Abstract
Background
Circulating tumor cells (CTCs) have been shown to predict reduced survival outcomes in metastatic breast cancer.
Methods
CTCs were analyzed in 2026 patients with early breast cancer before adjuvant chemotherapy and in 1492 patients after chemotherapy using the CellSearch System. After immuno-magnetic enrichment for cells expressing the epithelial-cell adhesion molecule, CTCs were defined as nucleated cells expressing cytokeratin and lacking CD45. The patients were followed for a median of 35 months (range = 0–54). Kaplan–Meier analyses and the log-rank test were used for survival analyses. All statistical tests were two-sided.
Results
Before chemotherapy, CTCs were detected in 21.5% of patients (n = 435 of 2026), with 19.6% (n = 136 of 692) of node-negative and 22.4% (n = 299 of 1334) of node-positive patients showing CTCs (P < .001). No association was found with tumor size, grading, or hormone receptor status. After chemotherapy, 22.1% of patients (n = 330 of 1493) were CTC positive. The presence of CTCs was associated with poor disease-free survival (DFS; P < .0001), distant DFS (P < .001), breast cancer-specific survival (P = .008), and overall survival (OS; P = .0002). CTCs were confirmed as independent prognostic markers in multivariable analysis for DFS (hazard ratio [HR] = 2.11; 95% confidence interval [CI] = 1.49 to 2.99; P < .0001) and OS (HR = 2.18; 95% CI = 1.32 to 3.59; P = .002). The prognosis was worst in patients with at least five CTCs per 30mL blood (DFS: HR = 4.51, 95% CI = 2.59 to 7.86; OS: HR = 3.60, 95% CI = 1.56 to 8.45). The presence of persisting CTCs after chemotherapy showed a negative influence on DFS (HR = 1.12; 95% CI = 1.02 to 1.25; P = .02) and on OS (HR = 1.16; 95% CI = 0.99 to 1.37; P = .06)
Conclusions
These results suggest the independent prognostic relevance of CTCs both before and after adjuvant chemotherapy in a large prospective trial of patients with primary breast cancer.
The prognostic relevance of disseminated tumor cells (DTCs) in the bone marrow of patients with early breast cancer has been confirmed with the highest level of evidence. A pooled analysis of 4703 patients reported poor outcomes in patients with DTCs before the initiation of primary therapy (1), and 726 patients with persistent DTCs during recurrence-free follow-up showed an increased risk for distant relapse and a shortened overall survival (OS) (2). Based on these results, it was hypothesized that DTCs may underlie subsequent metastatic spread (3).
Increasing evidence suggests that circulating tumor cells (CTCs) in the peripheral blood are associated with reduced progression-free survival and OS in metastatic disease (4–8). Whereas the detection of CTCs before the start of a new treatment has been associated with poor prognosis, the enumeration of CTCs shortly after the initiation of therapy provides additional information regarding treatment response (4,7).
Although conclusive data for the prognostic relevance of CTCs are available for metastatic disease, only a few prospective trials in smaller patient cohorts have been performed for early breast cancer that suggest the prognostic relevance for CTC detection (9–16). In the SUCCESS (Simultaneous Study of Gemcitabine-Docetaxel Combination adjuvant treatment, as well as Extended Bisphosphonate and Surveillance-Trial) trial (EUDRA-CT No. 2005-000490-21), CTCs were statistically significantly associated with node-positive disease. The presence of CTCs both before the start of systemic adjuvant treatment and after completion of chemotherapy was associated with deteriorated survival. Prognostic relevance independent of lymph node metastases was confirmed in multivariable analysis.
Methods
Patients
Eligible patients were defined as women with breast cancer (stages pT1–T4, pN0–N3, M0) who agreed to participate in the phase III SUCCESS study. SUCCESS was a prospective, randomized adjuvant study comparing three cycles of fluorouracil-epirubicin-cyclophosphamide (FEC; 500/100/500mg/m2) followed by 3 cycles of docetaxel (100mg/m2) every 3 weeks vs three cycles of FEC followed by 3 cycles of gemcitabine (1000mg/m2 d1,8)-docetaxel (75mg/m2) every 3 weeks. After the completion of chemotherapy, the patients were further randomized to receive either 2 or 5 years of zoledronate. Hormone receptor–positive women received adequate endocrine treatment. The research questions associated with CTC analysis, the blood sampling time points, and the methodology were prospectively designed, and the prognostic value of the CTCs was defined as a scientific objective of the study protocol. The study was approved by 37 German ethical boards (lead ethical board: Ludwig-Maximilians-University Munich) and conducted in accordance with the Declaration of Helsinki.
Blood samples for CTC enumeration were collected from 2090 consecutive patients after complete resection of the primary tumor and before adjuvant chemotherapy after written informed consent was obtained. Sixty-four patients were excluded because of test failure or a time interval of more than 96 hours between the blood collection and sample preparation. A follow-up evaluation after chemotherapy and before the start of endocrine or bisphosphonate treatment was available for a subgroup of 1492 patients (Supplementary Figure 1, available online).
The primary surgery consisted of either breast conservation (n = 1414 of 2012; 70.3%) or mastectomy (n = 598 of 2012; 29.7%) leading to R0 resection in all case patients. Sentinel node dissection was performed in all cN0 patients (sentinel node dissection as the only axillary intervention; n = 692 of 2026; 34.2%) followed by complete axillary node dissection in case patients with positive sentinel nodes. The cN1 patients primarily received axillary node dissection (n = 1334 of 2026; 65.8%). Radiotherapy was performed according to national guidelines (17–19) and was used in all case patients that received breast-conserving treatment.
Preparation of Blood Samples and Detection of CTCs
CTCs were analyzed using the CellSearch System (Veridex, Raritan, NJ). Peripheral blood was drawn into three CellSave tubes (30mL), shipped at room temperature to the central laboratory at the University of Munich, and analyzed within 96 hours of collection.
The samples were centrifuged for 10 minutes at 800 × g. The plasma was removed, and a dilution buffer was added. This mixture was overlaid on 6mL of Histopaque (Sigma, Steinheim, Germany) and centrifuged for 10 minutes at 400 × g. Subsequently, 7.5mL of this sample containing the buffy coat was processed on the CellTracks AutoPrep system using the CellSearch Epithelial Cell Kit (Veridex). After immuno-magnetic enrichment with an anti-Epcam antibody, the cells were labeled with fluorescent anticytokeratin (CK8,18,19–phycoerythrin) and anti-CD45 antibodies (CD45–allophycocyan), and 4,6-diamidino-2-phenylindoledihydrochloride was used to detect the intact cells.
The identification and enumeration of CTCs were performed using the CellTracks Analyzer II. CTCs were defined as nucleated cells lacking CD45 and expressing cytokeratin. All positive samples were reviewed by two independent investigators. The samples with at least one CTC per 30mL of blood were regarded as CTC positive.
The blood from 84 individuals with no clinical evidence of malignant disease was processed blinded and used as a negative control. Four of these negative controls (4.9%) included cells that fit the definition of epithelial cells and could be interpreted as CTCs (one control had one epithelial cell, two controls had two, and one control had three epithelial cells).
Follow-up and Patient Evaluation
The median follow-up was 35 months (range = 0–54 months). The patients were followed at the study sites at 3-month intervals for the first 3 years and every 6 months thereafter. Follow-up included clinical examination (each visit), mammography (every 6 months), and symptom-driven examinations if necessary. All data were obtained from the electronic case record forms of the SUCCESS study. The quality of the data was ensured by electronic data management, including automated plausibility checks and regular monitoring visits to the study site by an independent clinical research organization (Alcedis, Gießen, Germany).
Statistical Analyses
The endpoints were defined according to the STEEP criteria, with disease-free survival (DFS) as the primary endpoint (20). The product-limit method according to Kaplan–Meier was used to estimate survival (21). The survival estimates in different groups were compared using the log-rank test. The Cox proportional hazards regression model was used for the analyses taking into account all variables simultaneously (22). The assumption of proportional hazards was checked by plotting the log(-log(S(t)) against time on study. In both endpoints, OS and DFS, the lines were parallel and no influence of time could be seen.
The χ2 and Cochran–Armitage tests for trends in cases of more than two categories were used to analyze and compare frequencies for categorical variables. Continuous variables were compared using a t test. P less than .05 was considered significant in two-sided tests. No adjustment of the error probability for multiple testing was performed. SAS software,version 8.02 (SAS Institute, Cary, NC) was used.
Results
Prevalence of CTCs in Early Breast Cancer
Patient characteristics of 2026 patients with primary breast cancer are shown in Table 1. CTCs were detected in 21.5% of the patients (n = 435 of 2026) after the complete resection of the primary tumor and before the start of systemic treatment (median = 1.0 cell; range = 0–827 per 30mL of blood). The patients with lymph node metastases were statistically significantly more often CTC-positive than node-negative patients. The frequency of CTC positive patients was 19.6% (n = 136 of 692) in the N0 group and 22.4% (n = 299 of 1334) in the N1 to N3 group (P < .001), whereas the presence of any CTC was not statistically significantly associated with other clinico-pathological characteristics or local and systemic treatment. High CTC numbers of five or more were more frequent in postmenopausal patients (P = .02) (Table 1).
Table 1.
Patient characteristics at baseline for circulating tumor cell count before chemotherapy (n = 2026)*
| Characteristic | CTC ≥ 1† No. (%) | CTC = 0† No. (%) | P | CTC ≥ 5† No. (%) | CTC = 0–4† No. (%) | P |
|---|---|---|---|---|---|---|
| No. of patients | 435 (21.5) | 1591 (78.5) | 63 (3.1) | 1963 (96.9) | ||
| Age in years (mean ± SD) | 53.8±10.3 | 53.2±10.5 | .26‡ | 55.03+9.87 | 53.30+10.52 | .19‡ |
| Tumor size¶ | ||||||
| pT1a | 1 (0.2) | 16 (1.0) | .19§ | 0 (0) | 17 (0.8) | .31§ |
| pT1b | 19 (4.4) | 86 (5.4) | 3 (4.8) | 102 (5.2) | ||
| pT1c | 139 (32.0) | 561 (35.3) | 20(31.8) | 680 (34.6) | ||
| pT2–4 | 268 (61.6) | 906 (56.9) | 40 (63.5) | 1134 (57.8) | ||
| pTx | 7 (1.6) | 22 (1.4) | 0 (0) | 29 (1.5) | ||
| Lymph node metastases¶ | ||||||
| Absent (pN0)/ pNX | 136 (31.3) | 556 (35.0) | <.001§ | 15 (23.8) | 659 (33.6) | <.001§ |
| 1–3 axillary (pN1) | 178 (40.9) | 747 (47.0) | 23 (36.5) | 921 (46.9) | ||
| 4–9 axillary (pN2) | 72 (16.5) | 208 (13.0) | 16 (25.4) | 257 (13.1) | ||
| ≥10 axillary (pN3) | 49 (11.3) | 80 (5.0) | 9 (14.3) | 126 (6.4) | ||
| Grading# | ||||||
| G1 | 14 (3.2) | 85 (5.3) | .19‡ | 1 (1.6) | 98 (5.0) | .12‡ |
| G2 | 206 (47.4) | 740 (46.5) | 37 (58.7) | 909 (46.3) | ||
| G3 | 212 (48.7) | 753 (47.3) | 25 (39.7) | 940 (47.9) | ||
| Gx | 3 (0.7) | 13 (0.8) | 0 (0) | 16 (0.8) | ||
| Hormone receptor status | ||||||
| Negative | 128 (29.4) | 450 (28.3) | .64ǁ | 13 (20.6) | 565 (28.8) | .16ǁ |
| Positive | 307 (70.6) | 1141 (71.7) | 50 (79.4) | 1398 (71.2) | ||
| Her2-neu status | ||||||
| Undefined | 10 (2.3) | 41 (2.6) | .54ǁ | 3 (4.8) | 48 (2.4) | .95ǁ |
| Negative | 322 (74.0) | 1152 (72.4) | 45 (71.4) | 1429 (72.8) | ||
| Positive | 103 (23.7) | 398 (25.0) | 15 (23.8) | 486 (24.8) | ||
| Histological type | ||||||
| Undefined | 12 (.8) | 2 (0.5) | .15§ | 0 (0) | 14 (0.7) | .13§ |
| Ductal | 344 (79.1) | 1285 (80.8) | 45 (71.4) | 1584 (80.7) | ||
| Lobular | 62 (14.3) | 176 (11.1) | 12 (19.0) | 226 (11.5) | ||
| Mixed ductal-lobular | 27 (6.2) | 118 (7.4) | 6 (9.5) | 139 (7.1) | ||
| Menopausal status | ||||||
| Premenopausal | 169 (38.9) | 672 (42.2) | .20ǁ | 17 (27.0) | 824 (42.0) | .02ǁ |
| Postmenopausal | 266 (61.1) | 919 (57.8) | 46 (73.0) | 1139 (58.0) | ||
| Primary operation | ||||||
| Breast conserving | 295 (67.8) | 1119 (70.3) | .27ǁ | 45 (71.4) | 1369 (69.7) | .84ǁ |
| Mastectomy | 138 (31.7) | 460 (28.9) | 18 (28.6) | 580 (29.5) | ||
| Radiotherapy | ||||||
| Performed | 341 (78.4) | 1211 (76.1) | .11ǁ | 46 (73.0) | 1506 (76.7) | .68ǁ |
| Not performed | 94 (21.6) | 380 (23.9) | 17 (27.0) | 457 (23.3) | ||
| Systemic therapy | ||||||
| Chemotherapy–FEC-D | 205 (47.1) | 820 (51.5) | .10ǁ | 26 (41.3) | 999 (50.9) | .13ǁ |
| Chemotherapy–FEC-DG | 230 (52.9) | 771 (48.5) | 37 (58.7) | 964 (49.1) | ||
| Endocrine treatment | 266 (61.2) | 967 (60.7) | .88ǁ | 32 (50.8) | 990 (50.4) | .78ǁ |
| Trastuzumab | 83 (19.4) | 329 (21.2) | .41ǁ | 9 (14.3) | 229 (11.7) | .52ǁ |
* CTC = circulating tumor cell; FEC-D = fluorouracil-epirubicin-cyclophosphamide (500/100/500 mg/m2, FEC) followed by docetaxel (100 mg/mg2); FEC-DG = fluorouracil-epirubicin-cyclophosphamide (500/100/500 mg/m2, FEC) followed by gemcitabine (1,000 mg/m2 d1,8)-docetaxel (75 mg/m2); SD = standard deviation.
† Per 30mL of blood.
‡ Two-sided t test.
§ Two-sided Cochran–Armitage test for trend.
ǁ Two-sided χ2 test.
¶ Tumor-node-metastasis (TNM) was classified according to the revised American Joint Committee on Cancer TNM classification (23).
# Histopathological grading of the primary tumors was performed according to Elston–Ellis (24).
CTC analysis after completion of adjuvant chemotherapy was performed in a subgroup of 1492 patients. At this time point, CTCs (median = 1 cell; range = 0–124 cells per 30mL of blood) were detected in 22.1% of the patients (n = 330 of 1493).There was no difference in CTC counts before and after chemotherapy (Supplementary Table 1, available online).
Prognostic Relevance of CTCs for DFS
One hundred fourteen patients (6%) relapsed, including 16 patients with locoregional disease and 98 patients with distant metastases. CTCs were detected in three patients (19%) with locoregional relapse and in 35 patients (30%) with distant metastases.
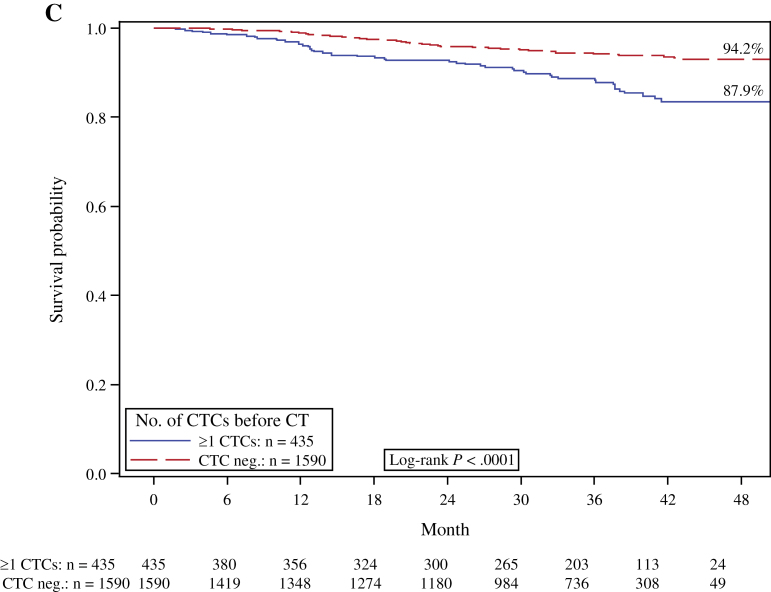
The disease-free probability at 36 months was 88.1% for patients with CTCs and 93.7% for patients without CTCs. The presence of CTCs was statistically significantly predictive of reduced DFS (log-rank test, P < .0001) (Figure 1A). The distant DFS at 36 months was 87.9% for CTC-positive patients and 94.2% for CTC-negative patients (log-rank test, P < .001).
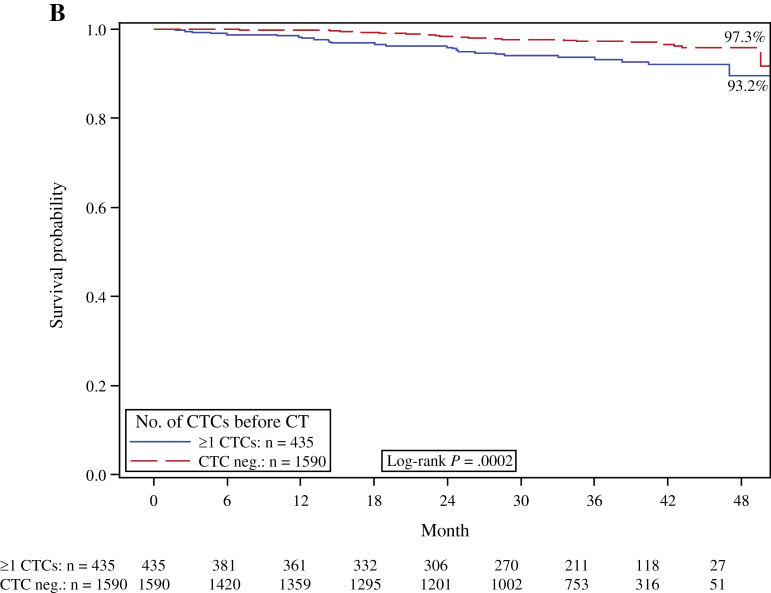
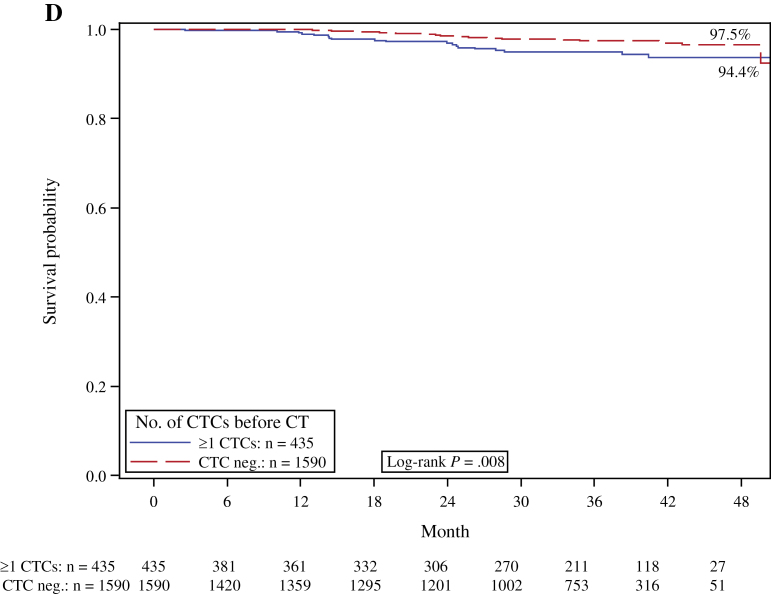
Figure 1.
Kaplan–Meier analysis according to the presence or absence (neg.) of peripheral blood circulating tumor cells (CTCs) before chemotherapy (CT). A) Disease-free survival. B) Overall survival. C) Distant disease-free survival. D) Breast cancer–specific survival. Two-sided log-rank test.
In the multivariable proportional hazards model, the presence of one or more CTCs was confirmed to be an independent prognostic factor for reduced DFS (hazard ratio [HR] = 2.11; 95% confidence interval [CI] = 1.49 to 2.99; P < .0001) in addition to negative hormone receptor status, lymph node involvement, unfavorable grading, and tumor size greater than 2cm (Table 2).
Table 2.
Univariate and multivariable proportional hazards model for disease-free survival for circulating tumor cell count before chemotherapy (n = 2026)*
| Variable | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| CTCs in blood, negative vs positive | 2.257 (1.595 to 3.195) | <.0001 | 2.107 (1.487 to 2.986) | <.0001 |
| Hormone receptor status, positive vs negative | 2.187 (1.559 to 3.066) | <.0001 | 1.972 (1.363 to 2.854) | .0003 |
| Lymph node involvement, N0 vs N1–3 | 1.780 (1.187 to 2.670) | .005 | 2.942 (1.922 to 4.505) | <.0001 |
| Grading, G1 vs G2–3 | 3.109 (2.124 to 4.551) | <.0001 | 3.254 (2.146 to 4.935) | <.0001 |
| Tumor size, T1 vs T2–4 | 2.205 (1.496 to 3.251) | <.0001 | 2.082 (1.405 to 3.083) | .0003 |
| Menopausal status, pre vs post | 1.221 (0.864 to 1.725) | .26 | 1.018 (0.717 to 1.445) | .92 |
| Histology, lobular/mixed vs ductal | 1.308 (0.822 to 2.083) | .26 | 0.931 (0.575 to 1.508) | .77 |
* Cox proportional hazards models. All statistical tests were two-sided. CI = confidence interval; CTC = circulating tumor cell; HR = hazard ratio.
In a subgroup analysis, the patients were stratified according to lymph node status. The presence of CTCs was associated with reduced DFS in all node-positive subgroups (ie, in patients with 1–3 [log-rank test, P = .008), 4–9 [log-rank test, P < .0001), and ≥10 involved lymph nodes [log-rank test, P = .001]), whereas no statistically significant difference was observed for DFS in node-negative patients (log-rank test, P = .23) (Supplementary Figure 2A, available online).
Prognostic Relevance of CTCs for Survival
Sixty-six patients died during follow-up, including 54 who died of breast cancer, and 12 patients who succumbed to other causes. The CTC positivity rate was 40.9% (n = 27 of 66) for the patients who died compared with 20.8% (n = 408 of 2026) for the patients who survived. The overall death rate and the breast cancer death rate were both statistically significantly higher in patients with CTCs. A total of 4.6% of the CTC-positive patients died of breast cancer compared with 2.2% of the CTC-negative patients. The Kaplan–Meier estimate for 36-month survival was 93.2% for CTC-positive patients and 97.3% for CTC-negative patients. The presence of CTCs was associated with reduced breast cancer–specific survival (log-rank test, P = .008) and OS (log-rank test, P = .0002) (Figure 1, D and B, respectively). In the multivariable analysis, CTC detection remained a statistically significant prognostic predictor of poor survival (HR = 2.18; 95% CI = 1.32 to 3.59; P = .002) (Table 3).
Table 3.
Univariate and multivariable proportional hazards model for overall survival for circulating tumor cell count before chemotherapy (n = 2026)*
| Variable | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR 95% CI | P | |
| CTCs in blood, negative vs positive | 2.447 (1.491 to 4.015) | .0004 | 2.177 (1.320 to 3.588) | .002 |
| Hormone receptor status, positive vs negative | 3.414 (2.098 to 5.556) | <.0001 | 2.997 (1.763 to 5.095) | <.0001 |
| Lymph node involvement, N0 vs N1–3 | 2.465 (1.290 to 4.709) | .006 | 4.254 (2.182 to 8.293) | <.0001 |
| Grading, G1 vs G2–3 | 4.097 (2.271 to 7.392) | <.0001 | 3.549 (1.864 to 6.760) | .0001 |
| Tumor size, T1 vs T2–4 | 2.969 (1.618 to 5.446) | .0004 | 2.665 (1.441 to 4.930) | .002 |
| Menopausal status, pre vs post | 1.990 (1.157 to 3.421) | .013 | 1.518 (0.876 to 2.629) | .14 |
| Histology, lobular/mixed vs ductal | 2.020 (0.923 to 4.423) | .08 | 1.262 (0.559 to 2.850) | .58 |
* Cox proportional hazards models. All statistical tests were two-sided. CI = confidence interval; CTC = circulating tumor cell; HR = hazard ratio.
Analysis of Different CTC Cutoff Values
An exploratory proportional hazard analysis was performed using several CTC levels as cutoffs to evaluate the influence of the cutoff on the hazard ratios of OS and DFS adjusted for standard risk factors and treatment. The patients were grouped and compared according to three different CTC cutoff values (0 vs ≥1; 0–1 vs ≥2; 0–4 vs ≥5 CTCs in 30mL of blood). DFS and OS were statistically significantly reduced in the group with the higher CTC levels for all three cutoff values (Table 4).
Table 4.
Multivariable proportional hazards model for disease-free survival and overall survival for different circulating tumor cell cutoff values*
| Variable | HRs (95% CI) adjusted for treatment | ||
|---|---|---|---|
| 0 vs ≥1 CTC per 30mL blood | 0–1 vs ≥2 CTC per 30mL blood | 0–4 vs ≥5 CTC per 30mL blood | |
| DFS | |||
| CTCs in blood, negative vs positive | 2.11† (1.487 to 2.986) | 3.19† (2.141 to 4.763) | 4.51† (2.586 to 7.864) |
| Hormone receptor status, positive vs negative | 1.97† (1.36 to 2.85) | 1.98† (1.366 to 2.861) | 1.98† (1.365 to 2.869) |
| Lymph node involvement, N0 vs N1–3 | 2.94† (1.92 to 4.51) | 2.77† (1.807 to 4.241) | 2.84† (1.859 to 4.349) |
| Grading, G1 vs G2–3 | 3.25† (2.15 to 4.94) | 3.39† (2.236 to 5.145) | 3.32† (2.186 to 5.026) |
| Tumor size, T1 vs T2–4 | 2.08† (1.41 to 3.08) | 2.13† (1.440 to 3.159) | 2.19† (1.485 to 3.246) |
| Menopausal status, pre vs post | 1.02 (0.88 to 2.63) | 1.00 (0.705 to 1.423) | 0.99 (0.699 to 1.410) |
| Histology, lobular/mixed vs ductal | 0.93 (0.58 to 1.51) | 0.91 (0.559 to 1.466) | 0.94 (0.579 to 1.516) |
| OS | |||
| CTCs in blood, negative vs positive | 2.18† (1.32 to 3.59) | 2.57† (1.416 to 4.659) | 3.60† (1.564 to 8.445) |
| Hormone receptor status, positive vs negative | 3.0† (1.76 to 5.10) | 3.04† (1.786 to 5.163) | 3.05† (1.790 to 5.190) |
| Lymph node involvement, N0 vs N1–3 | 4.25† (2.18 to 8.29) | 4.07† (2.085 to 7.947) | 4.19† (2.149 to 8.161) |
| Grading, G1 vs G2–3 | 3.55† (1.86 to 6.76) | 3.65† (1.920 to 6.954) | 3.66† (1.924 to 6.977) |
| Tumor size, T1 vs T2–4 | 2.67† (1.44 to 4.93) | 2.74† (1.479 to 5.058) | 2.85† (1.548 to 5.255) |
| Menopausal status, pre vs post | 1.52 (0.88 to 2.63) | 1.49 (0.856 to 2.580) | 1.49 (0.859 to 2.583) |
| Histology, lobular/mixed vs ductal | 1.26 (0.56 to 2.85) | 1.23 (0.546 to 2.779) | 1.25 (0.556 to 2.823) |
* CI = confidence interval; CTC = circulating tumor cell; DFS = disease free survival; HR = hazard ratio; OS = overall survival. Cox proportional hazards models. All statistical tests were two-sided.
† Statistically significant.
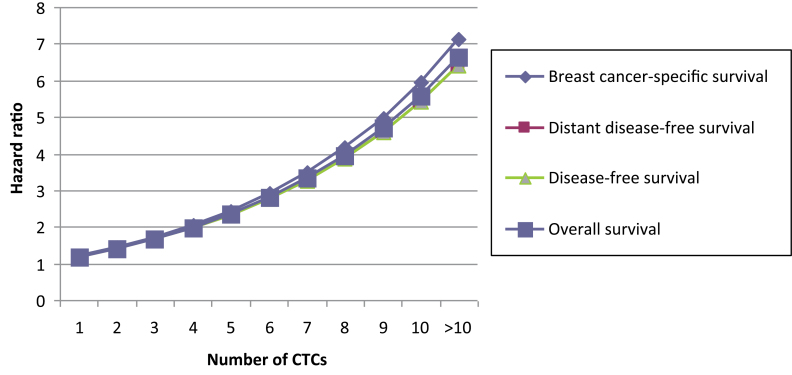
Patients with five or more CTCs were at highest risk for recurrence. At 36 months, 28.1% of patients presented with recurrent disease and 14.3% had died, compared with 7.1% and 3.4% of patients with less than five CTCs, respectively (log-rank test, P < .0001 and P = .005) (Figure 2). The results indicated that patient outcome was associated with the absolute number of CTCs because the hazard ratios consistently increased with increasing cutoff values. The risk of recurrence or death more than doubled when a cutoff value of five or more CTCs was used (DFS: HR = 4.51, 95% CI = 2.59 to 7.86; OS: HR = 3.60, 95% CI = 1.56 to 8.45) compared with a cutoff value of one or more CTCs (DFS: HR = 2.11; OS: HR = 2.18) (Table 4). To investigate the relationship between outcome and number of CTCs, the hazard ratio of the number of CTCs present compared with no CTCs was calculated, adjusted for the standard risk factors and treatment. For all clinical endpoints, patient prognosis deteriorated continuously with increasing CTC numbers (Figure 3).
Figure 2.
Kaplan–Meier analysis according to the presence or absence of five or more peripheral blood circulating tumor cells (CTCs) before chemotherapy (CT). A) Disease-free survival. B) Overall survival. Two-sided log-rank test.
Figure 3.
The correlation of hazard ratios with increasing numbers of circulating tumor cells (CTCs) per 30mL of blood according to survival endpoints.
CTC Detection in Different Breast Cancer Subtypes
Breast cancer is a heterogeneous disease and classified into molecular subtypes, which we analyzed with regard to the presence or absence of CTCs. We grouped the primary tumors according to their immunohistochemical phenotype. Luminal cancers were defined as estrogen receptor and/or progesterone receptor positive (n = 1155; 57.0%), basal-like tumors were defined as estrogen, progesterone, and HER2 negative (n = 347; 17.1%), and HER2-like tumors were defined as HER2 positive (n = 501; 24.7%). Following this classification, no association of CTC positivity with luminal, basal-like, or HER2-like tumors (χ2 test, all P ≥ .5) was found. In the largest subgroup of luminal patients, the presence of CTCs was associated with a reduced DFS (HR = 1.24; 95% CI = 1.16 to 1.33; P < .001) and OS (HR = 1.28; 95% CI = 1.16 to 1.44; P < .001).
Relevance of CTCs Persisting After Adjuvant Chemotherapy
A total of 85.7% of CTC-positive patients were free of recurrence at 36 months compared with 91.1% of CTC-negative patients. After chemotherapy, 22.1% of patients (n = 330 of 1493) were CTC positive. The presence of persisting CTCs after chemotherapy showed a negative influence on DFS (HR = 1.124; 95% CI = 1.02 to 1.25; P = .02) and on OS (HR = 1.162; 95% CI = 0.99 to 1.37; P = .06).
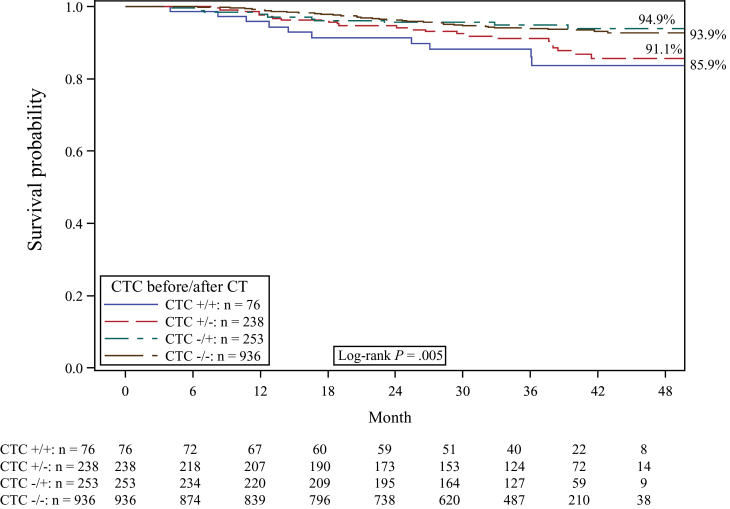
Four patient groups were formed according to their CTC status both before and after chemotherapy: persistently positive patients, persistently negative patients, patients with positive prechemotherapy CTC status changing to negative, and patients with negative prechemotherapy CTC status changing to positive. The Kaplan–Meier estimate for 36-month OS was 92.8% for persistently CTC-positive patients and 97.6% for persistently CTC-negative patients. For DFS, the estimates were 85.9% for persistently CTC-positive patients and 93.9% for persistently CTC-negative patients. The presence of CTCs both before and after chemotherapy compared with all other subgroups was associated with a statistically significantly reduced DFS (log-rank test, P = .005) (Figure 4) and a trend toward a reduced OS (log-rank test, P = .10).
Figure 4.
Kaplan–Meier analysis for disease-free survival according to the presence (+) or absence (−) of peripheral blood circulating tumor cells (CTCs) before and after chemotherapy (CT). Two-sided log-rank test.
Discussion
For the first time, we could show CTCs to be a prognostic marker for reduced DFS, distant DFS, breast cancer–specific survival, and OS before the start of systemic treatment and for DFS after completion of adjuvant chemotherapy in the setting of a large, multicenter, prospective, randomized trial. Prognostic relevance independent of other prognostic markers was confirmed in multivariable analysis both for DFS and OS. The strength of this prognostic effect increased with higher CTC levels.
The prevalence of at least one CTC per 30mL of blood was 21.5%, which is within the CTC positivity range found by other investigators (9,14,15). In smaller cohorts, CTCs were reported in 18% to 30% of patients with early breast cancer (9,12,14,15,25) and more frequently in patients with metastatic disease, with a prevalence of 70% (4,26). Lucci et al. recently published data on 302 breast cancer patients at the time of surgery: CTCs were detected in 24% of patients, and their presence predicted decreased progression-free survival and OS (15). Our trial confirmed these data in a much larger patient cohort, extending the data to patients after completion of chemotherapy. Based on the evaluation at sequential time points, we provided the prevalence, course, and prognostic relevance of CTCs before and after adjuvant chemotherapy within the same patients and could confirm our results in multivariable analysis. Because of the large number of patients, subgroup analyses taking into account the different CTC levels and biological breast cancer subtypes were performed. All patients were average-risk to high-risk early breast cancer patients for whom chemotherapy is recommended. Therefore, the observation that the presence of CTCs at primary diagnosis is associated with worse prognosis is likely to remain of limited impact for the modification of treatment algorithms in this group of patients. In contrast, the prognostic relevance of CTCs after chemotherapy could be especially valuable for individualized treatment approaches to allow for the identification of patients with tumor cells evading standard chemotherapy.
Although basal-like tumors are commonly treated with chemotherapy, decisions regarding adjuvant chemotherapy are much more difficult in the luminal subgroup. Despite recent advances in technology, such as the Oncotype DX or gene arrays, the benefit of a treatment with considerable side effects still remains unclear in the individual patient, leading to a general overtreatment in many cases. Because we observed an increased risk of recurrence, especially in the subgroup of luminal patients, the detection of CTCs can help select patients at risk by providing tumor biological information beyond the available diagnostic tests. Furthermore, because axillary operation will increasingly be confined to sentinel node biopsy, CTCs could be a helpful tool for selection of high-risk patients who might benefit from a more aggressive dose-dense chemotherapy regimen (27,28).
The limitations of this study include the short median follow-up of 35 months. This short follow-up in the context of a very good prognosis results in small absolute differences in the rate of recurrence and death. Despite this limited number of events in our data, as well as in the study published by Lucci et al., both trials consistently demonstrate a clear prognostic relevance of CTCs in early breast cancer. In addition, the number of cells detected by the CellSearch system is relatively low and limited to cells with expression of Epcam and cytokeratin 8/18/19. In contrast, basal-like tumors with low Epcam expression have been shown to contain a high frequency of stem cells (29–31) and are associated with very poor prognosis (32). CTCs with decreased epithelial marker expression as a result of the epithelial–mesenchymal transition could be missed by the CellSearch methodology (33). Epcam-independent detection approaches could increase the capacity to detect CTCs with stem cell phenotype. Nevertheless, the CellSearch system has shown highly reproducible and automated detection of CTCs in interlab validation trials (34,35).
Although the presence of persisting CTCs after chemotherapy was associated with worse outcome, survival of patients without CTCs before chemotherapy was the same irrespective of CTC status after chemotherapy. This might be explained by various effects of chemotherapy on CTCs. Tumor cell mobilization by chemotherapy or bone marrow stimulating agents such as granulocyte colony stimulating factor is a known phenomenon (36), whereas adjuvant chemotherapy reduces the number of proliferating CTCs (37,38). These differential effects could influence the metastatic potential of CTCs. The development of new techniques for CTC phenotyping could help to identify tumor cells responsible for subsequent metastatic disease.
Modern breast cancer treatment is tailored to the individual tumor characteristics (19,39). Changes in the tumor phenotype from the primary tumor to that of distant metastasis are a known phenomenon and may lead to treatment changes in up to 20% of patients (40,41). Given the chromosomal abnormalities and the overexpression of HER2 and stem cell markers in CTCs (9,24,42–44), improved phenotyping could help to identify treatment-relevant targets and resistance mechanisms (45). Clinical intervention trials are currently being performed to evaluate the predictive role of CTCs to tailor the treatment in primary and metastatic disease (SWOG S0500, TREAT CTC, and DETECT III) (46).
In conclusion, the SUCCESS study is the first trial to provide strong evidence for the prognostic relevance of CTCs in early breast cancer before and after adjuvant chemotherapy in a large patient cohort. Our data offer support for the clinical potential of CTCs to assess the individual risk of patients at the time of primary diagnosis and may be used for treatment tailoring in the absence of other strong quantitative markers. Future applications for CTCs will include the early assessment of treatment efficacy as well as the phenotyping of cells to individualize treatment strategies. Thus, in addition to established parameters, the use of CTCs may considerably contribute to the personalization of breast cancer treatment (36).
Funding
This translational research part of the SUCCESS trial was supported by AstraZeneca, Chugai, Lilly, Novartis, Sanofi-Aventis, and Veridex.
B. Rack, A. Schneeweiss, T. Fehm, M. W. Beckmann, and W. Janni have received research funding from AstraZeneca, Chugai, Lilly, Novartis, and Sanofi-Aventis. B. Rack, T. Fehm, K. Pantel, and W. Janni received research funding and speaker honoraria from Veridex. H. Tesch and M. W. Beckmann acted as advisors for Novartis and Sanofi-Aventis. A. Schneeweiss received speaker honoraria from AstraZeneca, Chugai, Lilly, Novartis, and Sanofi-Aventis. H. Tesch received speaker honoraria from Sanofi-Aventis and Novartis. P. Hepp received speaker honoraria from Chugai. C. P. A. Fasching received research funding and speaker honoraria from Novartis. C. Schindlbeck, U. Andergassen, J. Jückstock, T. Zwingers, W. Lichtenegger, and K. Friese have no conflicts of interest to declare.
References
- 1. Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802 [DOI] [PubMed] [Google Scholar]
- 2. Janni W, Vogl FD, Wiedswang G, et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse—a European pooled analysis. Clin Cancer Res. 2011;17(9):2967–2976 [DOI] [PubMed] [Google Scholar]
- 3. Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumor cells. Nat Rev Cancer. 2008;8(5):329–340 [DOI] [PubMed] [Google Scholar]
- 4. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791 [DOI] [PubMed] [Google Scholar]
- 5. Daskalaki A, Agelaki S, Perraki M, et al. Detection of cytokeratin-19 mRNA-positive cells in the peripheral blood and bone marrow of patients with operable breast cancer. Br J Cancer. 2009;101(4):589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bidard FC, Vincent-Salomon A, Sigal-Zafrani B, et al. Prognosis of women with stage IV breast cancer depends on detection of circulating tumor cells rather than disseminated tumor cells. Ann Oncol. 2008;19(3):496–500 [DOI] [PubMed] [Google Scholar]
- 7. Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12(14 Pt 1):4218–4224 [DOI] [PubMed] [Google Scholar]
- 8. Botteri E, Sandri MT, Bagnardi V, et al. Modeling the relationship between circulating tumor cells number and prognosis of metastatic breast cancer. Breast Cancer Res Treat. 2010;122(1):211–217 [DOI] [PubMed] [Google Scholar]
- 9. Riethdorf S, Muller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16(9):2634–2645 [DOI] [PubMed] [Google Scholar]
- 10. Xenidis N, Ignatiadis M, Apostolaki S, et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol. 2009;27(13):2177–2184 [DOI] [PubMed] [Google Scholar]
- 11. Ignatiadis M, Perraki M, Apostolaki S, et al. Molecular detection and prognostic value of circulating cytokeratin-19 messenger RNA-positive and HER2 messenger RNA-positive cells in the peripheral blood of women with early-stage breast cancer. Clin Breast Cancer. 2007;7(11):883–889 [DOI] [PubMed] [Google Scholar]
- 12. Sandri MT, Zorzino L, Cassatella MC, et al. Changes in circulating tumor cell detection in patients with localized breast cancer before and after surgery. Ann Surg Oncol. 2010;17(6):1539–1545 [DOI] [PubMed] [Google Scholar]
- 13. Pachmann K, Camara O, Kavallaris A, et al. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol. 2008;26(8):1208–1215 [DOI] [PubMed] [Google Scholar]
- 14. Bidard FC, Mathiot C, Delaloge S, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21(4):729–733 [DOI] [PubMed] [Google Scholar]
- 15. Lucci A, Hall CS, Lodhi AK, et al. Circulating tumor cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688–695 [DOI] [PubMed] [Google Scholar]
- 16. Serrano MJ, Rovira PS, Martinez-Zubiaurre I, et al. Dynamics of circulating tumor cells in early breast cancer under neoadjuvant therapy. Exp Ther Med. 2012;4(1):43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sautter-Bihl ML, Souchon R, Budach W, et al. DEGRO practical guidelines for radiotherapy of breast cancer II. Postmastectomy radiotherapy, irradiation of regional lymphatics, and treatment of locally advanced disease. Strahlenther Onkol. 2008;184(7):347–353 [DOI] [PubMed] [Google Scholar]
- 18. Sautter-Bihl ML, Budach W, Dunst J, et al. DEGRO practical guidelines for radiotherapy of breast cancer I: breast-conserving therapy. Strahlenther Onkol. 2007;183(12):661–666 [DOI] [PubMed] [Google Scholar]
- 19. Kreienberg R, Albert US, Follmann M., et al. Interdisziplinare S3-leitlinie fur die diagnostik, therapie und nachsorge des mammakarzinoms. Senologie. 2013;10(3):164–192 [Google Scholar]
- 20. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–2132 [DOI] [PubMed] [Google Scholar]
- 21. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481 [Google Scholar]
- 22. Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220 [Google Scholar]
- 23. Singletary ES, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer Staging System for Breast Cancer. J Clin Oncol. 2002;20:3628–3636 [DOI] [PubMed] [Google Scholar]
- 24. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410 [DOI] [PubMed] [Google Scholar]
- 25. Biggers B, Knox S, Grant M, et al. Circulating tumor cells in patients undergoing surgery for primary breast cancer: preliminary results of a pilot study. Ann Surg Oncol. 2009;16(4):969–971 [DOI] [PubMed] [Google Scholar]
- 26. Fehm T, Muller V, Aktas B, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010;124(2):403–412 [DOI] [PubMed] [Google Scholar]
- 27. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuemmel S, Kolberg H, Lueftner D, et al. Breast cancer 2011—new aspects. Geburtsh Frauenheilk. 2011;71:939–953 [Google Scholar]
- 29. Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–997 [DOI] [PubMed] [Google Scholar]
- 30. Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–88 [DOI] [PubMed] [Google Scholar]
- 31. Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11(4):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355(6):560–569 [DOI] [PubMed] [Google Scholar]
- 33. Wicha MS, Hayes DF. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J Clin Oncol. 2011;29(12):1508–1511 [DOI] [PubMed] [Google Scholar]
- 34. Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13(3):920–928 [DOI] [PubMed] [Google Scholar]
- 35. Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904 [DOI] [PubMed] [Google Scholar]
- 36. Viret F, Chabannon C, Sainty D, et al. Occult tumor cell contamination in patients with stage II/III breast cancer receiving sequential high-dose chemotherapy. Bone Marrow Transplant. 2003;32(11):1059–1064 [DOI] [PubMed] [Google Scholar]
- 37. Kallergi G, Konstantinidis G, Markomanolaki H, et al. Apoptotic circulating tumor cells (CTCs) in early and metastatic breast cancer patients. Mol Cancer Ther. 2013;12(9):1886–1895 [DOI] [PubMed] [Google Scholar]
- 38. Fehm T, Becker S, Becker-Pergola G, et al. Presence of apoptotic and nonapoptotic disseminated tumor cells reflects the response to neoadjuvant systemic therapy in breast cancer. Breast Cancer Res. 2006;8(5):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scharl A, Thomssen C, Harbeck N. AGO recommendations for diagnosis and treatment of patients with early and metastatic breast cancer: update 2012. Breast Care (Basel). 2012;7(4):322–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santinelli A, Pisa E, Stramazzotti D, et al. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int J Cancer. 2008;122(5):999–1004 [DOI] [PubMed] [Google Scholar]
- 41. Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20(9):1499–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fehm T, Sagalowsky A, Clifford E, et al. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res. 2002;8(7):2073–2084 [PubMed] [Google Scholar]
- 43. Swennenhuis JF, Tibbe AG, Levink R, et al. Characterization of circulating tumor cells by fluorescence in situ hybridization. Cytometry A. 2009;75(6):520–527 [DOI] [PubMed] [Google Scholar]
- 44. Reuben J, Lee B, Li C, et al. Genomics of circulating tumor cells in metastatic breast cancer. J Clin Oncol. 2007;25:18 [Google Scholar]
- 45. Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bidard FC, Fehm T, Ignatiadis M, et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2013;32(1–2):179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]