SUMMARY
We have previously demonstrated that mycobacterial lipoproteins engage TLR2 on human CD4+ T cells and up-regulate TCR triggered- IFN-γ secretion and cell proliferation in vitro. Here we examined the role of CD4+ T cell-expressed TLR2 in Mycobacterium tuberculosis (MTB) Ag-specific T cell priming and in protection against MTB infection in vivo. Like their human counterparts, mouse CD4+ T cells express TLR2 and respond to TLR2 co-stimulation in vitro. This Th1-like response was observed in the context of both polyclonal and Ag-specific TCR stimulation. To evaluate the role of T cell TLR2 in priming of CD4+ T cells in vivo, naïve MTB Ag85B specific TCR transgenic CD4+ T cells (P25 TCR-Tg) were adoptively transferred into Tlr2-/- recipient mice that were then immunized with Ag85B and with or without TLR2 ligand Pam3Cys-SKKKK (P3CSK4). TLR2 engagement during priming resulted in increased numbers of IFN-γ secreting P25 TCR-Tg T cells one week after immunization. P25 TCR-Tg T cells stimulated in vitro via TCR and TLR2 conferred more protection than T cells stimulated via TCR alone when adoptively transferred before MTB infection. Our findings indicate that TLR2 engagement on CD4+ T cells increases MTB-Ag specific responses and may contribute to protection against MTB infection.
Keywords: Mycobacterium tuberculosis, CD4+ T cells, toll-like receptor 2
INTRODUCTION
Mycobacterium tuberculosis (MTB) is one of the most successful pathogens, infecting one third of the world’s population and killing more adults than any other single microbe except for HIV-1 [1]. One of the major barriers to develop new tools to prevent and treat tuberculosis (TB) is our incomplete understanding of the factors required to steer the T cell response towards achieving sterilizing immunity. IFN-γ secreting CD4+ T cells have a central role in protection against TB. This was clearly demonstrated by the increased susceptibility to TB observed in HIV-1+ persons in addition to the poor control of MTB infection observed in IFN-γ- and MHC-II- deficient mouse strains and CD4+ T cell-depleted mice[2-4]. IFN-γ produced by CD4+ T cells synergizes with TNF-α to activate macrophage bactericidal and bacteriostatic functions and greatly contributes to long-lasting control of MTB infection [3]. Dysfunction of the CD4+ T cell- IFN-γ- macrophage axis significantly predisposes the host to mycobacterial diseases [5]. Since development and maintenance of strong CD4+ T cell responses are essential to MTB infection control, enhancement of CD4+ T cell function is likely critical to improve next generation TB vaccines and to develop novel TB immune therapies.
CD4+ T cell activation requires two signals, signal 1 elicited by MHC-II/peptide complex engagement of the TCR, and signal 2 triggered when the co-stimulatory receptor CD28 binds CD80 or CD86 on the Ag presenting cell (APC) (reviewed in [6]). T cell co-stimulation is an absolute requirement for naïve T cell priming and is also important for regulation of effector and memory T cells [7, 8]. Thus, both Ag availability and expression of co-stimulatory receptors may be limiting factors for T cell priming and memory maintenance. In addition to CD28, other costimulatory receptors have been described on CD4+ T cells [6]. Unlike CD28, which is constitutively expressed in both naïve and memory T cells, many co-stimulatory receptors are induced after activation. Toll-like receptor 2 (TLR2) has been recently recognized as a costimulatory receptor on CD4+ and CD8+ T cells [9-13]. TLR2 is unique among inducible costimulatory receptors in that it engages microbial ligands instead of receptors expressed by APCs. Recently, we reported on the ability of human CD4+ T cells to directly recognize mycobacterial lipoproteins via TLR2 [14]. In combination with TCR triggering, TLR2 engagement induced CD4+ T cell proliferation and secretion of IL-2 and IFN-γ. The role of this TLR2 ligand recognition system in CD4+T cell activation/differentiation in vivo and its impact on immune responses to MTB infection remains unexplored. CD4+ T cell expressed TLR2 may have a role in detection of MTB-infected macrophages by recognizing membrane-associated or extracellular TLR2 ligands and by providing additional co-stimulatory signals for naïve T cell priming or effector memory T cell re-stimulation. In this way, T cell TLR2 may amplify Ag specific CD4+ T cell responses and contribute to immune protection against MTB.
We tested the role of TLR2 ligand recognition by CD4+ T cells in the development of MTB Ag specific T cell responses in vitro and in vivo. Our findings indicate that engagement of CD4+ T cell-expressed TLR2 during priming increases Th1 cytokine secretion and T cell proliferation. Furthermore, adoptively transferred MTB Ag specific transgenic T cells primed via TCR and TLR2 conferred more protection against MTB aerosol infection compared to T cells primed via TCR alone. Thus simultaneous engagement of TCR and TLR2 by mycobacterial ligands may serve as a mechanism to amplify T cell responses to the pathogen. Failure of these mechanisms may explain deficient primary responses to MTB or loss of immune control during latency and subsequent reactivation. TCR/TLR2 co-engagement could be exploited to improve responses to immunization, thus increasing TB vaccine efficacy.
RESULTS
TLR2 expression is low in mouse naïve CD4+ T cells but increases after stimulation
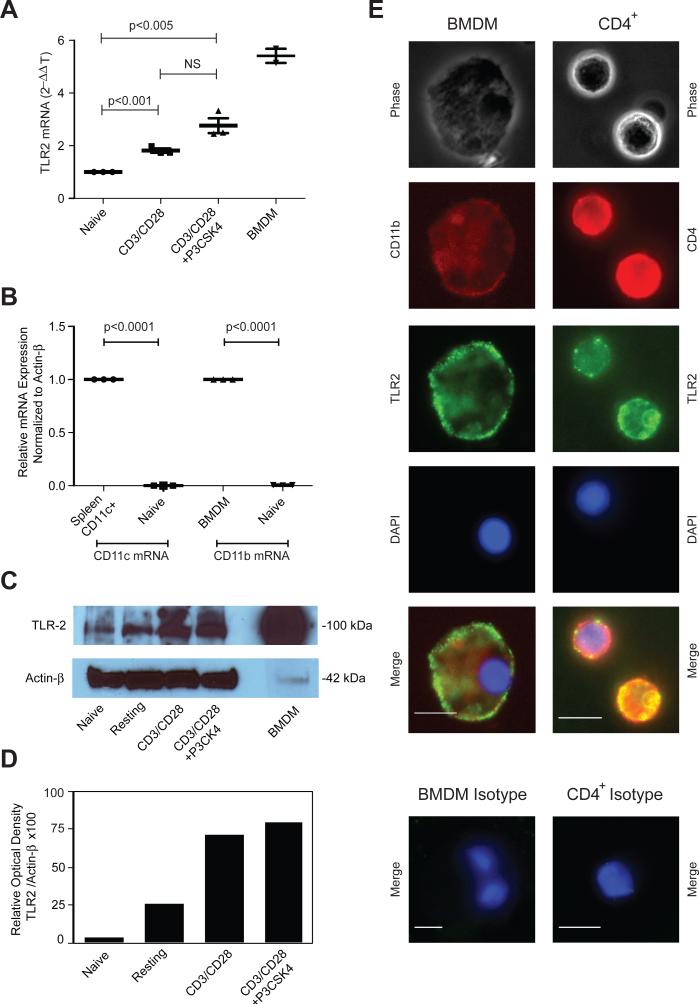
Several studies have demonstrated expression of TLR2 protein and mRNA in human CD4+ T cells and in mouse CD8+ T cells but few reports exist on the expression and function of TLR2 on mouse CD4+ T cells [10, 12, 15]. Thus, we tested if mouse naïve or in vitro-generated effector CD4+ T cells express TLR2. Although we were unable to detect surface TLR2 expression by flow cytometry (not shown), we detected TLR2 mRNA by RT-PCR (Fig. 1A). Neither CD11c- nor CD11b-mRNA could be detected in highly purified CD4+ T cells, indicating that detection of TLR2 mRNA in these samples was not due to contaminating APCs (Fig. 1B). TLR2 mRNA expression in cells activated with anti-CD3- and anti-CD28-mAbs alone was higher than in naïve T cells but not significantly different compared to cells activated with anti-CD3- and anti-CD28-mAbs in combination with the TLR2 agonist Pam3Cys-SKKKK (P3CSK4) (Fig. 1A, n=3). We confirmed TLR2 protein expression on CD4+ T cells by western blotting and by fluorescence microscopy (Fig. 1C-E). These data confirm that, like their human counterparts, mouse naïve CD4+ T cells express very low levels of TLR2 that increase after activation.
Figure 1. TLR2 expression on mouse CD4+ T cells increases after activation.
Highly purified naïve CD4+ T cells were either left untreated (naïve) or stimulated with anti-CD3/anti-CD28 mAbs alone or in combination with P3CSK4. BMDM or CD11c-purified spleen cells were used as positive controls for TLR2 and CD11b or CD11c expression respectively. (A, B) TLR2, CD11b and CD11c mRNA expression was determined by RT-PCR and expressed as a relative quantity using the 2-ΔΔCT method. Horizontal bars represent means ± SEM of three independent experiments. Each data point represents the mean of triplicates of one independent experiment. Each experiment was conducted with a separate pool of cells isolated from five animals. NS: differences not statistically significant. (C) TLR2 protein expression in cell lysates was determined by Western blotting. β-actin was used as the loading control. Image was cropped at the 100-kDa and 42-kDa MW ladder regions. (D) Western blots were analyzed in a VersaDoc Imaging system (Bio-Rad) and band intensity was expressed as a relative optical value. Shown is one representative experiment of three. (E) BMDM or activated CD4+ T cells (CD4+) were labeled with PE-CD11b or PE-CD4 Abs respectively, and with anti-TLR2 followed by a secondary FITC- anti-mouse IgG1 Ab and counterstained with DAPI. Control cells (Isotype, bottom panels) were labeled with correspondent isotype-matched IgG. Cells were analyzed by fluorescent microscopy on a Leica DMI 6000 B inverted microscope using 40x (BMDM) and 63x (CD4+) NA 1.4 objectives. Adobe Photoshop CS5.1 was used for RGB image overlays and minor adjustments to image contrast. Scale bar = 10μm. Shown is one representative experiment of three.
Synergism of TLR2- and TCR signals in mouse naïve CD4+ T cells triggers Th1 differentiation in vitro
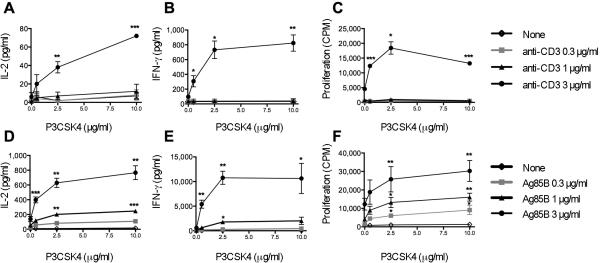
Before testing the role of CD4+ T cell-expressed TLR2 in vivo, we analyzed its role in the modulation of mouse CD4+ T cell activation and differentiation in vitro. Naïve CD4+ T cells were stimulated through the TCR with plate bound anti-CD3 mAb alone or combined with P3CSK4. As expected, naïve CD4+ T cells were unresponsive to TCR stimulation alone, with undetectable levels of IL-2 or IFN-γ and very low cell proliferation (Fig. 2 A-C). P3CSK4 increased significantly both cytokine and proliferative responses to anti-CD3 mAb in a dose-dependent manner. In absence of TCR stimulation, P3CSK4 did not trigger cytokine secretion or cell proliferation, confirming that TLR2 serves mainly as a co-stimulatory receptor.
Figure 2. TLR2 engagement on naïve CD4+ T cells up-regulates IL-2 and IFN-γ secretion and proliferation in response to both polyclonal and Ag-specific TCR stimulation.
Naïve CD4+ T cells from WT mice (A-C) or from P25 TCR Tg mice (D-F) were stimulated with plate-bound anti-CD3 mAb (A-C) or with Ag85B-pulsed TLR2neg BMDM (D-F) alone or in combination with P3CSK4 at indicated concentrations. IL-2 (A, D) and IFN-γ (B, E) were measured in culture supernatants by ELISA. Cell proliferation (C, F) was determined by [3H]thymidine incorporation and expressed as counts per minute (CPM). Means ± SEM of three independent experiments are shown. Each experiment was done in triplicates with a separate pool of cells isolated from five animals. * p < 0.05, ** p < 0.01, *** p < 0.005 compared with values obtained without P3CSK4.
To test the effect of triggering TLR2 on CD4+ T cells in Ag-specific responses, naïve CD4+ T cells from P25 TCR-Tg mice were stimulated with Ag85B-pulsed TLR2-negative (TLR2neg) BMDM. The use of BMDM isolated from Tlr2-/- mice ensures that observed effects result from interaction of P3CSK4 with T cell-expressed-TLR2 and not APC-expressed-TLR2. As shown in Fig. 2D-F, a dose-dependent up-regulation of both IL-2, IFN-γ and proliferative responses were observed in cells stimulated with Ag85B in the presence of P3CSK4 compared to cells stimulated with Ag85B alone. Neither IL-2/IFN-γ nor proliferative responses were detected after stimulation of naive CD4+ T cells with P3CSK4 in the absence of Ag85B (Fig. 2D-F). In addition to IL-2 and IFN-γ, we found increased TNF-γ and the IFN-γ-inducible chemokines IP-10 and MIG in supernatants of T cells activated in the presence of TLR2 ligand (Supporting information Table T1, Fig. S1). Importantly, we did not detect Th2 cytokines IL-4, IL-5 or IL-13. IL-17 also remained undetectable both at 24 and 48 h after Ag stimulation (Supporting information Table T1). This indicates that TLR2 engagement on mouse naïve CD4+ T cells synergizes with TCR triggering for the induction of Th1 differentiation. Considering that MTB TLR2 ligands can exit the phagosome and traffic outside the infected macrophage in small vesicles known as exosomes (Supporting information, Fig. S2), recognition of these ligands by naïve CD4+ T cells may have important implications for T cell priming and generation of MTB Ag-specific responses in vivo.
TLR2 engagement on effector CD4+ T cells up-regulates TCR- triggered effector functions
Since TLR2 expression increases with activation, we hypothesized that, in addition to regulating naïve CD4+ T cells during their first encounter with Ag, TLR2 could have a role in direct regulation of effector CD4+ T cell function. As shown in Fig. 3A and B, TLR2 triggering increased responses of in vitro-generated effector CD4+ T cells to anti-CD3 mAb. Furthermore, engagement of TLR2 on P25 TCR Tg effector cells increased MTB Ag85B specific- IL-2 and IFN-γ responses (Fig. 3C, D). In the absence of TCR triggering (no anti-CD3 mAb or Ag85B), P3CSK4 did not induce cytokine secretion by in vitro-generated effector CD4+ T cells (Fig. 3).
Figure 3. TLR2 engagement on in vitro-generated effector CD4+ T cells potentiates TCR-triggered IL-2 and IFN-γ secretion.
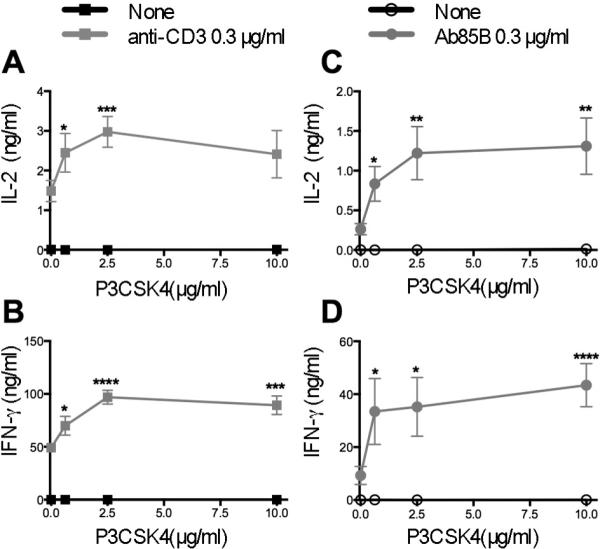
Effector CD4+ T cells were generated in vitro from naïve CD4+ T cells isolated from WT (A, B) or P25 TCR-Tg (C, D) mice, then re-stimulated with plate-bound anti-CD3 mAb or with Ag85B-pulsed TLR2neg BMDM respectively and without or with P3CSK4 at the indicated concentrations. IL-2 (A, C) and IFN-γ (B, D) were measured in culture supernatants by ELISA. Means ± SEM of three independent experiments are shown. Each experiment was conducted in triplicates with a separate pool of cells isolated from five animals. * p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.001 compared with values obtained without P3CSK4.
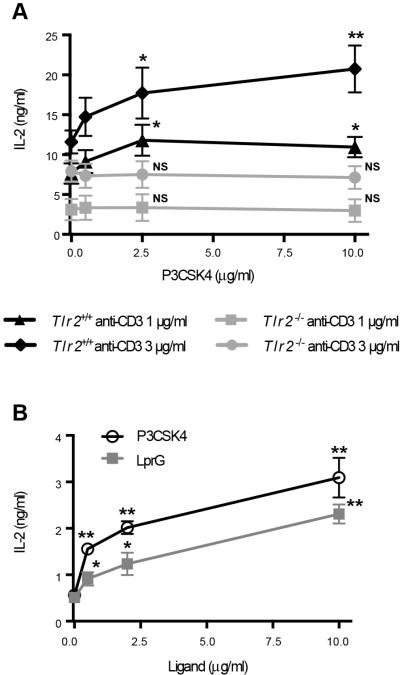
To confirm that P3CSK4 effect was mediated by TLR2, responses of in vitro-generated effector CD4+ T cells isolated from Tlr2-/- or from WT mice were compared. Unlike their TLR2+ counterparts, TLR2neg effector CD4+ T cells did not display increased IL-2 responses when stimulated with anti-CD3 mAb and P3CSK4 compared to anti-CD3 mAb alone (Fig. 4A). This demonstrates that P3CSK4 engages TLR2 on CD4+ T cells to trigger co-stimulatory signals. We also tested the effects of the natural MTB TLR2 ligand LprG and confirmed its co-stimulatory effect on mouse CD4+ T cell activation (Fig. 4B). These results demonstrate that TLR2 ligation on effector CD4+ T cells up-regulates TCR-triggered Th1 cytokine secretion and this may contribute to amplification of protective immune responses to MTB.
Figure 4. Co-stimulation of CD4+ T cells by P3CSK4 is TLR2 dependent and can also be triggered by mycobacterial LprG.
Effector T cells generated in vitro from naïve CD4+ T cells isolated from WT (A, B) or Tlr2-/- mice (A) were re-stimulated with plate-bound anti-CD3 mAb alone or with P3CSK4 at indicated concentrations (A, B) or LprG (B). IL-2 was measured in culture supernatants by ELISA. Means ± SEM of three independent experiments are shown. Each experiment was conducted in triplicates with a separate pool of cells isolated from five animals. * p < 0.05, ** p < 0.01 compared with values obtained without P3CSK4 or LprG. NS: not statistically significant.
In vivo engagement of TLR2 on CD4+ T cells increases T cell priming with MTB Ag85B
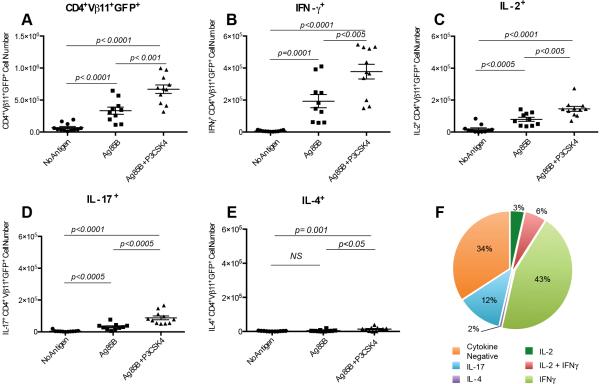
To test the impact of TLR2 engagement on CD4+ T cells in MTB Ag specific responses in vivo, we adoptively transferred GFP+ P25 TCR-Tg CD4+ T cells (Tlr2+/+) into Tlr2-/- recipient mice that were then immunized with Ag85B plus CpG ODN1826 alone or combined with P3CSK4. Increased percent and number of GFP+ P25 TCR-Tg CD4+ T cells were detected in spleens after immunization with Ag85B. This expansion of GFP+ P25 TCR-Tg CD4+ T cells was more pronounced in animals that received Ag85B in combination with P3CSK4 compared to those immunized with Ag85B alone (Fig. 5A). In the absence of cognate Ag, administration of P3CSK4 alone had no effect in the expansion of P25 TCR-Tg CD4+ T cells (not shown). Co-administration of P3CSK4 with Ag85B significantly increased the numbers of Ag-specific CD4+ T cells secreting IL-2, IFN-γ and IL-17 compared to Ag85B alone (Fig. 5, B-D). When compared to animals immunized with Ag85B only, those receiving Ag85B in combination with P3CSK4 have double the numbers of IFN-γ+ P25 TCR-Tg CD4+ T cells (Fig. 5C). IFN-γ-secreting cells represented the predominant phenotype after challenge with Ag85B plus P3CSK4 (Fig. 5F). Although IL-4-secreting cells were marginally increased by P3CSK4 co-administration, these cells represented less than 2% of P25 TCR-Tg cells (Fig. 5E-F). These results demonstrate that in the context of adequate DC activation (i.e. CpG ODN1826) expansion of MTB Ag-primed IFN-γ and IL-17 producing CD4+ T cells is enhanced by ligation of TLR2 on CD4+ T cells in vivo. Altogether these data suggest a role for T cell-expressed TLR2 in MTB specific-CD4+ T cell priming and Th1 and Th17 differentiation that could have implications in MTB infection and TB vaccine development.
Figure 5. Engagement of TLR2 on CD4+ T cells during priming increases mycobacterial Ag specific responses.
Naïve CD4+ T cells isolated from Tlr2+/+ GFP+ P25 TCR Tg mice were adoptively transferred into Tlr2-/- hosts (106 cells/mouse). Recipient animals were immunized with CpG-ODN1826 (No Antigen) or with CpG-ODN1826 plus Ag85B (5 μg/animal) alone (Ag85B) or in combination with P3CSK4 (30 μg/animal) (Ag85B+P3CSK4). Animals were sacrificed and spleens harvested seven days after immunization. (A) GFP+ P25 TCR-Tg cells in spleens were enumerated by assessing cell surface staining for CD4 and Vβ11 in combination with GFP expression using flow cytometry. (B- E) Numbers of GFP+ P25 TCR-Tg cells expressing intracellular IL-2, IFN-γ, IL-17 or IL-4 were determined in spleens after in vitro re-stimulation with Ag85B peptide 25 followed by intracellular staining and flow cytometry. Panels A-E represent a compilation of three experiments performed with 3-5 animals/group/experiment (no antigen, n=12; Ag85B, n=10; Ag85B + P3CSK4, n=11). Each symbol represents data from an individual mouse. Horizontal lines indicate the means ± SEM. NS: not statistically significant. F. Percent distribution of cytokine positive cells among GFP+ P25 TCR- Tg cells recovered from mice (n=11) immunized with Ag85B and P3CSK4 and measured after in vitro re-stimulation with Ag85B peptide 25.
Th1 cells generated in the presence of TLR2 ligand confer higher protection against MTB infection
Gallegos et al. demonstrated that adoptive transfer of ESAT-6 TCR-Tg Th1 effector cells confer protection against MTB aerosol infection [16]. We used a modified version of this model to assess the impact of T cell-expressed TLR2 signaling on CD4+ T cell effector function in vivo. Rested non-dividing GFP+ P25 TCR Tg CD4+ Th1 effector cells generated in vitro in the absence (P25 Th1) or the presence of P3CSK4 (P25 Th1-P3CSK4) were adoptively transferred into WT mice one day before MTB aerosol infection, and bacterial load and TCR transgenic cells assessed in the lungs 28 days later. As shown in Fig. 6A, animals transferred with Th1 cells activated in the presence of P3CSK4 had lower lung CFUs compared to animals transferred with Th1 cells generated in absence of TLR2 stimulation and even lower compared to control mice. Also, recipients of P25 Th1-P3CSK4 cells showed higher numbers of transgenic T cells in the lung (CD4+ Vβ11+ GFP+; Fig. 6B) compared to animals that received P25 Th1 cells stimulated in the absence of P3CSK4. Interestingly, we also observed an increase of total lung CD8+- (Fig. 6C) but not total CD4+- T cells (data not shown) in recipients of P25 Th1-P3CSK4 cells compared to controls. On the contrary, we did not observe an increase of CD8+ T cells in recipients of P25 Th1 cells compared to controls (Fig. 6C). These results suggest that engagement of TLR2 on CD4+ T cells during priming may enhance effector function upon encounter with cognate Ag in vivo and, in the case of MTB challenge, this might translate into better infection control.
Figure 6. TLR2 ligand up-regulates ability of Th1 effector cells to confer protection against MTB infection upon adoptive transfer.
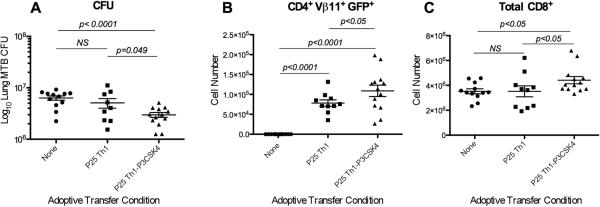
Th1 effector CD4+ T cells were generated in vitro by stimulating naïve CD4+ T cells from GFP+ P25 TCR-Tg mice with anti-CD3 mAb- and anti-CD28 mAbs, IL-12 and anti-IL-4 mAb in the absence (P25 Th1) or presence (P25 Th1-P3CSK4) of P3CSK4 (2 μg/ml). CD4+ Th1 effector cells were adoptively transferred to WT recipient mice (2 × 106 cells/mouse) that were subsequently infected with MTB H37Rv. (A) CFUs were determined in lung homogenates 28 days after infection. (B) GFP+ P25 TCR Tg (CD4+ Vβ11+GFP+) T cells and (C) total CD8+ T cells were determined in lungs 28 days after infection by flow cytometry. Figure represents a compilation of three experiments performed with 3-5 animals/group/experiment. Each symbol represents data from an individual mouse (no antigen, n=12; Ag85B, n=11; Ag85B + P3CSK4, n=13). Horizontal lines indicate the means ± SEM. NS: not statistically significant.
DISCUSSION
Microbial sensing by TLRs expressed on T cells has been described but the exact role of this recognition system in the immune response to infection is not well understood. We previously demonstrated that human CD4+ T cells recognize mycobacterial lipoproteins via TLR2 and this triggers co-stimulatory signals that up-regulate TCR-driven activation [14]. In this study, we developed a mouse model to further understand the effects of TLR2 ligation on CD4+ T cells in the immune response to mycobacterial Ags and its impact in the control of MTB infection in vivo. We found that ligation of TLR2 on mouse CD4+ T cells synergizes with TCR signals to increase the frequency of mycobacterial Ag specific T cells after immunization and to promote Th1 differentiation and effector functions. More importantly, simultaneous TCR and TLR2 ligation during in vitro priming confers Th1 effector CD4+ T cells with increased capacity to protect against MTB infection in vivo. These results suggest that TLR2 ligand recognition by CD4+ T cells in vivo could influence the quality and magnitude of the effector responses to MTB Ags.
MTB expresses two classes of TLR2 ligands, lipoproteins and glycolipids, that traffic in and out the infected macrophage, and have major effects on both innate and adaptive immune cells [17, 18]. The absence of TLR2 in mice results in a greater susceptibility to chronic or high-dose aerosol MTB infection, suggesting a defect in acquired immunity [19-21]. Polymorphisms in TLR2 have been associated with an increased risk of TB or with disseminated forms of TB in different populations [22, 23]. Although these genetic association studies cannot provide a direct causal relationship between TLR2/1 polymorphisms and risk of TB, they do suggest that TLR2 may have a role in development of protective immune responses to MTB.
The role of TLR2 in MTB infection remains controversial and this may be due to its multifaceted nature. First, TLR2 has a dual role in regulation of APC function. On one hand, TLR2 ligands stimulate innate immunity, i.e. trigger DC maturation, pro-inflammatory cytokines (IL-12, TNF-α) and antimicrobial activity [24, 25]. On the other hand, chronic exposure of APC to TLR2 ligands decreases MHC-II Ag processing and presentation in macrophages [26, 27]. Thus, the balance of up and down-regulation of APC function by TLR2 impacts CD4+ T cell responses. Second, TLR2 is also expressed on T cells, including CD4+ T cells isolated from TB pleural effusions, where it plays a co-stimulatory role [9, 14, 28]. T cell TLR2 may impact development of cell-mediated immunity to MTB, and defects of these mechanisms could be linked to susceptibility to develop active TB. Thus, novel in vivo models for the analysis of TLR2 in different immune compartments and at different phases of infection may provide a better insight on the role of TLR2 in infection than the traditional knockout mouse models. A tissue-specific conditional knockout mouse system is currently under development in our lab. The model used in this study is a preliminary attempt to address the role of T cell TLR2 and to confine the effects of TLR2 ligands in vivo to the CD4+ T cell compartment by using TLR2-deficient BMDM and mice in combination with TLR2+ MTB Ag specific T cells. Our results indicate that TLR2 expressed on CD4+ T cells may play an important role in T cell priming and regulation of effector function. These results will require validation in models like the one mentioned above, where expression of TLR2 in the APC compartment is preserved.
As demonstrated here, expression of TLR2 on T cells is several orders of magnitude lower than that of APCs. In spite of low receptor expression, TLR2 ligation on CD4+ T cells induces profound changes in T cell activation. Whether TLR2 engagement happens uniformly in all CD4+ T cells at a low level or is restricted to a small and specialized CD4+ T cell subset is not known and merits further investigation. Since detecting TLR2 on T cells by flow cytometry has proven difficult, this will require development of new reagents to improve the sensitivity of this assay to further explore regulation of TLR2 expression on T cells both in vitro and in vivo.
The role of TLR2 in CD4+ T cell priming and differentiation is controversial. TLR2 engagement promoted Th2 responses in some studies [29], induced IFN-γ secretion in others [10, 30] and either enhance [31] or down-regulate [32] T-reg activity. Also, TLR2 engagement has been shown to promote effector and memory IL-2/IL-17 secreting CD4+ T cells in responses to an influenza Ag [33]. In most of these studies effects were the result of TLR2 engagement on APCs. In agreement with Imanishi et al. [10], our results indicate that TLR2 engagement on CD4+ T cells induces predominantly Th1 differentiation after both polyclonal and Ag specific stimulation in vitro. Similarly, in vivo ligation of TLR2 on CD4+ T cells induced expansion of IFN-γ secreting T cells in response to MTB Ag. In accordance to Chandran et al. [33], we also observed a TLR2 driven increase of IL-17 secreting Ag specific CD4+ T cells in vivo but not in vitro. This suggests that Th17 differentiation in vivo may require a combination of stimuli and conditions (i.e. TGF-β, IL-6, IL-21, IL-23) not present in vitro but that TLR2 signaling in combination with these other stimuli may contribute to Th17 commitment. Thus, upon primary encounter with a cognate Ag, the role of CD4+ T cell-expressed TLR2 might be the amplification of signals initiated via TCR and CD28 and strengthening of the Th1 and Th17 differentiation programs.
Different strategies are being pursued to develop more effective TB vaccines, including subunit vaccines [34]. One of the roadblocks to progress in this field is the lack of adjuvants to induce potent cell mediated immunity [35]. TLRs 3-5 and 7-9 are attractive targets for new adjuvants because they activate DC, increase co-stimulation and induce IL-12 that promotes CD4+ T cell priming and Th1 differentiation required for protection against bacteria and viruses [36]. Lipoproteins have been tested as adjuvants for TB vaccines with mixed results, i.e. Th1 [30, 37] or Th2 induction [38]. When lipoproteins were administered as part of a subunit vaccine, they induced Th1 responses [30]. The emerging evidence that T cells themselves recognize TLR2 ligands and that this, in combination with TCR signals, triggers proliferation and IFN-γ secretion suggest this mechanism could be part of an adjuvant effect [9, 14]. In light of this, we propose that combining a potent DC adjuvant such as CpG, along with a TLR2 ligand, especially if delivery of the latter can be prolonged so as to target TLR2 expressing-activated T cells, may improve T cell priming during immunization by targeting both the innate and adaptive immune systems. Thus, TLR2 ligands used as vaccine adjuvants could improve CD4+ T cell responses by both indirect (DC activation, increased T cell priming) and direct (T cell co-stimulation, clonal expansion, amplification of cytokine responses) pathways to increase effector Th1 and memory responses to subunit vaccines.
Although IFN-γ secretion by effector CD4+ T cells is considered to be essential for activation of macrophages and MTB killing, recent work by Gallegos et al. supports that CD4+ T cells have IFN-γ independent mechanisms for controlling MTB infection in vivo [39]. In addition, Green et al. suggest that CD4+ T cell-derived IFN-γ might have a role in enhancing CD8+ T cell function during MTB infection [40]. Our data demonstrates that Th1 effector CD4+ T cells activated in vitro in the presence of TLR2 ligand have increased ability to expand and protect against MTB infection compared to Th1 cells generated in the absence of TLR2 ligand. This enhanced protection was associated not only with increased numbers of Ag-specific CD4+ T cells but also higher number of CD8+ T cells. Thus, it is possible that mechanisms other than IFN-γ activation of macrophages, including enhanced CD8+ T cell function, might be involved in this anti-MTB effect. Further studies will be required to confirm the protective effect of triggering TLR2 on CD4+ T cells in MTB infection and to establish if cytotoxicity, bystander regulation of CD8+ T cells, or other mechanisms are involved. Additionally, we speculate that engaging TLR2 on CD4+ T cells during priming may have long-term protective effects due to enhanced function and survival of effector and effector memory T cells. These cells could also have a lower TCR threshold of activation, contributing to maintaining memory responses to antigens that are expressed at low levels during chronic infection. Studies are in progress in our lab to address these issues in a mouse model of chronic MTB infection.
In contrast to observations made by Gallegos et al. [16] using an ESAT-6 TCR Tg system, we did not observe protection with P25 TCR Tg Th1 cells generated in the absence of P3CSK4. This is probably due to technical modifications we introduced in our experimental setup, namely the use of a different TCR Tg model, lower numbers of cells transferred, and the use of polyclonal- instead of peptide specific- stimulation for the generation of Th1 cells. Using Th1 cells of limited efficacy allowed us to demonstrate that TLR2 ligation during priming improves the capacity of resulting effector T cells to protect against infection.
In conclusion, our data strengthens the notion that microbial sensing by the adaptive immune system and more particularly via CD4+ T cell expressed TLR2, may play a role in the amplification of Ag specific Th1 responses. We also provide preliminary evidence that this mechanism may have a role in immune protection against MTB infection. Finally, we put forward the concept of direct T cell adjuvanticity via innate receptors and their use in novel vaccine designs to help improve Th1 effector and memory responses and protection against MTB.
MATERIALS AND METHODS
Mice
8-12 week old female, C57BL/6J mice (WT) were purchased from Charles River Laboratories (North Wilmington, MA). Tlr-2 gene knockout mice in the C57BL/6J background (Tlr2-/-) were generously provided by O. Takeuchi and S. Akira (Osaka University, Japan) [41]. Mycobacterial Ag 85B specific- TCR transgenic (P25 TCR-Tg) mice were provided by Kiyoshi Takatsu (University of Tokyo, Japan) [42]. P25 TCR-Tg were bred to GFP-expressing mice (C57BL/6-Tg(UBC-GFP)30Scha, Jackson laboratories) to generate homozygous GFP expressing P25 TCR-Tg mice (Supporting information Fig. S3). Mice were housed under specific-pathogen-free conditions in ventilated microisolator cages (Lab Products, Inc., Maywood, NJ). All studies were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
Bacteria, antigens and peptides
Mycobacterium tuberculosis
H37Rv (Colorado State University) was grown on 7H10 agar plates. Colonies were transferred to liquid GAS Media and grown to an absorbance of 1.1 (595 nm). Aliquots prepared in media supplemented with 20% glycerol were frozen at -80°C until use. Stock bacterial counts were determined by the colony-forming unit (CFU) method. Mycobacterial Ag 85B (Ag85B-Rv1886c) was cloned and expressed as described (TBVTRM Contract HHSN266200400091c website; http://csucvmbs.colostate.edu/academics/mip/Pages/Dobos-Laboratory-Manuals-and-SOPs.aspx). Endotoxin content in purified protein was 3.6 EU/ml of 1,000x stock solution, as measured by the LAL Chromogenic Endotoxin Quantitation Kit (Fisher). Ag85B, an immunodominant MTB Ag, was used as a model antigen in this study because its sequence encompasses the major epitope recognized by the P25 TCR-Tg T cells (peptide 25). The 15-mer sequence of Ag85B (aa 240-254), i.e. peptide 25 (FQDAYNAAGGHNAVF) was synthesized by Invitrogen. The synthetic analogue of mycobacterial lipoproteins Pam3Cys-SKKKK (EMC Microcollections GmbH, Germany) was used as a surrogate TLR2/1 agonist due to the cost and time required for cloning and expression of natural lipoproteins in the amounts required for these experiments. P3CSK4 has been extensively validated as a reference compound for TLR2/1 activation. We have previously demonstrated that the P3CSK4 effect mimics that of mycobacterial lipoproteins on human CD4+ T cell co-stimulation [14]. Mycobacterial lipoprotein LprG cloned and expressed as described [14] was used in a limited number of experiments to confirm results obtained with P3CSK4.
Isolation of naïve CD4+ T cells
Highly purified naïve CD4+ T cells devoid of APC contamination were isolated from spleens using a combination of immune-magnetic cell sorting (MACS) followed by fluorescence activated cell sorting (FACS) as described with some modifications [13, 14]. The average purity was 99.6 ± 0.15% CD3+, 93.87 ± 1.93 CD4+. See supporting information for complete protocol and cell purity (Fig. S4).
In vitro generation of effector CD4+ T cells
Naïve CD4+ T cells from WT or P25 TCR Tg mice were stimulated with anti-CD3/CD28 mAb coated-microbeads (Invitrogen) with 1 μg/ml of recombinant IL-2 (eBioscience) as per manufacturer instructions. After three days, microbeads were removed and cells rested overnight. On day 4, dead cells and debris were removed by Ficoll gradient centrifugation (Ficoll-Plaque Plus, GE Healthcare) and cell purity determined by FACS (100% CD3+, 93.9 ± 1.51 CD4+, n= 4; not shown).
In vitro T cell stimulation assays
Anti-CD3 mAb stimulation
Naïve- or in vitro-generated effector CD4+ T cells (105 cells/well) stimulated with different concentrations of plate-bound anti-mouse CD3 mAb (Fisher) in combination with different concentrations of P3CSK4 or media alone. Culture supernatants (50 μl) were collected at 24 h (IL-2) and 48 h (IFN-γ) for cytokine quantification. Cell proliferation was determined with the [3H]thymidine incorporation assay as described [14].
Ag85B-specific stimulation
Bone marrow derived macrophages (BMDM) from Tlr2-/- mice were isolated and differentiated for 7 days in L-929 conditioned media as a source of M-CSF (L-929 cells, ATCC). Forty eight hours prior to use, BMDM were incubated with 4 ng/ml IFN-γ (Peprotech, Rocky Hill, NJ) to upregulate MHC-II expression, then cultured overnight in complete media with or without Ag85B. BMDM were then washed and co-cultured with naïve- or with in vitro-generated effector P25 TCR-Tg CD4+ T cells. Culture supernatants were taken at 24 and 48 h for cytokine measurements and cell proliferation was determined as before.
Cytokine quantification
IL-2 was measured by ELISA with anti-IL-2 Ab pairs (eBioscience). IFN-γ was measured by ELISA (R&D Systems).
Adoptive transfer of naïve CD4+ T cells and Ag85B immunization
Age and sex-matched Tlr2-/- mice were inoculated with GFP+ P25 TCR-Tg naïve CD4+ T cells by retro-orbital injection (106 cells/mouse). One day later, mice were immunized in the base of the tail with CpG-ODN1826 (10 μg/mouse; Invivogen) alone or in combination with Ag85B (5 μg/mouse) or with Ag85B (5 μg/mouse) and P3CSK4 (30 μg/mouse) in incomplete Freund’s adjuvant (GibcoBRL; 100 μL emulsion/mouse). CpG-ODN1826 was used as a TLR9 agonist to generate optimal DC co-stimulatory function during priming. In preliminary experiments, a group of animals were immunized with P3CSK4 alone. Mice were sacrificed and spleens harvested 7 days after immunization. GFP+ P25 TCR-Tg cells (CD4+ Vβ11+ GFP+) in spleens were enumerated by flow cytometry.
Adoptive transfer of Th1 effector cells and aerosol infection with MTB
Generation of Th1 cells and adoptive transfer
Naïve CD4+ T cells from spleens of GFP+ P25 TCR-Tg mice were stimulated with anti-CD3 mAb/anti-CD28 mAb coated beads, 10 ng/ml IL-12, 5 μg/ml of neutralizing anti-mouse IL-4 mAb (R&D Systems) and with or without 2 μg/ml P3CSK4. Stimuli were removed on day 3 and cells rested overnight. On the next day, dead cells were eliminated as before and viable cells re-suspended in normal saline. Lack of cell proliferation before adoptive transfer was confirmed in a small sample of cells with the 3[H]thymidine incorporation assay (data not shown). Age and sex-matched WT mice were inoculated with non-dividing GFP+ P25 TCR-Tg effector Th1 CD4+ T cells (2 × 106 cells/mouse) as before.
Aerosol infection with MTB
Mice were exposed to aerosolized H37Rv for 30 min in the animal BSL-3 facility as previously described [43]. Lungs were removed and bacterial loads were determined with the CFU method as described [43]. The initial MTB inoculum was determined 1 day after infection and ranged between 100-200 CFUs/lung. GFP+ P25 TCR-Tg cells (CD4+ Vβ11+ GFP+) in lungs were detected by flow cytometry.
Cell surface receptors, intracellular cytokines, TLR2 protein and TLR2 mRNA quantification
Cell surface receptors and intracellular cytokines were measured by flow cytometry as described[43]. TLR2 protein expression in CD4+ T cell lysates was determined by western blotting and TLR2 mRNA was quantified by RT-PCR as described[14]. For additional details on these methods see Supporting information.
Statistical analysis
GraphPad Prism version 6 for MacOSX (GraphPad Software, San Diego CA) was used to test for differences in means between specific treatment groups. The null hypothesis of no difference in means between specific treatment groups was tested using a Student's t-test with a p-value <0.05 taken as evidence of a significant difference between groups (*p <0.05; **p <0.01; ***p <0.005; **** p<0.001).
Supplementary Material
ACKNOWLEDGEMENTS
We thank the CWRU/UH CFAR (NIH P30 AI036219) and the Cytometry Facility of the Case Comprehensive Cancer Center (NIH P30 CA43703) for access to core facilities that supported this research and Scott Howell and the Microscopy Core of the Visual Sciences Research center at CWRU (NIH P30 EY11373) for access to and technical assistance with fluorescence microscopy imaging. We thank W. Henry Boom for insightful discussions that improved this manuscript. This work was supported by NIH grants AI099494 (to R.E.R), CFAR Developmental Award (CWRU/UH CFAR, P30 AI036219) (to R.E.R), and AI034343 (to C.V.H).
Abbreviations
- BMDM
bone marrow derived macrophages
- TB
tuberculosis
- MTB
Mycobacterium tuberculosis
- P3CSK4
Pam3Cys-SKKKK
Footnotes
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.WHO Global Tuberculosis Report 2012. World Health Organization Press. 2012;1:8–28. [Google Scholar]
- 2.Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19:491–494. doi: 10.1016/s0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boesteanu AC, Katsikis PD. Memory T cells need CD28 costimulation to remember. Semin Immunol. 2009;21:69–77. doi: 10.1016/j.smim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imanishi T, Hara H, Suzuki S, Suzuki N, Akira S, Saito T. Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol. 2007;178:6715–6719. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- 11.Cottalorda A, Mercier BC, Mbitikon-Kobo FM, Arpin C, Teoh DY, McMichael A, Marvel J, et al. TLR2 engagement on memory CD8(+) T cells improves their cytokine-mediated proliferation and IFN-gamma secretion in the absence of Ag. Eur J Immunol. 2009;39:2673–2681. doi: 10.1002/eji.200939627. [DOI] [PubMed] [Google Scholar]
- 12.Mercier BC, Cottalorda A, Coupet CA, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J Immunol. 2009;182:1860–1867. doi: 10.4049/jimmunol.0801167. [DOI] [PubMed] [Google Scholar]
- 13.Lancioni CL, Thomas JJ, Rojas RE. Activation requirements and responses to TLR ligands in human CD4+ T cells: comparison of two T cell isolation techniques. J Immunol Methods. 2009;344:15–25. doi: 10.1016/j.jim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancioni CL, Li Q, Thomas JJ, Ding X, Thiel B, Drage MG, Pecora ND, et al. Mycobacterium tuberculosis lipoproteins directly regulate human memory CD4(+) T cell activation via Toll-like receptors 1 and 2. Infect Immun. 2011;79:663–673. doi: 10.1128/IAI.00806-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas A, Banerjee P, Biswas T. Porin of Shigella dysenteriae directly promotes toll-like receptor 2-mediated CD4+ T cell survival and effector function. Mol Immunol. 2009;46:3076–3085. doi: 10.1016/j.molimm.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, et al. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 18.Gilleron M, Nigou J, Nicolle D, Quesniaux V, Puzo G. The acylation state of mycobacterial lipomannans modulates innate immunity response through toll-like receptor 2. Chem Biol. 2006;13:39–47. doi: 10.1016/j.chembiol.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Reiling N, Holscher C, Fehrenbach A, Kroger S, Kirschning CJ, Goyert S, Ehlers S. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169:3480–3484. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 20.Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, Fremond C, et al. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velez DR, Wejse C, Stryjewski ME, Abbate E, Hulme WF, Myers JL, Estevan R, et al. Variants in toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum Genet. 2010;127:65–73. doi: 10.1007/s00439-009-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motsinger-Reif AA, Antas PR, Oki NO, Levy S, Holland SM, Sterling TR. Polymorphisms in IL-1beta, vitamin D receptor Fok1, and Toll-like receptor 2 are associated with extrapulmonary tuberculosis. BMC Med Genet. 2010;11:37. doi: 10.1186/1471-2350-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 26.Noss EH, Harding CV, Boom WH. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell Immunol. 2000;201:63–74. doi: 10.1006/cimm.2000.1633. [DOI] [PubMed] [Google Scholar]
- 27.Kincaid EZ, Wolf AJ, Desvignes L, Mahapatra S, Crick DC, Brennan PJ, Pavelka MS, Jr., et al. Codominance of TLR2-dependent and TLR2-independent modulation of MHC class II in Mycobacterium tuberculosis infection in vivo. J Immunol. 2007;179:3187–3195. doi: 10.4049/jimmunol.179.5.3187. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Zhang M, Zhu X, Deng Q, Liu H, Larmonier N, Graner MW, et al. Engagement of Toll-like receptor 2 on CD4(+) T cells facilitates local immune responses in patients with tuberculous pleurisy. J Infect Dis. 2009;200:399–408. doi: 10.1086/600075. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 30.Wang B, Henao-Tamayo M, Harton M, Ordway D, Shanley C, Basaraba RJ, Orme IM. A Toll-like receptor-2-directed fusion protein vaccine against tuberculosis. Clin Vaccine Immunol. CVI. 2007;14:902–906. doi: 10.1128/CVI.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 32.Oberg HH, Ly TT, Ussat S, Meyer T, Kabelitz D, Wesch D. Differential but direct abolishment of human regulatory T cell suppressive capacity by various TLR2 ligands. J Immunol. 2010;184:4733–4740. doi: 10.4049/jimmunol.0804279. [DOI] [PubMed] [Google Scholar]
- 33.Chandran SS, Verhoeven D, Teijaro JR, Fenton MJ, Farber DL. TLR2 engagement on dendritic cells promotes high frequency effector and memory CD4 T cell responses. J Immunol. 2009;183:7832–7841. doi: 10.4049/jimmunol.0901683. [DOI] [PubMed] [Google Scholar]
- 34.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dissel JT, Arend SM, Prins C, Bang P, Tingskov PN, Lingnau K, Nouta J, et al. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine. 2010;28:3571–3581. doi: 10.1016/j.vaccine.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 36.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 37.Sieling PA, Chung W, Duong BT, Godowski PJ, Modlin RL. Toll-like receptor 2 ligands as adjuvants for human Th1 responses. J Immunol. 2003;170:194–200. doi: 10.4049/jimmunol.170.1.194. [DOI] [PubMed] [Google Scholar]
- 38.Rao V, Dhar N, Shakila H, Singh R, Khera A, Jain R, Naseema M, et al. Increased expression of Mycobacterium tuberculosis 19 kDa lipoprotein obliterates the protective efficacy of BCG by polarizing host immune responses to the Th2 subtype. Scand J Immunol. 2005;61:410–417. doi: 10.1111/j.1365-3083.2005.01569.x. [DOI] [PubMed] [Google Scholar]
- 39.Gallegos AM, van Heijst JW, Samstein M, Su X, Pamer EG, Glickman MS. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 2011;7:e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green AM, Difazio R, Flynn JL. IFN-gamma from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J Immunol. 2013;190:270–277. doi: 10.4049/jimmunol.1200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugawara I, Yamada H, Li C, Mizuno S, Takeuchi O, Akira S. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol Immunol. 2003;47:327–336. doi: 10.1111/j.1348-0421.2003.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 42.Tamura T, Ariga H, Kinashi T, Uehara S, Kikuchi T, Nakada M, Tokunaga T, et al. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. Int Immunol. 2004;16:1691–1699. doi: 10.1093/intimm/dxh170. [DOI] [PubMed] [Google Scholar]
- 43.Anis MM, Fulton SA, Reba SM, Harding CV, Boom WH. Modulation of naive CD4+ T-cell responses to an airway antigen during pulmonary mycobacterial infection. Infect Immun. 2007;75:2260–2268. doi: 10.1128/IAI.01709-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.