Short abstract
Both mechanical load and elevated levels of proinflammatory cytokines have been associated with the risk for developing osteoarthritis (OA), yet the potential interaction of these mechanical and biological factors is not well understood. The purpose of this study was to evaluate the response of chondrocytes to the effects of dynamic unconfined compression, TNF-α, and the simultaneous effects of dynamic unconfined compression and TNF-α. The response to these three treatments was markedly different and, taken together, the response in the gene expression of chondrocytes to the different treatment conditions suggest a complex interaction between structure, biology, and mechanical loading.
Introduction
Mechanical loading and elevated levels of proinflammatory cytokines have been implicated in the risk for premature OA following traumatic knee injuries such as rupture of the anterior cruciate ligament (ACL) or meniscal injury [1–3]. Changes in the in vivo functional joint motion [4–6] alone or in combination with changes in joint biochemistry [7–12] after injury is one potential kinematic pathway to the initiation of post traumatic OA [13,14]. Evidence for the support of a kinematic pathway has been based on evidence of regional topological variations in the properties of knee articular cartilage structure that are thought to arise in response to the variation in local mechanical environment [15]. It has been shown that chondrocyte populations in different regions of the tibial plateau (covered by meniscus versus not covered by meniscus) respond differently to equivalent tissue level mechanical loading [16]. Thus alteration in the in vivo functional joint motion may result in subtle shifts in the load distribution at the knee altering the local mechanical environment and exposing chondrocytes to loading patterns they are unaccustomed to thereby contributing to degenerative changes.
While changes in the in vivo function of the joint may alone contribute to degenerative changes in articular cartilage of the knee, changes in joint biochemistry are also likely contributing factors. In the healthy knee, synovial fluid concentrations of proinflammatory cytokines are small [7,8,11] with moderate levels for the protective cytokine interleukin-1 receptor antagonist (IL-1ra) [8]. Following ACL injury the most consistent acute cytokine responses observed are increased levels of IL-6, IL-8, and TNF-α [7,8,10]. Although these levels decrease within weeks of the injury, they are still elevated relative to healthy subjects [7–9,11] and are similar to synovial fluid concentrations observed in patients with OA [10]. One cytokine of interest, TNF-α has the ability to induce chondrocyte-mediated cartilage degradation by elevating production of degradative enzymes [17] and inhibiting synthesis [18–20] and expression [20] of structural proteins, leading to increased matrix degradation [21–23].
In vitro studies [24,25] have demonstrated that the specific effects of certain cytokines may be modulated through mechanical loading. Moderate levels of dynamic compression appear to inhibit some of the catabolic effects of interleukin-1 on articular chondrocytes [24,25]. However, proteoglycan (PG) loss in cartilage explants subjected to injurious compression and cultured in the presence of TNF-α was greater than that produced by either injurious compression or TNF-α treatment alone [26]. The coactive effects of TNF-α and in vitro dynamic compression on chondrocyte gene expression are not well understood, particularly for cartilage from distinct regions of the tibial plateau.
Thus, the purpose of this study was to evaluate the regional response of chondrocytes to the coaction of load and biological stimuli by testing the following hypotheses: (1) There are significant differences in the gene expression of chondrocytes to a combined application of dynamic compression with exogenous proinflammatory cytokine (Load + TNF-α) relative to the gene expression in response to isolated Load or TNF-α stimuli. (2) Gene expression in response to Load, TNF-α and Load + TNF-α stimuli will be different in the central and peripheral regions of tibial cartilage.
Materials and Methods
Tissue Explant and Culture.
Articular cartilage was obtained from the 36 stifle joints of healthy 6–8 month juvenile pigs within 4 h of sacrifice; the animals were sacrificed for an unrelated study. This animal model has been used in a previous study of the effect of mechanical loading on chondrocyte gene expression [16]. The joints were opened under aseptic conditions and rinsed periodically with phosphate-buffered saline (pH 7.2, Invitrogen, Carlsbad, CA) during sample harvesting. Using an Osteochondral Autograft Transfer System fitted with a 6 mm harvester (Arthrex, Inc., Naples, FL), full-depth cylindrical cartilage explants were removed from four regions of the tibial plateau, specifically the medial central (not covered by meniscus), medial peripheral (covered by meniscus), lateral central, and lateral peripheral regions. Thus, a total of 36 explants per region were removed from a single limb of 36 different animals. Four samples were taken from each joint (medial uncovered, medial covered, lateral uncovered, and lateral covered). The underlying bone was removed at the subchondral interface with a razor blade.
Cartilage explants were subsequently initiated in serum-supplemented culture. Explants were incubated for 48 h at 37 °C and 5% CO2 in individual wells of a 12-well plate containing 4 ml Dulbecco's modified Eagle's medium supplemented with 10 mM N-2 hydroxyethylpiperazine-N-2-ethanosulphonic acid buffer (Invitrogen, Carlsbad, CA), 10% qualified fetal bovine serum (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA).
Experimental Groups.
After the 48 h free-swelling equilibration period, explants from both central and peripheral regions were subjected to one of four treatment conditions for 6 h (Fig. 1). Approximately one quarter of the explants (n = 20 central, n = 20 peripheral) were given fresh media and subjected to dynamic unconfined compression, termed the “Load” group. Half of the explants were given fresh media, but with the addition of recombinant TNF-α at a concentration of 100 ng/ml; those maintained in free-swelling conditions were termed the “TNF-α” group (n = 16 central, n = 16 peripheral) while those subjected to dynamic unconfined compression were termed the “Load + TNF-α” group (n = 16 central, n = 16 peripheral). The remaining explants served as untreated controls (n = 20 central, n = 20 peripheral), and were given fresh media with no TNF-α and maintained in free-swelling culture conditions.
Fig. 1.

Time course of study. Explants equilibrate in free-swelling culture for ∼48 h prior to being subjected to one of four treatment groups. After 6 h in the assigned treatment (e.g., dynamic unconfined compression), explants are digested and total RNA is isolated.
Mechanical Loading.
Dynamic unconfined compression was applied using a custom fabricated, incubator-housed, pneumatic loading device [16]. Briefly, the compressor was designed with four cylindrical thermoplastic polymer wells for simultaneous loading of multiple explants, similar to compression systems utilized in other studies [27–29]. Each well was separated from a common pressure chamber by flexible polyurethane film such that when the chamber was pressurized the film would deflect. Cartilage explants were centered on the polyurethane foundation of each well; radial position of explants was maintained with stainless steel spacers while stainless steel loading posts with polished flat ends were lowered into contact with the articular surface of each sample. Thus, positive pressurization of the chamber compressed samples between the foundation and the stationary platen. Chamber pressure was regulated using a DC actuated, three-way ball valve connected to a regulated pressure supply at one terminal and vented to ambient pressure at the other. Load magnitude was adjusted from the regulated air supply and monitored with an inline manometer, while loading frequency could be altered by varying the DC power to the valve actuator.
In order to assess the dynamic strains and creep consolidation experienced by cartilage explants under the applied loading, eight additional cartilage explants (four central region, four peripheral region) were subjected to dynamic unconfined compression using an MTS 858 Mini Bionix II (MTS, Eden Prarie, MN) device fitted with flat impermeable stainless steel indenter and base. Specimen thickness was measured under application of a 0.01 N tare load, after which a nominal square wave (0–100 kPa at 0.5 Hz) was applied for 6 h. Cyclic strain was measured as the normal strain that occurred over a single 0–100 kPa loading cycle while creep consolidation was measured by as the change in sample height at a specific point in time (during the 0 kPa portion of the load cycle) compared with the original sample height measured under the 0.01 N tare load.
Cytokine Treatment.
Recombinant porcine TNF-α was obtained from R&D Systems (Minneapolis, MN) and reconstituted with sterile phosphate-buffered saline and 0.1% bovine serum albumin solution (Sigma-Aldrich, St. Louis, MO) at 50 μg/ml. Reconstituted TNF-α was added to the culture medium such that the final concentration was 100 ng/ml, a dosage similar to that employed by previous studies [26,27].
Ribonucleic Acid (RNA) Isolation, Reverse Transcription, and Real-Time PCR.
Immediately following treatment, cartilage explants were minced into ∼1 mm3 pieces, placed in 1 ml TRIzol Reagent (Invitrogen, Carlsbad, CA), and allowed to incubate at room temperature for 10 min according to manufacturer's protocol. Chloroform was added (20% v/v) and following 20 min of incubation at 20 °C the mixture was centrifuged at 12,000 g for 25 min at 4 °C. To precipitate the RNA, the aqueous phase was transferred to a fresh tube containing 0.5 ml isopropanol, incubated at 20 °C for 15 min, and centrifuged 12,000 g for 15 min at 4 °C. The supernatant was removed, after which the RNA pellet was washed with an addition of 1 ml 75% ethanol. Brief vortexing dislodged the pellet and the samples were centrifuged at 7500 g for 7 min at 4 °C. Finally, the RNA pellet was allowed to air-dry for 5 min and resuspended in 10 μl UltraPure DNase/RNase-free distilled water (Invitrogen, Carlsbad, CA). RNA concentration and purity were determined by absorbance measurements at 260 nm and 280 nm.
Reverse transcription was performed with 1.5 μg RNA from each sample using the GeneAmp RNA PCR Core Kit (Applied Biosystems, Foster City, CA) and Eppendorf Mastercycler thermocycler (Eppendorf, Westbury, NY). Real-time polymerase chain reaction (PCR) for gene expression quantitation was performed with the Applied Biosystems 7900HT system using SYBR Green master mix (Applied Biosystems, Foster City, CA) and each reaction was run in triplicate. Porcine-specific primers for type II collagen, aggrecan, MMP-1, MMP-3, MMP-13, ADAM-TS4, TIMP-1, TIMP-2, and TNF-α were taken from literature, while primers for the porcine 18 s rRNA (Accession number AY265350) sequence were designed with Primer Express (Applied Biosystems, Foster City, CA). No appropriate sequence could be located for porcine ADAM-TS5, thus primers for human ADAM-TS5 [30] were used. Standard curves were generated for each gene on each plate, from which cycle threshold values from the linear region of amplification were transformed to relative copy numbers. Copy numbers were normalized by 18 s rRNA copy numbers to account for potential variability in starting cDNA content. Mechanically compressed samples were always run on the same plate as their site-matched unloaded controls and the platens were in place for the loaded and unloaded conditions. Experiments included no template controls, in which cDNA was omitted, and no enzyme controls, in which reverse transcriptase was omitted, for each primer pair.
Data Analysis and Statistics.
All statistics were performed with Minitab 15 (Minitab Inc., State College, PA). The 2(Delta Delta C(T)) [31] method was used to determine the fold difference between experimental groups and pooled (combined central and peripheral) untreated free-swelling controls. In this method, ΔCt is calculated as the difference between cycle threshold (Ct) for a target gene and Ct for 18 s for each sample; ΔΔCt then represents the difference between corresponding ΔCt values between treatment and control groups. Fold difference data was analyzed using a four factor general linear model, in which region (central, peripheral), cytokine treatment (+TNF-α, −TNF-α), and mechanical loading (dynamic unconfined compression, free-swelling) were treated as fixed factors and donor was treated as a random factor. Bonferonni-adjusted two tailed Student's t-tests were used for post hoc pairwise comparison. A gene was deemed to be differently expressed for fold differences ≥2 and p < 0.05. Fold differences are presented as mean ± SEM relative to pooled (central and peripheral) free-swelling controls.
Validation of 2(Delta Delta C(T)).
Plots of ΔCt (target gene relative to 18 s) for a series of fivefold dilutions of stock cDNA show that the amplification efficiencies of 18 s and each of the ten target genes were approximately equal (Fig. 2). That is, the slopes of the least-squares regression of the data were less than 0.1, indicating that the amplification of a target gene relative to 18 s is relatively insensitive to template dilution. Note that the average amplification efficiency was close to optimal, with a value of 92%.
Fig. 2.

Validation of the 2−ΔΔCt method. ΔCt calculated as Ct ,target gene − Ct ,18 s for each cDNA dilution. Data were fit using a least-squares regression analysis (with each reaction run in triplicate) to calculate the slope of the best fit line. A slope < 0.1 indicates that amplification efficiencies are adequately similar to use the 2−ΔΔCt method [31].
The ΔC't method [31] was used to determine the suitability of 18 s as a housekeeping gene and there was no statistically significant difference in 18 s levels when treating region, mechanical loading, and cytokine treatment as factors.
Results
No differences in mRNA levels for the ten genes examined were observed between central and peripheral explants in the untreated control group, supporting the conclusion that pooling these samples provided a meaningful common baseline for comparison with central and peripheral regions.
Analysis of the pooled sample response indicated that isolated Load had a significant effect on the gene expression of matrix structural proteins. CII and aggrecan were significantly up regulated relative to untreated free-swelling controls irrespective of TNF-α treatment (Fig. 3(a)). Isolated TNF-α was not a significant factor in the expression of either collagen (p = 0.158) or aggrecan (p = 0.157).
Fig. 3.
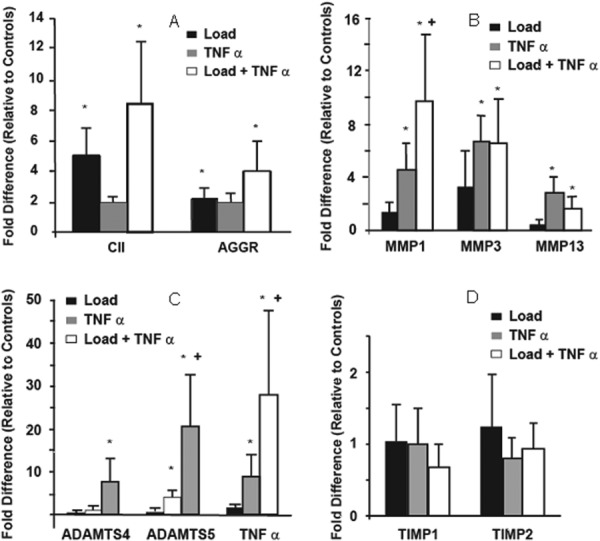
Relative expression levels (averaged central and peripheral) of (a) type II collagen and aggrecan, (b) MMPs 1, 3, and 13, (c) ADAM-TS4, ADAM-TS5, and TNF-α. (d) TIMPs 1 and 2. * indicates significant difference (p < 0.05) from free-swelling control values, + indicates Load/TNF-α interaction term significant (MMP1, ADAM-TS5, TNF).
There was a substantial catabolic response to the combined TNF-α + Load stimulus as compared to isolated TNF-α. An isolated TNF-α stimulus resulted in a significant upregulation of MMP1 (p < 0.01), MMP3 (p < 0.01), MMP13 (p = 0.012), and ADAM-TS5 (p = 0.028) (Fig. 3(b)). However, the significant upregulation of MMP1 (p = 0.028) to the combined TNF-α + Load was approximately four fold greater than produced by the isolated TNF-α treatment (Figs. 3(b) and 3(c)). Similarly, the aggrecanase ADAM-TS4 (p = 0.011) was only upregulated with the combined TNF-α + Load stimulus. It is also important to note that TNF-α expression was also significantly increased by the combination of Load + TNF-α (Fig. 3(c)) and this upregulation was significantly greater than that elucidated by isolated exogenous TNF-α administration. Finally, there was not a significant response in TIMP1 and TIMP2 with TNF-α with any of the external stimuli (Fig. 3(d)).
Region-Dependent Response.
The most striking region-dependent response (Fig. 4) was the large increase (approximately 13-fold) in the expression of ADAM-TS4 mRNA in the central region to the combined Load + TNF-α stimulus relative to the peripheral regions (approximately threefold) (p = 0.0466). Neither isolated Load nor TNF-α stimulus produced an increased response in ADAM-TS4 in the central or peripheral regions.
Fig. 4.
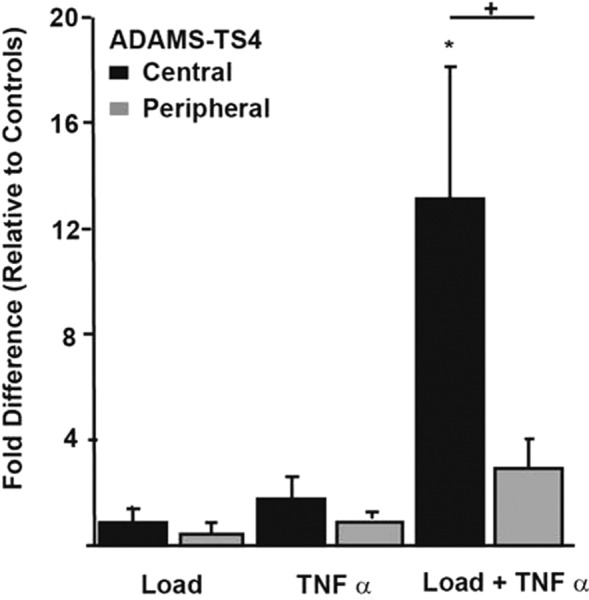
Relative expression of ADAM-TS4, stratified by region. * indicates significant difference relative to free-swelling controls and + indicates significant regional differences (p < 0.05).
There were also significant regional differences in the response to isolated Load and isolated TNF-α. In particular, there was a strong regional effect of Load on the expression of type II collagen, aggrecan, and MMP3 with significantly greater upregulation of these genes in central region explants compared with peripheral region explants (p = 0.015, p = 0.010, p = 0.020, respectively), (Figs. 5 and 6). While isolated Load was not a significant factor in the overall expression of MMP3 (p = 0.27), there was a significant upregulation of MMP3 in central region explants that was not observed in peripheral region explants when subjected to the isolated load conditions (Fig. 6). The upregulation of MMP1 by TNF-α treatment also exhibited a region-dependent response (Fig. 6). Specifically, the increase in MMP1 mRNA level was significantly greater in peripheral region explants compared with central region explants when subjected to TNF-α in the absence of dynamic unconfined compression (p = 0.037).
Fig. 5.

Relative expression levels of CII (left) and aggrecan (right) stratified by region. * indicates significant difference from free-swelling controls and + indicates significant regional differences in the upregulation of mRNA (p < 0.05).
Fig. 6.
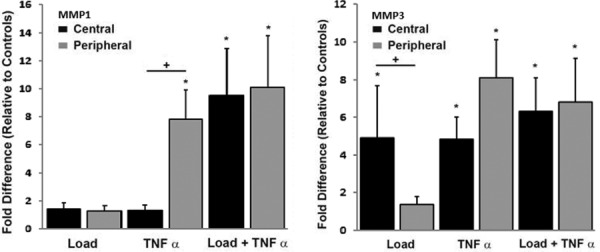
Relative expression of MMP1 (left) and MMP3 (right) stratified by region. * indicates significant difference from free-swelling controls, + indicates significant regional differences in the upregulation of mRNA.
Cartilage Thickness and Mechanical Loading Response.
Tissue thickness was significantly greater in central (1.41 ± 0.04 mm) compared with peripheral region (1.05 ± 0.09 mm) explants (p = 0.011). Cyclic strain (on the order of 2–3%) did not differ significantly between regions at any time during the loading, but creep strain was markedly greater in peripheral compared with central region explants. Creep strains after 600 s of loading were 5.8% ± 2.5% and 13.5% ± 2.0% in central and peripheral regions, respectively (p = 0.052); after 6 h, creep strains rose to 10.8% ± 2.9% and 19.3% ± 1.2% in central and peripheral regions, respectively (p = 0.037).
Discussion
The results of this study support the hypothesis that there is an altered response in the gene expression of chondrocytes to a combined application of dynamic unconfined compression and an exogenous proinflammatory cytokine stimulus relative to the response to isolated application of load or isolated proinflammatory cytokine stimuli alone. In addition, the finding that chondrocytes in the central regions respond differently than chondrocytes from peripheral regions to the same stimuli suggests that there may be important variations in the local regional biological and mechanical cartilage properties that can influence cartilage health. It is also important to note that applying a mechanical load stimulus in the absence of an exogenous proinflammatory cytokine produced an anabolic response while addition of TNF-α resulted in upregulation of both anabolic and catabolic markers. Thus conditions that produce elevated levels of proinflammatory cytokines in combination with altered mechanical loads in vivo may represent a stimulus for extracellular matrix turnover. Given the low adaptation potential of mature articular cartilage, such a stimulus might then lead to cartilage degeneration and OA [15].
The interactive effects of mechanical stimuli and cytokines on articular cartilage have been investigated in in vivo [32] and in vitro studies [24,25,33] and the interaction may be dependent on the duration and magnitude of mechanical loading. Continuous passive motion, a low-magnitude load stimulus, attenuated cartilage degradation relative to immobilization in an in vivo model of ag-induced arthritis in rabbits [32]. In that investigation, interleukin-1 beta expression was lowest at late time points in the study, suggesting that the suppressive effects of continuous passive motion on catabolic activity might be cumulative and time-dependent [32]. Importantly, the two-week time course of the study by Ferretti et al. [32] was much longer than the time course of this study. Further, low levels of in vitro dynamic compression seem to inhibit the catabolic effects of cytokines on articular chondrocytes [24,25,33], but a loading threshold may exist above which the protective effects of mechanical load are not observed [33]. Consistent with the findings of Li et al. [33], dynamic unconfined compression was observed to upregulate CII and aggrecan expression relative to unloaded samples treated with TNF-α and TNF-α treatment was observed to upregulate ADAM-TS expression even in the presence of dynamic compression. Thus, it appears that the interaction between mechanical and biochemical stimuli is complex and it may be an oversimplification to view mechanical loading simply as an anticatabolic stimulus.
The results of this study may have implications for understanding conditions associated with increased risk for knee OA. For example, conditions such as soft tissue trauma (e.g., ACL, meniscus) and obesity are associated with elevated levels of proinflammatory cytokines [7–9,11] as well as alteration in ambulatory joint mechanics [6,14,15,32]. Thus, the results showing increased expression of matrix proteases (MMP1, ADAMTS4, and ADAM-TS5) to the combined load and TNF-α stimulus helps provide a basis for understanding how specific biological and mechanical conditions converge to elevate the risk for OA. Perhaps the most striking catabolic regional effect was in response to the combined Load + TNF-α stimulus with a significantly greater upregulation of the aggrecanases ADAM-TS4 in central region relative to the peripheral region. Regional responses were also observed in response to the isolated TNF-α stimuli for MMP1 and to the isolated load condition for MMP3. These combined regional catabolic responses may represent a pathway to a breakdown of predominant regional structural proteins. The upregulation of the aggrecanase ADAM-TS4 was greater in central region cartilage where PG concentration is greater [34–37] and upregulation of the collagenase MMP1 was greater in peripheral region cartilage where the CII network appears to be a more vital component of the matrix mechanical function [38].
The regional sensitivity in both the catabolic and anabolic response also provides important insight into the phenotypic variations in knee cartilage morphology that suggest both the biological and mechanical properties are highly conditioned to the local mechanical environment. The thicker cartilage in the central region relative to the thinner cartilage peripheral regions [14,39,40] are consistent with the central region experiencing greater load and the findings of increased expression of CII and aggrecan in response to load. Also these findings support reports that kinematic changes associated with soft tissue changes at the knee can initiate a degenerative pathway as these changes can shift loading to regions that cannot adapt to the altered loading [39,41]. The decreased cartilage thickness reported [39,41] in regions where kinematic changes moved loads away from a specific area is consistent with the findings in this study that expression of CII and aggrecan occurs response to load irrespective of levels of exogenous TNF-α. These observation are also consistent with previous reports that dynamic unconfined compression was a more potent regulator of CII and aggrecan expression than proinflammatory cytokines [25].
It is possible that differences in matrix mechanics might have accounted for the regional differences in response to dynamic unconfined compression, as application of equivalent magnitude (stress-controlled) dynamic compression resulted in different creep strains in central and peripheral region explants [16]. It should also be noted that the suppressive effect of static compression on the expression [42,43] and synthesis [44–49] of structural proteins by chondrocytes has been well-documented, and may well provide a partial explanation for the regional variations in response to equivalent mechanical loading. That is, the greater upregulation of both CII and aggrecan in central region compared with peripheral region cartilage explants may simply reflect the lesser creep consolidation experienced by those samples under dynamic unconfined compression, suggesting that the regional differences in gene expression might reflect the regional differences in mechanical properties as well as thickness.
The goal of this study was to examine chondrocyte gene expression in the presence or absence of TNF-α rather than explore a dose effect. Although diffusion into the cartilage explants was not quantified, boundary conditions were similar between treatment groups and the differences in gene expression between treatment groups suggest that the treatment time was sufficient for transport of TNF-α into the cartilage samples. One question becomes whether dynamic unconfined compression served to either impede or enhance transport of the TNF-α protein into the tissue relative to the unloaded condition. If dynamic unconfined compression only affected transport of the TNF-α molecule but did not actually have an interactive effect, then it would be expected that expression of all genes affected by TNF-α treatment alone would be uniformly increased or decreased in the Load + TNF-α treatment group. However, increased expression of only three of the six genes that were upregulated by TNF-α alone was observed in the Load + TNF-α group (MMP1, ADAM-TS5, and TNF-α). Based on these findings it would be of interest to focus next on the dose-response relationship of exogenous TNF-α and mechanical loading on chondrocyte gene expression.
The results of this study should be considered in light of the experimental design. The TNF-α dosage applied in this study was greater than physiologic concentrations even following joint injury or in OA [7,8,10], although the duration of treatment was relatively short. Similar concentrations have been used in a number of other in vitro studies examining the effects of TNF-α on the chondrocyte [26,27]. The effects of TNF-α did not appear to vary significantly between applied concentrations ranging from 20 ng/ml to 100 ng/ml [27]. It was not the aim of this study to assess a dose effect but rather to assess the effects of the presence or absence of exogenous TNF-α. Thus the amount of TNF-α that penetrated into the cartilage implants was not quantified. However, given the consistency of the protocol for all samples and the use of both the Load only and TNF-α only controls in addition to the free-swelling control the comparison of the relative changes in expression levels between groups appears appropriate. Caution should also be exercised in the interpretation of the changes in gene expression levels as translational and posttranslational events as well as mechanisms involved in the activation of proenzymes make it difficult to correlate mRNA and protein level; however, the mRNA level may be indicative of differences in the factors that can initiate responses. Finally healthy cartilage was used in this study and some results might not reflect conditions in OA cartilage. Specifically, the lack of a significant influence on the expression of CII and aggregan in response to TNF-α in the absence of dynamic unconfined compression (Fig. 3) might reflect the difference between healthy and OA cartilage as described by Kobayashi et al. [50].
In conclusion, the response in the gene expression of chondrocytes to a combined application of dynamic unconfined compression and an exogenous proinflammatory cytokine stimulus highlights the importance of considering potential interactions between mechanical and biochemical factors in the joint. Taken together with the unique regional response that was observed, these results suggest a complex interaction between structure, biology, and mechanical loading that converge at a systems level in a manner that can influence cartilage health and breakdown. This supports the idea that a comprehensive approach [51] that integrates mechanics, structure, and biology at an in vivo systems level is needed to understand the complexity of OA.
Acknowledgment
This study was supported in part by the Office of Research and Development (Rehabilitation R&D Service), Department of Veterans Affairs Pre-Doctoral Associated Health Rehabilitation Research Fellowship and National Institutes of Health grant AR039421.
Contributor Information
S. L. Bevill, Mem. ASME , Department of Physical and , Environmental Sciences, , Colorado Mesa University, , 1100 North Avenue, , Grand Junction, CO 81501 , e-mail: sbevill@coloradomesa.edu .
K. A. Boyer, Department of Kinesiology, , University of Massachusetts-Amherst, , 110 Totman Building, , 30 Eastman Lane, , Amherst, MA 01003-9258 , e-mail: kboyer@kin.umass.edu
T. P. Andriacchi, Department of Mechanical Engineering, , Stanford University, , 227 Durand Building, , Stanford, CA 94305-4038; Department of Orthopedic Surgery, , Stanford University Medical Center, , 227 Durand Building, , Stanford, CA 94305-4038; Bone and Joint Center of Excellence, , Palo Alto, CA , e-mail: tandriac@stanford.edu
References
- [1]. Kannus, P. , and Jarvinen, M. , 1989, “Posttraumatic Anterior Cruciate Ligament Insufficiency as a Cause of Osteoarthritis in a Knee Joint,” Clin. Rheumatol, 8(2), pp. 251–260. 10.1007/BF02030082 [DOI] [PubMed] [Google Scholar]
- [2]. Lohmander, L. S. , Englund, P. M. , Dahl, L. L. , and Roos, E. M. , 2007, “The Long-Term Consequence of Anterior Cruciate Ligament and Meniscus Injuries: Osteoarthritis,” Am. J. Sports Med., 35(10), pp. 1756–1769. 10.1177/0363546507307396 [DOI] [PubMed] [Google Scholar]
- [3]. Roos, H. , Adalberth, T. , Dahlberg, L. , and Lohmander, L. S. , 1995, “Osteoarthritis of the Knee After Injury to the Anterior Cruciate Ligament or Meniscus: The Influence of Time and Age,” Osteoarthritis Cartilage, 3(4), pp. 261–267. 10.1016/S1063-4584(05)80017-2 [DOI] [PubMed] [Google Scholar]
- [4]. Andriacchi, T. P. , and Dyrby, C. O. , 2005, “Interactions Between Kinematics and Loading During Walking for the Normal and ACL Deficient Knee,” J. Biomech., 38(2), pp. 293–298. 10.1016/j.jbiomech.2004.02.010 [DOI] [PubMed] [Google Scholar]
- [5]. Li, G. , Moses, J. M. , Papannagari, R. , Pathare, N. P. , DeFrate, L. E. , and Gill, T. J. , 2006, “Anterior Cruciate Ligament Deficiency Alters the in Vivo Motion of the Tibiofemoral Cartilage Contact Points in Both the Anteroposterior and Mediolateral Directions,” J. Bone Jt. Surg. Am., 88(8), pp. 1826–1834. 10.2106/JBJS.E.00539 [DOI] [PubMed] [Google Scholar]
- [6]. Scanlan, S. F. , Chaudhari, A. M. , Dyrby, C. O. , and Andriacchi, T. P. , 2010, “Differences in Tibial Rotation During Walking in ACL Reconstructed and Healthy Contralateral Knees,” J. Biomech., 43(9), pp. 1817–1822. 10.1016/j.jbiomech.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Cameron, M. L. , Fu, F. H. , Paessler, H. H. , Schneider, M. , and Evans, C. H. , 1994, “Synovial Fluid Cytokine Concentrations as Possible Prognostic Indicators in the ACL-Deficient Knee,” Knee Surg. Sports Traumatol. Arthroscopy, 2(1), pp. 38–44. 10.1007/BF01552652 [DOI] [PubMed] [Google Scholar]
- [8]. Cameron, M. , Buchgraber, A. , Passler, H. , Vogt, M. , Thonar, E. , and Fu, F. , 1997, “The Natural History of the Anterior Cruciate Ligament-Deficient Knee. Changes in Synovial Fluid Cytokine and Keratan Sulfate Concentrations,” Am. J. Sports Med., 25(6), pp. 751–754. 10.1177/036354659702500605 [DOI] [PubMed] [Google Scholar]
- [9]. Dahlberg, L. , Friden, T. , Roos, H. , Lark, M. W. , and Lohmander, L. S. , 1994, “A Longitudinal Study of Cartilage Matrix Metabolism in Patients With Cruciate Ligament Rupture—Synovial Fluid Concentrations of Aggrecan Fragments, Stromelysin-1 and Tissue Inhibitor of Metalloproteinase-1,” Br. J. Rheumatol., 33(12), pp. 1107–1111. 10.1093/rheumatology/33.12.1107 [DOI] [PubMed] [Google Scholar]
- [10]. Irie, K. , Uchiyama, E. , and Iwaso, H. , 2003, “Intraarticular Inflammatory Cytokines in Acute Anterior Cruciate Ligament Injured Knee,” Knee, 10(1), pp. 93–96. 10.1016/S0968-0160(02)00083-2 [DOI] [PubMed] [Google Scholar]
- [11]. Higuchi, H. , Shirakura, K. , Kimura, M. , Terauchi, M. , Shinozaki, T. , and Watanabe, H. , 2006, “Changes in Biochemical Parameters After Anterior Cruciate Ligament Injury,” Int. Orthop., 30(1), pp. 43–47. 10.1007/s00264-005-0023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Marks, P. H. , and Donaldson, M. L. , 2005, “Inflammatory Cytokine Profiles Associated With Chondral Damage in the Anterior Cruciate Ligament-Deficient Knee,” Arthroscopy, 21(11), pp. 1342–1347. 10.1016/j.arthro.2005.08.034 [DOI] [PubMed] [Google Scholar]
- [13]. Andriacchi, T. P. , Mundermann, A. , Smith, R. L. , Alexander, E. J. , Dyrby, C. O. , and Koo, S. , 2004, “A Framework for the in Vivo Pathomechanics of Osteoarthritis at the Knee,” Ann. Biomed. Eng., 32(3), pp. 447–457. 10.1023/B:ABME.0000017541.82498.37 [DOI] [PubMed] [Google Scholar]
- [14]. Andriacchi, T. P. , Koo, S. , and Scanlan, S. F. , 2009, “Gait Mechanics Influence Healthy Cartilage Morphology and Osteoarthritis of the Knee,” J. Bone Jt. Surg. Am., 91(Suppl. 1), pp. 95–101. 10.2106/JBJS.H.01408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Chaudhari, A. M. , Briant, P. L. , Bevill, S. L. , Koo, S. , and Andriacchi, T. P. , 2008, “Knee Kinematics, Cartilage Morphology, and Osteoarthritis After ACL Injury,” Med. Sci. Sports Exercise, 40(2), pp. 215–222. 10.1249/mss.0b013e31815cbb0e [DOI] [PubMed] [Google Scholar]
- [16]. Bevill, S. L. , Briant, P. L. , Levenston, M. E. , and Andriacchi, T. P. , 2009, “Central and Peripheral Region Tibial Plateau Chondrocytes Respond Differently to in Vitro Dynamic Compression,” Osteoarthritis Cartilage, 17(8), pp. 980–987. 10.1016/j.joca.2008.12.005 [DOI] [PubMed] [Google Scholar]
- [17]. Dozin, B. , Malpeli, M. , Camardella, L. , Cancedda, R. , and Pietrangelo, A. , 2002, “Response of Young, Aged and Osteoarthritic Human Articular Chondrocytes to Inflammatory Cytokines: Molecular and Cellular Aspects,” Matrix Biol., 21(5), pp. 449–459. 10.1016/S0945-053X(02)00028-8 [DOI] [PubMed] [Google Scholar]
- [18]. Saklatvala, J. , 1986, “Tumour Necrosis Factor Alpha Stimulates Resorption and Inhibits Synthesis of Proteoglycan in Cartilage,” Nature, 322(6079), pp. 547–549. 10.1038/322547a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Lefebvre, V. , Peeters-Joris, C. , and Vaes, G. , 1990, “Modulation by Interleukin 1 and Tumor Necrosis Factor Alpha of Production of Collagenase, Tissue Inhibitor of Metalloproteinases and Collagen Types in Differentiated and Dedifferentiated Articular Chondrocytes,” Biochim. Biophys. Acta, 1052(3), pp. 366–378. 10.1016/0167-4889(90)90145-4 [DOI] [PubMed] [Google Scholar]
- [20]. Reginato, A. M. , Sanz-Rodriguez, C. , Diaz, A. , Dharmavaram, R. M. , and Jimenez, S. A. , 1993, “Transcriptional Modulation of Cartilage-Specific Collagen Gene Expression by Interferon Gamma and Tumour Necrosis Factor Alpha in Cultured Human Chondrocytes,” Biochem. J., 294(Pt. 3), pp. 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Saklatvala, J. , and Bird, T. , 1986, “A Common Class of Receptors for the Two Types of Porcine Interleukin-1 on Articular Chondrocytes,” Lymphokine Res., 5(Suppl. 1), pp. S99–S104. [PubMed] [Google Scholar]
- [22]. Campbell, I. K. , Piccoli, D. S. , Roberts, M. J. , Muirden, K. D. , and Hamilton, J. A. , 1990, “Effects of Tumor Necrosis Factor Alpha and Beta on Resorption of Human Articular Cartilage and Production of Plasminogen Activator by Human Articular Chondrocytes,” Arthritis Rheum., 33(4), pp. 542–552. 10.1002/art.1780330412 [DOI] [PubMed] [Google Scholar]
- [23]. Bunning, R. A. , and Russell, R. G. , 1989, “The Effect of Tumor Necrosis Factor Alpha and Gamma-Interferon on the Resorption of Human Articular Cartilage and on the Production of Prostaglandin E and of Caseinase Activity by Human Articular Chondrocytes,” Arthritis Rheum., 32(6), pp. 780–784. 10.1002/anr.1780320618 [DOI] [PubMed] [Google Scholar]
- [24]. Chowdhury, T. T. , Bader, D. L. , and Lee, D. A. , 2006, “Dynamic Compression Counteracts IL-1Beta Induced iNOS and COX-2 Activity by Human Chondrocytes Cultured in Agarose Constructs,” Biorheology, 43(3–4), pp. 413–429. [PubMed] [Google Scholar]
- [25]. Torzilli, P. A. , Bhargava, M. , Park, S. , and Chen, C. T. , 2010, “Mechanical Load Inhibits IL-1 Induced Matrix Degradation in Articular Cartilage,” Osteoarthritis Cartilage, 18(1), pp. 97–105. 10.1016/j.joca.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Patwari, P. , Cook, M. N. , DiMicco, M. A. , Blake, S. M. , James, I. E. , and Kumar, S. , 2003, “Proteoglycan Degradation After Injurious Compression of Bovine and Human Articular Cartilage in Vitro: Interaction With Exogenous Cytokines,” Arthritis Rheum., 48(5), pp. 1292–1301. 10.1002/art.10892 [DOI] [PubMed] [Google Scholar]
- [27]. Kuroki, K. , Stoker, A. M. , and Cook, J. L. , 2005, “Effects of Proinflammatory Cytokines on Canine Articular Chondrocytes in a Three-Dimensional Culture,” Am. J. Vet. Res., 66(7), pp. 1187–1196. 10.2460/ajvr.2005.66.1187 [DOI] [PubMed] [Google Scholar]
- [28]. Fermor, B. , Weinberg, J. B. , Pisetsky, D. S. , Misukonis, M. A. , Banes, A. J. , and Guilak, F. , 2001, “The Effects of Static and Intermittent Compression on Nitric Oxide Production in Articular Cartilage Explants,” J. Orthop. Res, 19(4), pp. 729–737. 10.1016/S0736-0266(00)00049-8 [DOI] [PubMed] [Google Scholar]
- [29]. Fermor, B. , Haribabu, B. , Weinberg, J. B. , Pisetsky, D. S. , and Guilak, F. , 2001, “Mechanical Stress and Nitric Oxide Influence Leukotriene Production in Cartilage,” Biochem. Biophys. Res. Commun., 285(3), pp. 806–810. 10.1006/bbrc.2001.5237 [DOI] [PubMed] [Google Scholar]
- [30]. Bau, B. , Gebhard, P. M. , Haag, J. , Knorr, T. , Bartnik, E. , and Aigner, T. , 2002, “Relative Messenger RNA Expression Profiling of Collagenases and Aggrecanases in Human Articular Chondrocytes in Vivo and in Vitro,” Arthritis Rheum., 46(10), pp. 2648–2657. 10.1002/art.10531 [DOI] [PubMed] [Google Scholar]
- [31]. Livak, K. J. , and Schmittgen, T. D. , 2001, “Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method,” Methods, 25(4), pp. 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- [32]. Ferretti, M. , Gassner, R. , Wang, Z. , Perera, P. , Deschner, J. , Sowa, G. , Salter, R. B. , and Agarwal, S. , 2006, “Biomechanical Signals Suppress Proinflammatory Responses in Cartilage: Early Events in Experimental Antigen-Induced Arthritis,” J. Immunol., 177, pp. 8757–8766. 10.4049/jimmunol.177.12.8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Li, Y. , Frank, E. H. , Wang, Y. , Chubinskaya, S. , Huang, H. H. , and Grodzinsky, A. J. , 2013, “Moderate Dynamic Compression Inhibits Pro-Catabolic Response of Cartilage to Mechanical Injury, Tumor Necrosis Factor-α and Interleukin-6, but Accentuates Degradation Above a Strain Threshold,” Osteoarthritis Cartilage, 21, pp. 1933–1941. 10.1016/j.joca.2013.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Noyes, F. R. , Schipplein, O. D. , Andriacchi, T. P. , Saddemi, S. R. , and Weise, M. , 1992, “The Anterior Cruciate Ligament-Deficient Knee With Varus Alignment. An Analysis of Gait Adaptations and Dynamic Joint Loadings,” Am. J. Sports Med., 20(6), pp. 707–716. 10.1177/036354659202000612 [DOI] [PubMed] [Google Scholar]
- [35]. Little, C. B. , Ghosh, P. , and Bellenger, C. R. , 1996, “Topographic Variation in Biglycan and Decorin Synthesis by Articular Cartilage in the Early Stages of Osteoarthritis: An Experimental Study in Sheep,” J. Orthop. Res., 14(3), pp. 433–444. 10.1002/jor.1100140314 [DOI] [PubMed] [Google Scholar]
- [36]. Little, C. B. , and Ghosh, P. , 1997, “Variation in Proteoglycan Metabolism by Articular Chondrocytes in Different Joint Regions is Determined by Post-Natal Mechanical Loading,” Osteoarthritis Cartilage, 5(1), pp. 49–62. 10.1016/S1063-4584(97)80031-3 [DOI] [PubMed] [Google Scholar]
- [37]. Appleyard, R. C. , Burkhardt, D. , Ghosh, P. , Read, R. , Cake, M. , and Swain, M. V. , 2003, “Topographical Analysis of the Structural, Biochemical and Dynamic Biomechanical Properties of Cartilage in an Ovine Model of Osteoarthritis,” Osteoarthritis Cartilage, 11(1), pp. 65–77. 10.1053/joca.2002.0867 [DOI] [PubMed] [Google Scholar]
- [38]. Briant, P. L. , 2008, “Structural Variations in Cartilage Make it Sensitive to Shifts in Joint Kinematics,” Ph.D. thesis, Stanford University, Stanford, CA.
- [39]. Koo, S. , Rylander, J. H. , and Andriacchi, T. P. , 2001, “Knee Joint Kinematics During Walking Influences the Spatial Cartilage Thickness Distribution in the Knee,” J. Biomech., 44(7), pp. 1405–1409. 10.1016/j.jbiomech.2010.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Li, G. , Park, S. E. , DeFrate, L. E. , Schutzer, M. E. , Ji, L. , and Gill, T. J. , 2005, “The Cartilage Thickness Distribution in the Tibiofemoral Joint and Its Correlation With Cartilage-to-Cartilage Contact,” Clin. Biomech. (Bristol, Avon), 20(7), pp. 736–744. 10.1016/j.clinbiomech.2005.04.001 [DOI] [PubMed] [Google Scholar]
- [41]. Andriacchi, T. P. , and Mundermann, A. , 2006, “The Role of Ambulatory Mechanics in the Initiation and Progression of Knee Osteoarthritis,” Curr. Opin. Rheumatol., 18(5), pp. 514–518. 10.1097/01.bor.0000240365.16842.4e [DOI] [PubMed] [Google Scholar]
- [42]. Ragan, P. M. , Badger, A. M. , Cook, M. , Chin, V. I. , Gowen, M. , and Grodzinsky, A. J. , 1999, “Down-Regulation of Chondrocyte Aggrecan and Type-II Collagen Gene Expression Correlates With Increases in Static Compression Magnitude and Duration,” J. Orthop. Res., 17(6), pp. 836–842. 10.1002/jor.1100170608 [DOI] [PubMed] [Google Scholar]
- [43]. Leipzig, N. D. , and Athanasiou, K. , 2008, “Static Compression of Single Chondrocytes Catabolically Modifies Single Cell Gene Expression,” J. Biophys., 94(6), pp. 2412–2422. 10.1529/biophysj.107.114207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Torzilli, P. A. , Grigiene, R. , Huang, C. , Friedman, S. M. , Doty, S. B. , and Boskey, A. L. , 1997, “Characterization of Cartilage Metabolic Response to Static and Dynamic Stress Using a Mechanical Explant Test System,” J. Biomech., 30(1), pp. 1–9. 10.1016/S0021-9290(96)00117-0 [DOI] [PubMed] [Google Scholar]
- [45]. Palmoski, M. J. , and Brandt, K. D. , 1984, “Effects of Static and Cyclic Compressive Loading on Articular Cartilage Plugs in Vitro,” Arthritis Rheumtol., 27(6), pp. 675–681. 10.1002/art.1780270611 [DOI] [PubMed] [Google Scholar]
- [46]. Sah, R. L. , Kim, Y. J. , Doong, J. Y. , Grodzinsky, A. J. , Plaas, A. H. , and Sandy, J. D. , 1989, “Biosynthetic Response of Cartilage Explants to Dynamic Compression,” J. Orthop. Res., 7(5), pp. 619–636. 10.1002/jor.1100070502 [DOI] [PubMed] [Google Scholar]
- [47]. Gray, M. L. , Pizzanelli, A. M. , Grodzinsky, A. J. , and Lee, R. C. , 1988, “Mechanical and Physiochemical Determinants of the Chondrocyte Biosynthetic Response,” J. Orthop. Res., 6(6), pp. 777–792. 10.1002/jor.1100060602 [DOI] [PubMed] [Google Scholar]
- [48]. Wong, M. , Siegrist, M. , and Cao, X. , 1999, “Cyclic Compression of Articular Cartilage Explants is Associated With Progressive Consolidation and Altered Expression Pattern of Extracellular Matrix Proteins,” Matrix Biol., 18(4), pp. 391–399. 10.1016/S0945-053X(99)00029-3 [DOI] [PubMed] [Google Scholar]
- [49]. Li, K. W. , Williamson, A. K. , Wang, A. S. , and Sah, R. L. , 2001, “Growth Responses of Cartilage to Static and Dynamic Compression,” Clin. Orthop. Relat. Res., 391(Suppl. 1), pp. S34–S48. 10.1097/00003086-200110001-00005 [DOI] [PubMed] [Google Scholar]
- [50]. Kobayashi, M. , Squires, G. R. , Mousa, A. , Tanzer, M. , Zukor, D. J. , and Antoniou, J. , 2005, “Role of Interleukin-1 and Tumor Necrosis Factor Alpha in Matrix Degradation of Human Osteoarthritic Cartilage,” Arthritis. Rheum., 52(1), pp. 128–135. 10.1002/art.20776 [DOI] [PubMed] [Google Scholar]
- [51]. Andriacchi, T. P. , 2012, “Osteoarthritis: Probing Knee OA as a System Responding to a Stimulus,” Nat. Rev. Rheumatol., 8(7), pp. 371–372. 10.1038/nrrheum.2012.59 [DOI] [PubMed] [Google Scholar]


