Abstract
Endoscopic submucosal dissection is an effective treatment modality for early gastric cancer (EGC), though the submucosal fibrosis found in ulcerative EGC is an obstacle for successful treatment. This report presents two cases of ulcerative EGC in two males, 73- and 80-year-old, with severe fibrosis. As endoscopic ultrasonography suggested that the EGCs had invaded the submucosal layer, the endoscopic submucosal tunnel dissection salvage technique was utilized for complete resection of the lesions. Although surgical gastrectomy was originally scheduled, the two patients had severe coronary heart disease, and surgeries were refused because of the risks associated with their heart conditions. The endoscopic submucosal tunnel dissection salvage technique procedures described in these cases were performed under conscious sedation, and were completed within 30 min. The complete en bloc resection of EGC using endoscopic submucosal tunnel dissection salvage technique was possible with a free resection margin, and no other complications were noted during the procedure. This is the first known report concerning the use of the endoscopic submucosal tunnel dissection salvage technique salvage technique for treatment of ulcerative EGC. We demonstrate that endoscopic submucosal tunnel dissection salvage technique it is a feasible method showing several advantages over endoscopic submucosal dissection for cases of EGC with fibrosis.
Keywords: Endoscopy, Early gastric cancer, Endoscopic submucosal dissection, Endoscopic submucosal tunnel dissection, Fibrosis
Core tip: The fibrosis found in ulcerative early gastric cancer (EGC) has been an obstacle to successful endoscopic submucosal dissection (ESD). This report demonstrates the first use of the endoscopic submucosal tunnel dissection (ESTD) salvage technique for treatment of ulcerative EGC with fibrosis. ESTD involves the use of an endoscopic cap under the tunnel flap, which enables a clear view of the submucosal dissection line, making it easier than conventional ESD to resect an EGC lesion without complication. Therefore, the ESTD salvage technique has several advantages for resection of EGC with severe fibrosis due to previous ESD or severe ulceration.
INTRODUCTION
Early gastric cancer (EGC) is defined by gastric carcinomas confined to the mucosa or submucosa regardless of the presence of regional lymph node metastases[1]. Endoscopic submucosal dissection (ESD) is an effective treatment modality for EGC, though the fibrosis found in ulcerative EGC has been an obstacle to successful treatment[2,3]. A recent study demonstrated that the severity of endoscopic submucosal fibrosis correlated with lower en bloc resection rates and incomplete resection[2]. Following the publication of a report detailing the use of peroral endoscopic myotomy to treat achalasia[4], an endoscopic submucosal tunnel dissection (ESTD) method has been described for the treatment of submucosal tumors in the esophagus and gastric cardia[4-6]. In two cases reported here, the ESTD salvage technique was used to overcome the procedural limitations associated with fibrosis. These cases represent the first report in which ESTD was used to treat ulcerative EGC with fibrosis.
CASE REPORT
Case 1
A 73-year-old asymptomatic man was referred to our hospital for the treatment of EGC detected by esophagogastroduodenoscopy in a local clinic. Upper endoscopy revealed an ulcerative lesion, 30 mm in diameter, covered with a white coat and partial erythema on the anterior wall of the proximal antrum. The surrounding mucosa was irregular and slightly elevated (Figure 1A). The patient was diagnosed with type III EGC that included a depressed lesion in the center with swollen surrounding mucosa. Radial endoscopic ultrasonography (EUS) (GF-UCT 240; Olympus Co., Tokyo, Japan) revealed an irregular heterogeneous hypoechoic lesion with smooth tapering in the third layer of the gastric wall and indicated that the EGC had invaded the submucosal layer (Figure 1B). No lymph node enlargement was observed around the stomach by EUS or abdominal computed tomography. ESD was not indicated due to invasion of the EGC above the submucosal 1 layer, as determined by esophagogastro-duodenoscopy and EUS. As the patient also suffered from severe coronary heart disease, the scheduled surgical gastrectomy was refused because of the risks associated with his condition. Therefore, an endoscopic resection was proposed as an alternative and performed following the patient’s informed consent.
Figure 1.
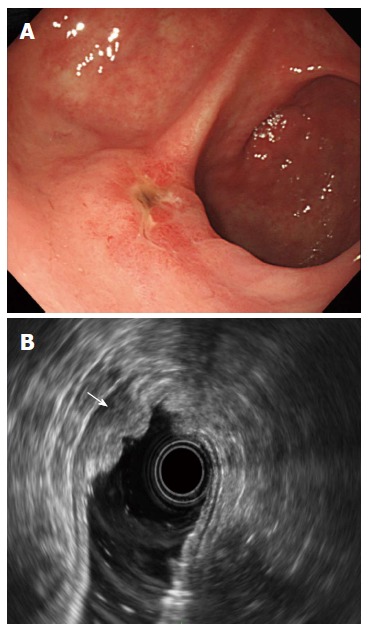
Endoscopic images. A: Ulcerative early gastric cancer on the anterior wall of the proximal antrum; B: Radial endoscopic ultrasonography revealed an irregular heterogeneous hypoechoic lesion with smooth tapering in the third layer of the gastric wall with indication that the cancer had invaded the superficial submucosal layer (arrow).
For the endoscopic procedure, propofol was administered intravenously to induce sedation, and supplemental oxygen (2 L/min) was administered nasally throughout sedation. Additional propofol was used to maintain anesthesia, and cardiorespiratory function was monitored during the 30-min procedure. ESTD was performed using carbon dioxide insufflation. Severe fibrosis was revealed with an ESD endoscope (GIF-Q260J; Olympus Co.). A submucosal injection with saline and sodium hyaluronate into the EGC lesion failed to induce mucosal elevation. Therefore, the ESTD salvage technique (Figure 2) was used for resection of the EGC. For this procedure, marking dots were placed on the surrounding EGC using argon plasma coagulation (APC-300; ERBE Elektromedizin, Tübingen, Germany), and markings for the tunnel gate were placed 4 cm from the proximal side of the lesion. A solution of saline and sodium hyaluronate with diluted epinephrine and indigo carmine was injected into the submucosal layer of the tunnel area and surrounding the EGC lesion to raise the mucosal layer. The tunnel gate was incised with a hook knife (Olympus Co.) and a circumferential incision around the lesion, excluding the tunnel side, was performed. The hook knife was inserted through the tunnel gate for direct dissection of the EGC following confirmation of the lesion margin. A standard water-jet endoscope cap (D-201-11804; Olympus Co.) was used to facilitate hook knife dissection below the tunnel flap and EGC lesion. The resected lesion was removed completely from the muscle layer, and the overlying tunnel mucosal flap from the tunnel gate to the lesion was also removed (Figure 3). A complete en bloc resection was successfully performed with no adverse events.
Figure 2.
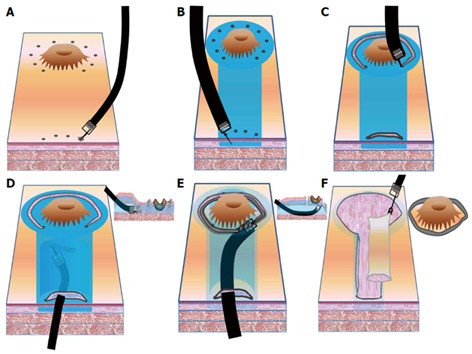
Key procedures of the endoscopic submucosal tunnel dissection salvage technique. A: An area surrounding the early gastric cancer (EGC) lesion and tunnel gate is marked with argon plasma coagulation; B: A solution containing saline and sodium hyaluronate with diluted epinephrine and indigo carmine is injected into the submucosal layer around the EGC lesion and tunnel area; C: A hook knife is used to make an incision at the tunnel gate and a circumferential incision surrounding the lesion; D: A tunnel dissection of the EGC lesion is made with a hook knife and an endoscopic submucosal dissection cap; E: The EGC margin is confirmed and the lesion is directly dissected; F: The resected lesion and the overlying tunnel mucosae are removed without clipping.
Figure 3.
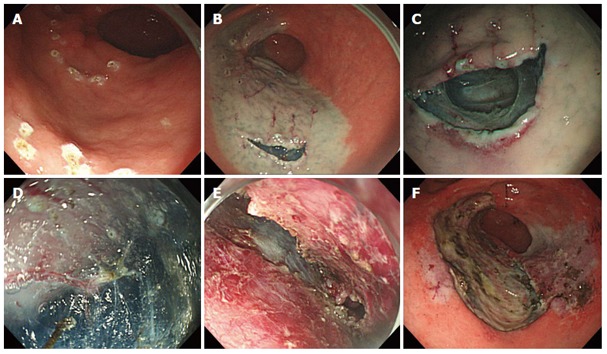
Endoscopic submucosal tunnel dissection salvage technique. Endoscopic images showing; A: Marking of the surrounding the early gastric cancer (EGC) lesion and tunnel gate; B: Submucosal injection and incision of the tunnel gate; C: Initiation of tunnel dissection from tunnel gate; D: Tunnel dissection with hook knife and an endoscopic submucosal dissection cap; E: Direct dissection of EGC; F: En bloc resection.
The histopathology of the excised tissue indicated a well-differentiated tubular carcinoma that was defined as an intestinal type according to Lauren’s classification. The tumor measured 30 mm × 27 mm, and invasion was determined to be up to the muscularis mucosae (pT1a) (Figure 4). Lymphatic, venous, and perineural invasion was not evident, and the removed tissue had a free resection margin with no lateral or deep invasion. An esophagogastroduodenoscopy performed three months after the operation showed no significant findings other than some scarring as a result of the ESTD. Examination of a biopsy specimen of the resection scar showed no remnants of the tumor. The patient had no evidence of cancer recurrence after one year and is scheduled for periodic checkups.
Figure 4.
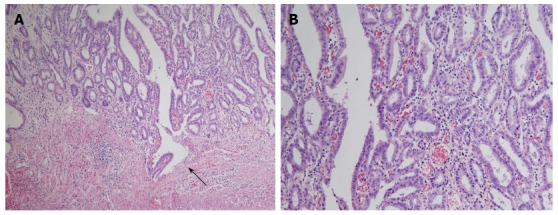
Histopathology findings. Hematoxylin and eosin staining of a biopsy sample (case 1) revealed the presence of a well-differentiated tubular carcinoma invading the muscularis mucosa (arrow) (magnification × 100) (A) and lesion was composed of predominantly discrete glands with pseudostratified hyperchromatic nuclei and some fused glands (magnification × 200) (B).
Case 2
An 80-year-old man was admitted to our hospital for the treatment of EGC that was detected in a local clinic. Esophagogastroduodenoscopy showed an ulcerative EGC lesion 30 mm in diameter on the anterior wall of the distal antrum. The patient underwent radial EUS, which revealed an irregular hypoechoic lesion that appeared to invade the submucosal layer. This patient also refused surgical gastrectomy due to a coronary heart problem. Upper endoscopy showed ulcerative EGC with severe fibrosis. After explaining the risks associated with endoscopic resection, the patient provided informed consent for an ESTD procedure. A complete en bloc resection was completed by the same endoscopist using a similar method as described in case 1, and there were no adverse events. The histopathology of the resected tissue indicated a well-differentiated tubular carcinoma of an intestinal type according to Lauren’s classification. The EGC measured 27 mm × 15 mm in size with invasion to the muscularis mucosae (pT1a). There was a free resection margin with no lymphatic, venous, or perineural invasion. Esophagogastroduodenoscopy performed six months after the procedure showed no significant findings other than the scar from ESTD. The patient is scheduled for follow-up in an outpatient clinic.
DISCUSSION
ESD is gaining recognition as the preferred treatment option for EGC[7]. Nevertheless, fibrosis accompanying large ulcerative EGC remains an obstacle to successful ESD, resulting in a difficulty in achieving complete and en bloc resection[2,3,8]. To overcome these limitations, we used an ESTD salvage technique to treat EGC with features that exceeded the expanded indications for ESD.
When performing an ESTD, the endoscopist must create the tunnel gate on the proximal side of the lesion. Hence, ESTD can be performed on the body or antrum along the greater curvature side of the stomach. There is currently no consensus concerning the distance of the tunnel gate from the proximal side of the lesion. In the cases reported here, the tunnel gate was placed 4-5 cm from the proximal side of the EGC, as previously described[5]. However, we suggest that a distance of 2-3 cm from the proximal side may produce a sufficient submucosal tunnel to treat EGC.
The use of ESTD to remove EGC has limited indications, such as EGC with severe fibrosis due to previous ESD or severe ulceration, and achievement of a sufficient resection margin because of submucosal invasion. In the present cases, ulcerative EGC with severe fibrosis was confirmed, and submucosal invasion was suspected from preoperative EUS. Current indications for ESD based on the criteria of Gotoda et al[9] include an ulcerative mucosal EGC < 3 cm in diameter or a submucosal 1 invasion depth of the EGC ≤ 3 cm. As the EGC in our patients could exceed these criteria before the procedure due to invasion above the submucosal 1 layer, gastrectomy was recommended as the preferred treatment option. However, this treatment was refused by both patients, and the amount of observed fibrosis indicated that a complete en bloc resection was unlikely using the standard ESD method. Therefore, the ESTD salvage technique was performed, with favorable results. Although the EUS indicated invasion of the submucosal layer, histopathology revealed the invasion was only to the muscularis mucosae (pT1a). It is possible that previous biopsies may have induced ulceration and fibrosis, which appeared as an invasion of the submucosal layer on EUS.
The use of ESTD to treat EGC has several advantages over ESD. First, it is easier to establish a safety margin when complete en bloc resection is difficult to perform due to severe fibrosis or when submucosal invasion is suspected. Second, the higher risk of bleeding that is associated with severe fibrosis can be prevented. As the dissection in ESTD is parallel to the lesion, the vessels are exposed, allowing easier control of bleeding. In addition, ESD can be a time-consuming and technically difficult procedure if the resected mucosa cannot be pulled up and the resection line cannot be fully seen. In ESTD, the endoscopic cap under the tunnel flap enables a clear view of the submucosal dissection line, making it easier to resect an EGC lesion and shortening the overall procedure time. Despite the severe fibrosis in the present cases, the ESTD procedures were performed within 30 min. Although general anesthesia has been used to perform ESTD, conscious sedation sufficed in these cases. However, further research is required to assess the safety and effectiveness of the ESTD salvage technique for resection of EGC due to the limited number of patients and short follow-up time of cases using this procedure.
COMMENTS
Case characteristics
Two males, 73- and 80-year-old, with histories of early gastric cancer with fibrosis were resected by endoscopic submucosal tunnel dissection.
Clinical diagnosis
Both patients were asymptomatic, with no specific physical exams.
Laboratory diagnosis
Complete blood counts, electrolytes, metabolic panels, and liver function tests were within normal limits.
Imaging diagnosis
Abdominal computed tomography scans showed no metastases to lymph nodes.
Pathological diagnosis
Histopathology indicated intestinal tumors as defined by Lauren’s classification, and revealed well-differentiated tubular carcinomas, measuring 30 mm × 27 mm (case 1) and 27 mm × 15 mm (case 2), with invasion depths up to muscularis mucosae (pT1a); lymphatic, venous, and perineural invasion were not evident in either case, and the removed tissues had free resection margins with no lateral or deep margin invasion.
Treatment
The patients were treated by complete en bloc resection by endoscopic submucosal tunnel dissection.
Term explanation
Endoscopic submucosal tunnel dissection is an endoscopic technique that creates a submucosal tunnel to provide working space for direct dissection of a submucosal area.
Experiences and lessons
Complete resection by endoscopic submucosal dissection is often difficult to perform in large ulcerative early gastric cancer due to fibrosis and this report describes the first known use of endoscopic submucosal tunnel dissection to complete en bloc resection for ulcerative early gastric cancer with fibrosis.
Peer review
In this case report, endoscopic submucosal tunnel dissection was used to treat two cases of ulcerative early gastric cancer with submucosal fibrosis that were not suited for treatment by conventional endoscopic submucosal dissection.
Footnotes
Supported by Korea Healthcare Technology R and D Project, Ministry of Health and Welfare, South Korea, No. A111182; A grant from Korea University
P- Reviewers: Chen JQ, Hamashima C, Ihara E S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
References
- 1.Chun HJ, Keum B, Kim JH, Seol SY. Current status of endoscopic submucosal dissection for the management of early gastric cancer: a Korean perspective. World J Gastroenterol. 2011;17:2592–2596. doi: 10.3748/wjg.v17.i21.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeong JY, Oh YH, Yu YH, Park HS, Lee HL, Eun CS, Han DS. Does submucosal fibrosis affect the results of endoscopic submucosal dissection of early gastric tumors? Gastrointest Endosc. 2012;76:59–66. doi: 10.1016/j.gie.2012.03.172. [DOI] [PubMed] [Google Scholar]
- 3.Higuchi K, Tanabe S, Azuma M, Sasaki T, Katada C, Ishido K, Naruke A, Mikami T, Koizumi W. Double-endoscope endoscopic submucosal dissection for the treatment of early gastric cancer accompanied by an ulcer scar (with video) Gastrointest Endosc. 2013;78:266–273. doi: 10.1016/j.gie.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 5.Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225–230. doi: 10.1055/s-0031-1291659. [DOI] [PubMed] [Google Scholar]
- 6.Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231–235. doi: 10.1055/s-0031-1291720. [DOI] [PubMed] [Google Scholar]
- 7.Choi HS, Kim KO, Chun HJ, Keum B, Seo YS, Kim YS, Jeen YT, Um SH, Lee HS, Kim CD, et al. The efficacy of transdermal fentanyl for pain relief after endoscopic submucosal dissection: a prospective, randomised controlled trial. Dig Liver Dis. 2012;44:925–929. doi: 10.1016/j.dld.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Yun WK, Min BH, Lee JH, Rhee PL, Kim KM, Rhee JC, Kim JJ. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2011;25:1985–1993. doi: 10.1007/s00464-010-1499-7. [DOI] [PubMed] [Google Scholar]
- 9.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]


