |
| ALT (U/L) | -0.119 | 0.107 | -0.102 | 0.198 | 0.102 | 0.215 | -0.057 | 0.422 | -0.093 | 0.445 |
| AST (U/L) | 0.060 | 0.535 | -0.072 | 0.161 | 0.034 | 0.681 | -0.011 | 0.880 | -0.082 | 0.499 |
HBV: Hepatitis B virus; IT: Immune tolerance phase; IC: Immune clearance phase; ENH: Hepatitis B e antigen negative hepatitis; LR: Low-replicative; LC: Liver cirrhosis induced by HBV.
DISCUSSION
This study aimed to determine baseline HBsAg levels during the different phases of CHB infection in a Chinese cohort. Both serum HBsAg levels and HBsAg/HBV DNA ratios were obviously different during the five phases of CHB. This finding was previously clearly demonstrated by Jaroszewicz et al[16] in a European cohort, by Nguyen et al[17] in an Asian cohort and by Ramachandran et al[18] in an Indian cohort. However, the present study is the largest to characterize HBsAg levels in the different phases of CHB infection.
Our results showed that median HBsAg titer was highest in the IT phase (4.85 log10 IU/mL) followed by the IC phase (4.36 log10 IU/mL), and both of these phases were HBeAg positive. As indicated in Table 3, HBsAg titers were higher in the HBeAg positive phase compared with the HBeAg negative phase, including the ENH (2.95 log10 IU/mL) and LR (3.18 log10 IU/mL) phases. The lowest HBsAg titers were observed in the LC phase (2.69 log10 IU/mL). All patients were genotype B or C. In both viral genotypes, the median HBsAg titers were also different among the five phases of CHB; however, there were no differences in median HBsAg titers between genotype B and C in all five phases.
Quantification of HBsAg was introduced more than 20 years ago, but has only recently been significantly improved by new automated quantitative assays. Several studies have suggested that quantitative HBsAg is an effective tool for identifying patients who are candidates for therapy, and for monitoring response to pegylated interferon (Peg-IFN), telbivudine and entecavir treatment, and HBV DNA levels[8,19-21]. Our results demonstrated that there was a positive correlation between serum HBsAg and serum HBV DNA levels in the IC phase, a modest correlation in the ENH and IT phases, and a poor correlation between serum HBsAg titers and serum HBV DNA in the LR and LC phases. In a recent study by Nguyen et al[17], Asian patients with HBV infection also showed a strong correlation between HBsAg levels and HBV DNA during the IC phase. In agreement with the results of Nguyen et al[17], our study also indicated that the serum HBsAg/HBV DNA ratio was significantly higher in the LR phase compared with the IT, IC, ENH and LC phases (1.17 vs 0.56, 0.56, 0.54 and 0.57, respectively, P < 0.001). The reason why HBsAg levels and HBV DNA titers appear unrelated in different stages of HBV infection is unclear. A possible explanation may be that HBsAg production far exceeds that required for the production of virions and is secreted in the form of noninfectious particles. Commercially available HBsAg quantification methods may detect all forms of HBsAg; however, the clinical significance of the different HBsAg forms and their ratios is unknown. Another possible explanation is that the S gene of HBV may integrate into host genome in CHB patients.
Several studies have suggested that there is a relationship between serum HBsAg titers and intrahepatic markers of HBV infection, such as covalently closed circular DNA (cccDNA) and intrahepatic HBV DNA[22-24]. Compared to both serum HBV DNA and intrahepatic cccDNA, the costs for determining serum HBsAg are less. This is very important in a developing country, such as China. However, the usefulness of serum HBsAg levels as a substitute for both cccDNA and serum HBV DNA needs to be further elucidated, as studies have indicated a poor correlation with cccDNA[25], and only a positive correlation with HBV DNA in HBeAg positive CHB[26].
Our results also indicated that the levels of HBsAg were lowest in the LC phase, representing long-term immune clearance of HBV infection. A possible mechanism for this may be long-term vigorous immune action, but not complete clearance of HBV in the LC phase. Another possible explanation is a reduction in healthy hepatocytes which can synthesize HBsAg, due to liver fibrosis.
This was a cross-sectional study. A longitudinal study designed to follow the different phases of HBV infection is required. However, HBV infection is a progressive disease in most phases, such as the IC, ENH and LC phases. On the other hand, according to recent guidelines, CHB patients in the IC, ENH and LC phases should receive antiviral treatment. Therefore, it is very difficult to design a longitudinal follow-up study to evaluate HBsAg levels in different phases of HBV infection.
In conclusion, this Chinese cohort study demonstrated that (i) HBsAg titers are different in the five phases of HBV infection; HBsAg levels are highest in the IT phase and lowest in the LC phase; HBsAg shows a strong correlation with HBV DNA in the IC phase; and there were no differences in the median HBsAg titers between genotype B and C in all five phases. Our findings have provided evidence of the pathophysiology and natural history of hepatitis B infection. Future longitudinal studies should be conducted to confirm these results.
COMMENTS
Background
The quantification of serum hepatitis B surface antigen (HBsAg) has recently been advocated as a reliable marker of disease activity in chronic hepatitis B (CHB). Knowledge of HBsAg in the natural history of chronic hepatitis B is important for disease management. Despite the usefulness of HBsAg quantification as a predictor for CHB, there is a lack of corresponding data on the baseline level of HBsAg during the nature history of CHB in China.
Research frontiers
As the natural course of hepatitis B virus (HBV) infection proceeds, liver histology may progressively worsen and some patients with CHB may develop liver failure or hepatocellular carcinoma. Furthermore, serum HBsAg levels are strongly correlated with intrahepatic HBV DNA and covalently closed circular DNA (cccDNA); cccDNA is superior to serum HBV DNA as a predictor of the sustained response to antiviral therapy.
Innovations and breakthroughs
Quantification of serum HBsAg helps in the management of patients with chronic HBV infection. Median HBsAg levels differ significantly during the natural history of HBV infection, progressively declining from immune tolerance to the inactive phase. The combination of HBsAg < 1000 IU/mL and HBV DNA < 2000 IU/mL at a single time point accurately identifies true inactive carriers. During antiviral treatment, HBsAg levels decline more rapidly in patients treated with pegylated interferon (Peg-IFN) than in those treated with nucleos(t)ide analogues, and in responders to Peg-IFN compared to non-responders, suggesting that a response-guided therapy in both HBeAg-positive and -negative patients treated with Peg-IFN could improve the cost-effectiveness of this therapeutic approach. This is the first study to determine the baseline HBsAg levels during each phase of the natural history of chronic hepatitis B.
Applications
As the population of hepatitis B carriers and CHB patients in China is approximately 1/3 to 1/2 of patients worldwide, baseline HBsAg quantification data could be used for further cohort studies in China.
Peer review
This is a good clinical study in which the authors, for the first time, determine the baseline HBsAg levels during each phase of the natural history of chronic hepatitis B. The results of this study help further understand the pathophysiology and the natural history of hepatitis B infection.
Footnotes
Supported by National Key Program for Infectious Diseases of China to Yang YD, No. 2013ZX10002001; and the 12th Five-Year Significant New Drugs Creation Plan of the Ministry of Science and Technology of China to Li LJ, No. 2011ZX09302-003-03
P- Reviewers: Borgia G, Choe BH, Filik L, Ozenirler S S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Zhang DN
References
- 1.Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen DH, Ludgate L, Hu J. Hepatitis B virus-cell interactions and pathogenesis. J Cell Physiol. 2008;216:289–294. doi: 10.1002/jcp.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Martinot-Peignoux M, Lapalus M, Asselah T, Marcellin P. The role of HBsAg quantification for monitoring natural history and treatment outcome. Liver Int. 2013;33 Suppl 1:125–132. doi: 10.1111/liv.12075. [DOI] [PubMed] [Google Scholar]
- 5.Martinot-Peignoux M, Asselah T, Marcellin P. HBsAg quantification to predict natural history and treatment outcome in chronic hepatitis B patients. Clin Liver Dis. 2013;17:399–412. doi: 10.1016/j.cld.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Chevaliez S. Is HBsAg quantification ready, for prime time? Clin Res Hepatol Gastroenterol. 2013;37:559–563. doi: 10.1016/j.clinre.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, Bonino F. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol. 2002;36:263–270. doi: 10.1016/s0168-8278(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 8.Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer N, Bedossa P, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151–1157. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- 9.Rijckborst V, Hansen BE, Cakaloglu Y, Ferenci P, Tabak F, Akdogan M, Simon K, Akarca US, Flisiak R, Verhey E, Van Vuuren AJ, Boucher CA, ter Borg MJ, Janssen HL. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology. 2010;52:454–461. doi: 10.1002/hep.23722. [DOI] [PubMed] [Google Scholar]
- 10.Xun YH, Zang GQ, Guo JC, Yu XL, Liu H, Xiang J, Liu J, Shi JP. Serum hepatitis B surface antigen quantification as a useful assessment for significant fibrosis in hepatitis B e antigen-positive hepatitis B virus carriers. J Gastroenterol Hepatol. 2013;28:1746–1755. doi: 10.1111/jgh.12304. [DOI] [PubMed] [Google Scholar]
- 11.Kuo A, Gish R. Chronic hepatitis B infection. Clin Liver Dis. 2012;16:347–369. doi: 10.1016/j.cld.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369:1582–1583. doi: 10.1016/S0140-6736(07)60723-5. [DOI] [PubMed] [Google Scholar]
- 13.Borgia G, Gentile I. Treating chronic hepatitis B: today and tomorrow. Curr Med Chem. 2006;13:2839–2855. doi: 10.2174/092986706778521995. [DOI] [PubMed] [Google Scholar]
- 14.Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline on prevention and treatment of chronic hepatitis B in China (2005) Chin Med J (Engl) 2007;120:2159–2173. [PubMed] [Google Scholar]
- 15.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514–522. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, Levy M, Locarnini SA. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52:508–513. doi: 10.1016/j.jhep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran J, Ismail AM, Chawla G, Fletcher GJ, Goel A, Eapen CE, Abraham P. Serum HBsAg quantification in treatment-naïve Indian patients with chronic hepatitis B. Indian J Gastroenterol. 2014;33:131–135. doi: 10.1007/s12664-013-0395-1. [DOI] [PubMed] [Google Scholar]
- 19.Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, Wong GL, Sung JJ. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol. 2007;5:1462–1468. doi: 10.1016/j.cgh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Wursthorn K, Jung M, Riva A, Goodman ZD, Lopez P, Bao W, Manns MP, Wedemeyer H, Naoumov NV. Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology. 2010;52:1611–1620. doi: 10.1002/hep.23905. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Ahn SH, Kim HS, Park H, Chang HY, Kim do Y, Hwang SG, Rim KS, Chon CY, Han KH, et al. Quantitative hepatitis B surface antigen and hepatitis B e antigen titers in prediction of treatment response to entecavir. Hepatology. 2011;53:1486–1493. doi: 10.1002/hep.24221. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AJ, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, Slavin J, Bowden S, Gane EJ, Abbott W, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933–1944. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Ahn SH. Quantification of HBsAg: basic virology for clinical practice. World J Gastroenterol. 2011;17:283–289. doi: 10.3748/wjg.v17.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson SB, Eilard A, Malmström S, Hannoun C, Dhillon AP, Norkrans G, Lindh M. HBsAg quantification for identification of liver disease in chronic hepatitis B virus carriers. Liver Int. 2013:Epub ahead of print. doi: 10.1111/liv.12345. [DOI] [PubMed] [Google Scholar]
- 25.Papatheodoridis GV, Manesis EK, Manolakopoulos S, Elefsiniotis IS, Goulis J, Giannousis J, Bilalis A, Kafiri G, Tzourmakliotis D, Archimandritis AJ. Is there a meaningful serum hepatitis B virus DNA cutoff level for therapeutic decisions in hepatitis B e antigen-negative chronic hepatitis B virus infection? Hepatology. 2008;48:1451–1459. doi: 10.1002/hep.22518. [DOI] [PubMed] [Google Scholar]
- 26.Fried MW, Piratvisuth T, Lau GK, Marcellin P, Chow WC, Cooksley G, Luo KX, Paik SW, Liaw YF, Button P, et al. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology. 2008;47:428–434. doi: 10.1002/hep.22065. [DOI] [PubMed] [Google Scholar]


