Abstract
Elastin is a structural protein that provides resilience to biological tissues. We examined the contributions of elastin to the quasi-static tensile response of porcine medial collateral ligament through targeted disruption of the elastin network with pancreatic elastase. Elastase concentration and treatment time were varied to determine a dose response. Whereas elastin content decreased with increasing elastase concentration and treatment time, the change in peak stress after cyclic loading reached a plateau above 1 U/ml elastase and 6 hr treatment. For specimens treated with 2 U/ml elastase for 6 hr, elastin content decreased approximately 35%. Mean peak tissue strain after cyclic loading (4.8%, p≥0.300), modulus (275 MPa, p≥0.114) and hysteresis (20%, p≥0.553) were unaffected by elastase digestion, but stress decreased significantly after treatment (up to 2 MPa, p≤0.049). Elastin degradation had no effect on failure properties, but tissue lengthened under the same pre-stress. Stiffness in the linear region was unaffected by elastase digestion, suggesting that enzyme treatment did not disrupt collagen. These results demonstrate that elastin primarily functions in the toe region of the stress-strain curve, yet contributes load support in the linear region. The increase in length after elastase digestion suggests that elastin may pre-stress and stabilize collagen crimp in ligaments.
Keywords: ligament, elastin, elastase, tensile, quasi-static
Introduction
The elastin network in biological tissues provides a restorative force after deformation.1–6 Elastin is assembled in the extracellular space and consists of a core of tropoelastin molecules surrounded by a fibrillin-rich microfibril scaffold.1,2 Repeating α-helix segments composed of alanine and lysine oxidize to form highly stable covalent crosslinks between tropoelastin molecules.7,8 Elastin stretches and recoils through both entropic and hydrophobic mechanisms.1,2 Via selective degradation with elastin specific proteases like elastase9, the mechanical contributions of elastin in ligament can be quantified. Elastase cleaves tropoelastin, creating fragments of the network that vary in size and number of intact crosslinks.9 Elastin fragments may contribute in some part to the tissue response while trapped in the extracellular matrix, so the relative level of degradation may incrementally affect the mechanics of the tissue.
Elastin dominates the mechanical response of highly extensible tissues like artery, heart valve, and nuchal ligament, which consist of up to 70% elastin.3,4,10 Elastase degradation decreases stiffness and peak stress, and increases energy dissipation in these tissues.3,4 These changes are not as pronounced in tissues with lower elastin content, like skin and tendon, which are composed of < 7% elastin.5,6,8,11,12
The influence of elastin on major structural ligaments is not fully understood. It is believed that elastin stabilizes the collagen crimp in ligament13,14, given that it is localized along the surface of collagen.2,12,15 The physiologic scale (e.g. fibril, fiber, fascicle, tissue) on which elastin functions is not well understood. Characterization of the macro-scale mechanical contributions of elastin in ligament is necessary to provide a baseline from which the origins of elasticity and failure of the tissue can be studied. Additionally, ligament repair and replacement strategies may benefit once the influence of elastin is characterized.
We investigated the contributions of elastin to the quasi-static tensile response of ligament along the primary collagen fiber direction. Porcine medial collateral ligament (MCL) was tested before and after selective degradation of elastin, and dose-response tests were performed to quantify the degree of elastin degradation required to alter the material behavior.
Methods
Experimental Design
55 porcine knees (age 5–8 mo, mixed sex, Innovative Medical Device Solutions, UT) were fresh frozen until testing. Knees were thawed, and the MCL was fine dissected to remove overlying fascia. The ligament was hydrated with PBS during preparation. Isolated MCLs were frozen to −70 °C, and a dogbone shaped specimen was punched from the mid-substance, with the axis of the punch aligned with the primary collagen fibers.16 Specimen width and thickness were measured 3 times at the center of the gauge length with a micrometer (accuracy ±0.02 mm). To visually monitor applied strain, 3 beads (300 μm diam) were evenly spaced down the axis of the gauge length and affixed with cyanoacrylate.
Specimens served as their own controls via repeated testing before and after treatment.16 Specimens were divided between two groups: control treatment or elastase digestion. Quasi-static tensile testing was performed pre- and post-treatment for each specimen, and tissue was then assayed to determine the elastin content. Dose response experiments (enzyme concentration, treatment time) were performed to determine if elastin provided a graded contribution to the mechanical response of ligament.
15 MCLs were used to examine the influence of elastase concentration. Each specimen was treated for 3 hr under one of the following conditions: control (buffer only) or 0.1, 1.0, 10.0, or 20.0 U/ml elastase (3 specimens/dose). Subsequently, 24 ligaments were used to examine the influence of treatment time. Specimens were treated with either control buffer or 1 U/ml elastase 1, 3, 6, or 24 hr (3 specimens/treatment/time period). Based on the results of the graded concentration and treatment time period, 16 additional specimens underwent the test sequence with either control buffer (8) or elastase (8) for 6 hr at 2 U/ml.
Uniaxial Tensile Testing
Testing was adapted from a protocol used to study the effect of Chondroitinase B treatment on the tensile response of ligament.16 Briefly, specimens were randomly assigned to treatment groups and clamped in a custom testing system. A servo-driven positioning stage (Tol-O-Matic, MN, accuracy ±0.1 μm) applied displacement to the tissue along its primary collagen axis. Motion analysis software (DMAS v6, Spicatek, HI, accuracy ±0.5 μm) was used to record the position of the fiducial markers and measure applied strain.16,17
A 0.05 MPa pre-stress was applied to each specimen before preconditioning at 10% strain (based on clamp to clamp distance,) which was applied at 1 %/sec and held for 1 min. 10% clamp strain ensured that the tissue strain was below the sub-structural failure limit of ligament (5–6%18), where tissue strain is about half of clamp strain.19 Load was removed, and the tissue was allowed to recover for 5 min while wrapped in PBS soaked gauze.
11 cycles of 10% clamp strain were applied with a triangle displacement waveform (1 %/sec). Force and displacement were monitored continuously via a load cell (LSB200 5 lb., Futek, CA, accuracy ±0.1% FS) and LVDT (ATA 2001, Schaevitz, VA, accuracy ±0.05% FS), respectively. The 11th cycle was used to calculate peak stress and tissue strain after cyclic loading. Tangent modulus was calculated from linear regression of the last 1% tissue strain in the linear region of the stress-strain curve. Hysteresis was calculated as the area enclosed by the loading and unloading curves. Three metrics were examined to characterize the toe region. First, λ* defined the clamp strain and resultant stress where crimp was removed and collagen was fully engaged, starting the linear region of the stress-strain curve.20 The integrated stress (strain energy) up to λ* was also reported. Next, to separate the influence of elastase treatment on the toe and linear regions, a transition point was defined from the intersection of a bilinear curve fit of the stress-strain curve.21 Finally, a local modulus was calculated as the slope of the linear regression of points in the toe region, incorporating 3 points on either side of the applied strain in question.
Treatment protocol
All specimens were equilibrated for 15 min in control buffer (15 ml 1x PBS, 0.1 mg/ml soybean trypsin inhibitor (SBTI) at room temperature). SBTI is suggested to block proteolytic activity against collagen5,6, likely if the elastase is contaminated with trypsin. Mechanical testing was carried out, then the clamps were locked together and removed from the machine to ensure no deformation of the tissue during enzyme treatment. The clamp/tissue assembly was bathed in 15 ml of control buffer or control buffer + elastase (trypsin-free porcine pancreatic elastase, EPC134, Elastin Products Co., MO). Elastase concentration (U/ml) was based on manufacturer-stated activity of >9 U/mg of protein on the substrate Suc-Ala-Ala-Ala-pNA, and that 1 U will hydrolyze 1 μmole of substrate/min at pH 8.3 and 25°C. Treatment time and elastase concentration were altered during the dose response experiments. For 24 hr samples, 0.01% thiomersal was added as an antiseptic and antifungal agent. Conditions for the final 16 specimens were based on the results of the dose response study (6 hr, 2 U/ml elastase).
The clamp assembly was returned to the test machine after treatment, and the locking mechanism was removed. Post-treatment testing was the same as pre-treatment, with the exception that the stress relaxation step performed prior to setting the initial length was omitted to preserve the length of the tissue between trials.
A new initial length was established after post-treatment testing based on the 0.05 MPa pre-stress. The tissue was then loaded to failure at 1 %/sec clamp strain. Specimens were removed from the clamps, and tissue between the clamps was retained for elastin quantification. Samples were frozen at −70° C until biochemical analysis.
Elastin Quantification
Specimens were thawed, and the wet weight was recorded. Specimens were lyophilized, and then dry weight was recorded to calculate water content. To facilitate removal of digested elastin fragments trapped in the tissue macrostructure, tissue was rinsed with 200 mM glycine, pH 2.8, at room temperature for 30 min under gentle agitation, 3 times. Specimens were washed 3 times with PBS for 10 min prior to being lyophilized.
The Fastin Elastin colorimetric assay (Biocolor Ltd., UK) was used to quantify elastin content according to manufacturer’s instructions.22,23 Briefly, tissue was treated twice in 30 volumes of 0.25 M oxalic acid at 100° C. The soluble fraction was retained, and elastin was precipitated from a 100 μl aliquot, then allowed to bind the elastin-specific dye for 1.5 hr under gentle agitation at room temperature. Bound dye was suspended in a 250 μl volume and then transferred to a 96-well plate. Absorbance was measured on a plate reader (Synergy HT, Bio-TEK, VT) and compared to a standard curve generated from known concentrations of alpha-elastin. Alpha-elastin originates from oxalic acid solubilization and differs from beta-elastin in that it precipitates.24,25 Elastin content was normalized to wet weight.
Statistical Analysis
All comparisons were carried out with paired (repeated measures) or independent (between treatment groups) t-tests. Holm’s step-down correction was used, allowing for multiple comparisons de in lieu of analysis of variance and post hoc analysis. Significance was set at p ≤ 0.05 for all tests. Data are presented as mean ± SD unless otherwise noted.
Results
Dose Response - Elastase Concentration
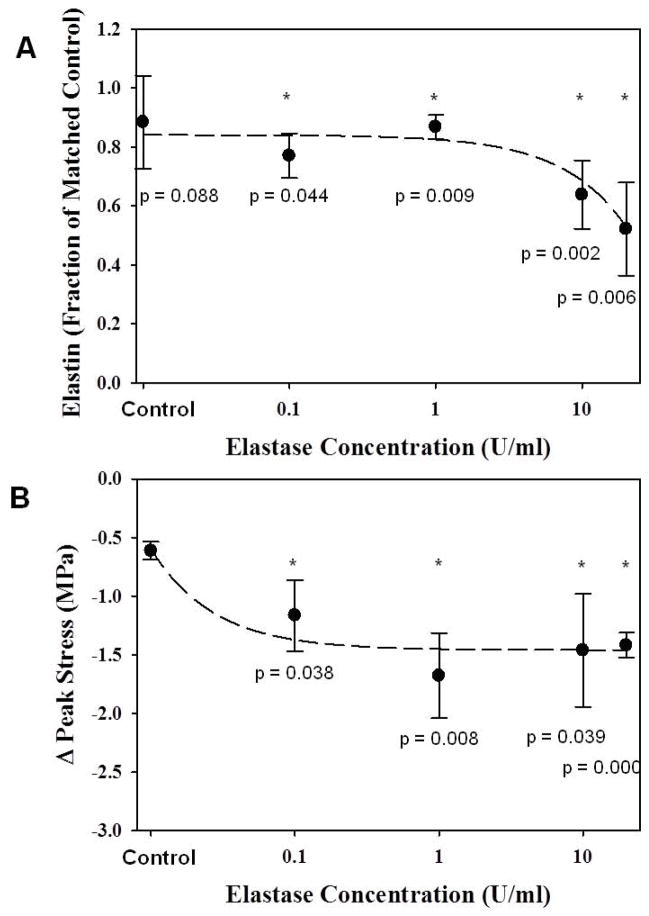
No differences were found in initial length or cross-sectional area between control and elastase groups (Table 1, p≥0.33). Water content was higher for elastase treated tissue (p=0.004). Elastin content, as a fraction of matched control samples, was relatively constant at low elastase concentrations, but decreased significantly as elastase concentration increased beyond 1 U/ml (Fig. 2A). Conversely, the peak stress decreased as elastase concentration increased, but the change in stress reached a plateau above 1 U/ml elastase (Fig. 2B). Peak tissue strain and modulus were not significantly different between pre- and post-treatment, and no differences were detected between treatment groups for any concentration (Table 1, p≥0.19). Therefore, test specimens (N=8) were treated with 2 U/ml elastase to ensure treatment was above the threshold for changes in peak stress.
Table 1.
Properties of porcine MCL specimens (Mean ± SD).
| Specimen | Length (mm) | Area (mm2) | (%) Water Content | Peak Tissue Strain (%) | Pre-treatment Modulus (MPa) | Post-treatment Modulus (MPa) |
|---|---|---|---|---|---|---|
| Concentration dose (3 hr) | ||||||
| Control (N = 3) | 19.8 ± 0.7 | 3.5 ± 0.3 | 69.8 ± 5.5 | 4.7 ± 1.4 | 267.0 ± 123.6 | 290.8 ± 153.3 |
| Elastase (N = 12) | 19.3 ± 0.8 | 4.0 ± 0.6 | 75.2 ± 3.8* | 5.6 ± 0.9 | 228.2 ± 128.0 | 256.6 ± 154.0 |
|
| ||||||
| Time dose (1 U/ml) | ||||||
| Control (N = 12) | 20.1 ± 0.7 | 3.8 ± 0.3 | 71.7 ± 4.6 | 5.2 ± 2.2 | 255.4 ± 86.2 | 338.8 ± 137.2 |
| Elastase (N = 12) | 20.0 ± 1.0 | 3.5 ± 0.6 | 77.2 ± 3.2* | 4.6 ± 1.7 | 238.0 ± 86.3 | 279.1 ± 117.0 |
|
| ||||||
| Specimens (6 hr, 2 U/ml) | ||||||
| Control (N=8) | 19.9 ± 0.8 | 3.7 ± 0.2 | 72.3 ± 3.4 | 4.2 ± 0.5 | 279.3 ± 92.7 | 344.8 ± 111.2 |
| Elastase (N=8) | 19.7 ± 1.0 | 3.8 ± 0.8 | 77.6 ± 2.2* | 4.9 ± 1.7 | 262.0 ± 92.8 | 293.1 ± 155.2 |
significant with respect to control group
Figure 2.
A: Elastin content decreased as elastase concentration increased. * - significant w.r.t. matched control; dashed line - linear curve fit. B: The change in peak tensile stress upon elastase degradation increased up to 1 U/ml elastase, above which the change remained constant. * - significant w.r.t. control; dashed line - inverse polynomial curve fit.
Dose Response - Treatment Time
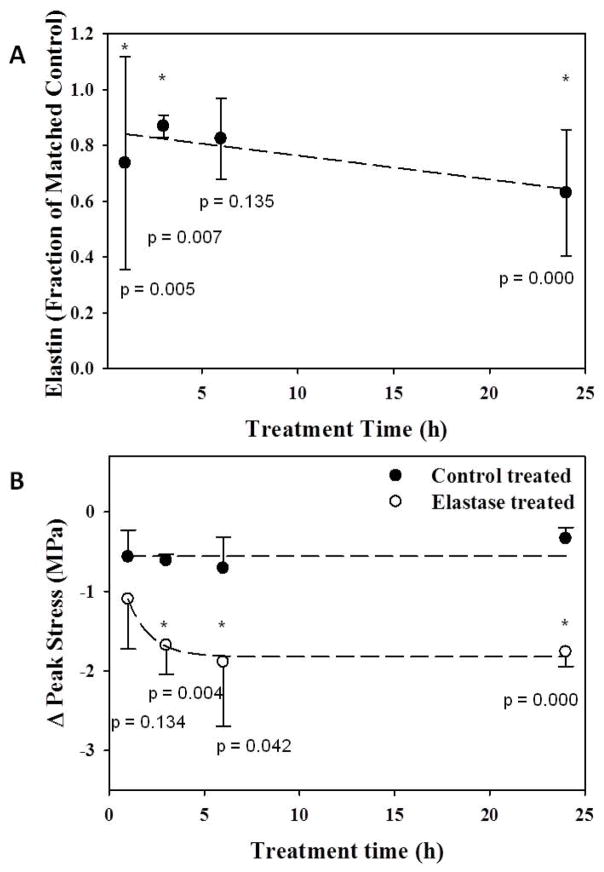
No differences were found in initial length or cross-sectional area between control and elastase groups (Table 1, p≥0.35). Water content was again higher for elastase treated tissue (p=0.000). Elastin content, as a fraction of matched control samples, significantly decreased as digestion time increased, except at 6 hrs (Fig. 3A). Similarly, the peak stress decreased as digestion time increased, but this trend reached a plateau above 6 hrs (Fig. 3B). Peak tissue strain and modulus were not significantly different between pre- and post-treatment, and no differences were detected between treatment groups for any treatment time (Table 1, all p≥0.11). Therefore, test specimens (N=8) were treated for 6 hrs to ensure treatment time was above the threshold for changes in peak stress.
Figure 3.
A: Elastin content decreased as treatment time increased. * - significant w.r.t. matched control; dashed line - linear curve fit. B: The change in peak tensile stress upon elastase degradation increased up to 6 hr of treatment, above which the change remained constant. Change in peak stress for control tissues remained constant regardless of treatment time. * - significant w.r.t. control treated; dashed line - inverse polynomial curve fit.
Influence of Elastin on the Tensile Response of Porcine MCL
No differences were found in initial length or cross-sectional area between control and elastase groups for the final 16 specimens (Table 1, p≥0.61). Water content was higher for elastase treated tissue (p=0.016). Elastin content in control tissue was 11.5±4.0 μg/mg, which dropped to 7.4±1.5 μg/mg after elastase digestion.
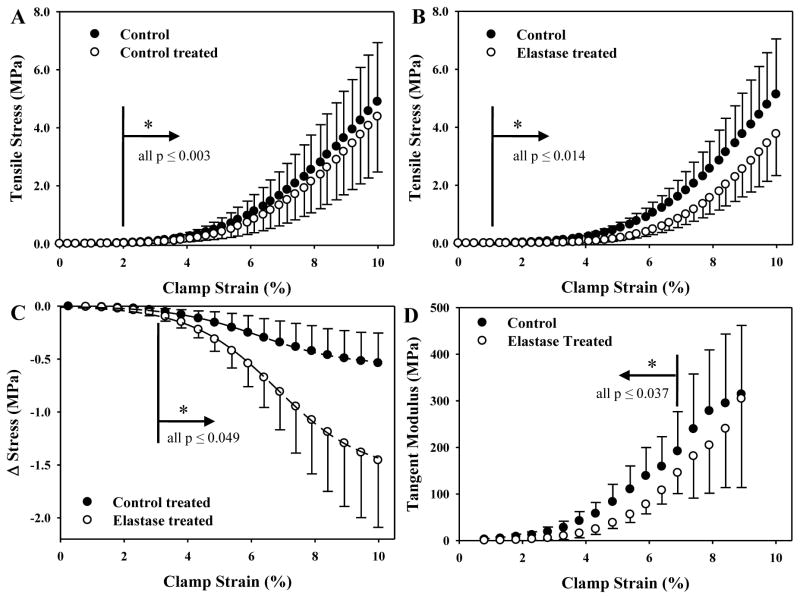
Post-treatment, stress dropped with respect to pre-treatment for both control and elastase treated tissue (Figs. 4A, B), but the change was significantly greater for elastase treated samples (Fig. 4C). Pre- and post-treatment hystereses for control tissue were 19.6±3.1% and 18.9±2.7%, respectively (p=0.63). Pre- and post-treatment hystereses for elastase treated tissue were 20.8±2.6% and 20.0±3.1%, respectively (p=0.53). There were no differences between groups for hysteresis (p≥0.41) or peak tissue strain and modulus (Table 1, p≥0.22).
Figure 4.
Tensile stress-strain curves as a function of control (6 h, buffer only) or elastase treatment (6 h, 1 U/ml). A: Control and control treated samples (N = 8). B: Control and elastase treated samples (N = 8). C: Sigmoid curves fit the stress differential between treatment cases and their respective controls. D: The instantaneous tangent modulus for elastase treated samples was significantly smaller than matched controls up to 7% clamp strain.
The strain denoting the point between the toe and linear regions of the stress-strain curve did not differ between control and elastase treated samples (p = 0.081, λ*, Table 2). Stress at λ* for elastase samples was lower than control (p = 0.000), as was the integrated stress up to λ* (p = 0.000). The transition strain, as defined by the intersection of the bilinear curve fit, increased after elastase treatment (p = 0.000) whereas the transition stress decreased similar to λ* (p = 0.009). The local moduli for elastase treated samples were significantly lower than their matched control counterparts for all points up to 7% clamp strain (Fig. 4D). Beyond 7% strain, the local moduli for control and elastase samples qualitatively converged.
Table 2.
Toe region analysis (Mean ± SD).
| Specimen | λ* Strain (%) | λ* Stress (MPa) | Integrated Stress (MPa) | Transition Strain (%) | Transition Stress (MPa) |
|---|---|---|---|---|---|
| Specimens (6 hr, 2 U/ml) | |||||
| Control (N=8) | 8.5 ± 0.2 | 3.13 ± 0.92 | 25.1 ± 6.8 | 5.5 ± 0.4 | 0.37 ± 0.17 |
| Elastase (N=8) | 8.7 ± 0.1 | 2.26 ± 0.81† | 15.4 ± 5.4† | 6.3 ± 0.4† | 0.24 ± 0.11† |
significant with respect to control group
Upon establishing a new initial length, elastase treated tissue elongated more than control treated tissue (Table 3, p=0.027). No differences were detected between treatment groups for failure stress, tissue strain or modulus (Table 3, all p≥0.14). Neither peak tissue strain nor modulus of the failure tests were different than the cyclic tests (all p≥0.16).
Table 3.
Failure properties after new initial length was established (Mean ± SD).
| Specimen | Δ Length (%) | Failure Stress (MPa) | Failure Strain (%) | Failure Modulus (MPa) |
|---|---|---|---|---|
| Specimens (6 hr, 2 U/ml) | ||||
| Control (N=8) | 2.9 ± 1.0 | 9.1 ± 4.1 | 5.4 ± 2.8 | 243.5 ± 84.2 |
| Elastase (N=8) | 3.9 ± 0.6* | 10.2 ± 6.3 | 4.7 ± 2.2 | 252.5 ± 132.8 |
significant with respect to control group
Discussion
We assessed the contributions of elastin to the quasi-static tensile response of porcine MCL. Enzymatic degradation of elastin reduced tissue stress, originating in the toe-region of the stress-strain curve, but did not alter any other cyclic or failure properties of the ligament. Increases in elastase concentration and treatment time caused graded decreases in elastin content, whereas changes in the stress reached a plateau before maximal elastin degradation. This shows that elastin provides pre-strain and load support within the tissue, regardless of degradation beyond an effective maximum.
Elastase treatment of porcine MCL decreased the stress starting in the toe region of the stress-strain curve, and this change was maintained in the linear region. Elastin provided up to 2 MPa of load support, equivalent to 20–30% of total tissue stress. This is a large contribution from elastin, which makes up ~1% of the tissue wet weight. This contrasts with prior reports in ligament and tendon of 4–6% by wet weight5,6,8,11,12. The literature on elastin in cruciate and collateral knee ligaments is sparse, with studies focusing on visualizing the elastic network26. Our study is a first report of elastin in porcine MCL, so differences could be driven by species or the relative extensibility of the tissue, which is related to overall elastin content. Also, the Fastin assay quantifies α-elastin fragments, which may contain multiple (iso)desmosine crosslinks. Other methods to measure elastin content, like high performance liquid chromatography, isolate the crosslinks and could result in a relative mismatch in elastin content per unit wet weight.
Longer treatment times and higher enzyme concentration did not alter this offset. The influence of elastin was initially detected at low strains, where it is hypothesized to stabilize collagen crimp.13,14 Less stress was supported (Table 2, Figs. 4B, 4C) and modulus decreased (Fig. 4D) after elastase treatment, though collagen was fully engaged at the same point (Table 2, λ*). Transition strain increased after treatment, which corroborates the nearly 1% increase in tissue length (Table 3). These data indicate that elastin is likely coupled to the phenomenon of collagen crimp, where sequential fiber recruitment in the toe region extinguishes the characteristic waveform of the collagen fibers13. The presence of elastin between collagen fibers27–29 could create a pre-stress along the primary tissue axis and transmit forces between adjacent fibers/fiber bundles to reduce shear/sliding between neighbors. This could stabilize the crimp waveform and recoil it after deformation14, softening the sequential recruitment of fibers and potentially limiting micro-failure during repeated loading. Of note, the failure stress was higher for elastase treated tissues (Table 3), though not significantly. This likely arose from the increased initial length, placing the tissue further into the toe region before testing began.
Peak tissue strain during cyclic testing, on average 4.8%, was unaffected by any treatment condition (Table 1). The magnitude of tissue strain was comparable to that measured in human MCL,16 which is below the 5–6% threshold for sub-structural failure.18 The start of the linear region where collagen fibers fully engaged (Table 2, λ*) and the linear stiffness were unaffected by any treatment. Modulus only decreased in the toe region after elastase treatment (Fig. 4D). These data support that digestion of elastin did not compromise the integrity of the collagen network.
Our findings contrast with studies of tendon and palmar aponeuroses, where the stiffness was markedly lower after elastase digestion.6,11 This difference may arise in that the tendon and aponeuroses underwent extraction of 30–70 μg/mg elastin, up to 80% of the total.6,11 Porcine MCL contained <20 μg/mg elastin, of which <50% was extracted, though the plateau in dose response curves suggests elastin had a finite tensile contribution. Also, prior studies did not quantify tissue strain, utilize a consistent pre-stress, or use a repeated measures experimental design. These inherent differences may have obscured outcomes from elastin degradation in prior studies. Alternatively, tissue construction/organization may have contributed to the perceived differences. Given that tendon is exposed to cyclic loading from muscle, higher elastin content may be required to shield the tissue from micro-failure under repeated loading.
Hysteresis of control tissues (20%) was similar to previous studies in human MCL16, but nearly double that of palmar tendon.6,11 Energy dissipation was unaffected by elastase digestion, contrary to palmar tendon where it increased up to 60%. Hysteresis did not increase after 24 hr treatment or with higher elastase concentration in our study (data not shown), suggesting that quasi-static energy dissipation in ligament is unaffected by elastin. This could indicate that a secondary source of elastic recoil is present, or that residual elastin fragments provide a viscoelastic contribution not detected herein. Higher loading rates, harmonic oscillation, or confined compression experiments may elucidate the influence of elastin’s entropic and hydrophobic mechanisms in the tissue.
Water content was lower in control tissue than in elastase treated tissue, suggesting that treatment lowered the dry weight due to elastin fragments leaching and/or increased tissue swelling during treatment. There were no significant differences between treatment groups for wet weight, but there were for dry weight (data not shown), which justified the normalization to wet weight. Otherwise there were no differences in tissue preparation between specimens in this repeated measures study, except as noted for elastase treatment. Therefore the differences detected arose from the elastin degradation.
This study has limitations. First, elastase removed <50% of elastin from the tissue, and residual fragments may have contributed to the mechanical response. Given that the change in stress reached a plateau in dose response testing, even as elastin content decreased, the contribution of elastin was probably limited beyond this nominal level of digestion. Second, quantifying elastin content with the Fastin assay presented challenges. The tissue was solubilized under conditions of high temperature and low pH (100° C, oxalic acid) to disrupt the chemical stability of elastin.1,2,9 This allowed differentiation of fragments arising from elastase degradation from those arising from hydrolyzation. Elastin fragmentation may have been higher than detected by the Fastin assay, but final tests were based on the results of the dose response where mechanical changes reached a plateau. Finally, elastase could have had proteolytic activity against other molecular populations. Early elastase purifications may have been contaminated with trypsin, explaining why SBTI was effective in blocking activity against collagen.5,6,11 Our study used trypsin-free EC134 elastase, chromatographically purified. Elastase has been used to isolate collagen monomers30,31, but this mechanism was likely driven by degradation of the lysyl oxidase bonds between the collagen and elastin networks, not direct action against structural collagen. Our data support that elastase had limited, if any, influence on collagen. Each of the major tissue properties was unaffected: modulus, tissue strain, λ*, and failure stress and strain. Of note, pilot data indicated that modulus did not change even without SBTI in the treatment solution, and elastase treatment of collagen gels yielded no proteolytic fragments as quantified by SDS-PAGE.
In conclusion, elastin resists tensile elongation of porcine MCL. Elastin provides up to 30% of tissue load support at high strains, but its influence can be detected in the toe region of the stress-strain curve. Tissue lengthening after elastase digestion suggests elastin transitions the recruitment of collagen crimp to the linear region of the stress-strain curve and may shield the tissue from potential for micro-structural failure during loading. Energy dissipation was unaffected by elastin degradation, so additional tests are required to determine the viscoelastic contributions of elastin in ligament. Failure properties were unaffected by elastase treatment, indicating that collagen supports a majority of applied tensile load at high strains.
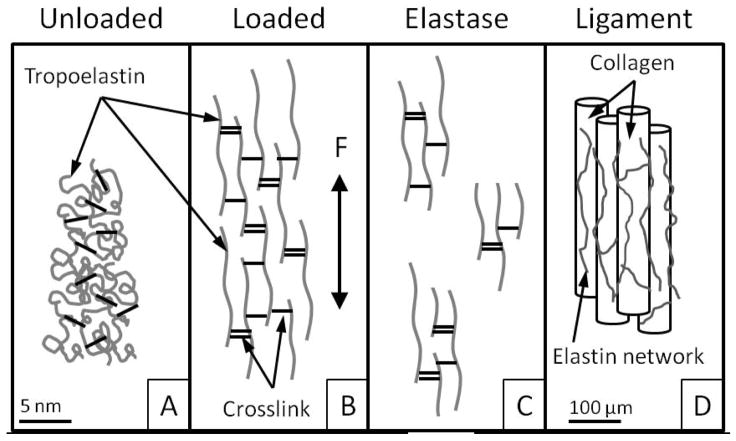
Figure 1.
A: The unloaded elastin network recoils through entropic and hydrophobic mechanisms. B: Upon loading (F), elastin can significantly elongate. C: Elastase digestion may leave fragments of various sizes, with crosslinks intact. D: Elastin is localized between, along and around collagen fibers and fascicles in ligament and tendon.
Acknowledgments
Financial support from NIH #AR047369 is gratefully acknowledged.
Footnotes
The authors have no professional or financial conflicts of interest to disclose.
References
- 1.Gacko M. Elastin: Structure, properties, and metabolism. Cell & Mol Biol Lett. 2000;5:327–348. [Google Scholar]
- 2.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 3.Lee TC, Midura RJ, Hascall VC, Vesely I. The effect of elastin damage on the mechanics of the aortic valve. J Biomech. 2001;34:203–210. doi: 10.1016/s0021-9290(00)00187-1. [DOI] [PubMed] [Google Scholar]
- 4.Miskolczi L, Guterman LR, Flaherty JD, Szikora I, Hopkins LN. Rapid saccular aneurysm induction by elastase application in vitro. Neurosurgery. 1997;41:220–228. doi: 10.1097/00006123-199707000-00034. discussion 228–229. [DOI] [PubMed] [Google Scholar]
- 5.Oxlund H, Manschot J, Viidik A. The role of elastin in the mechanical properties of skin. J Biomech. 1988;21:213–218. doi: 10.1016/0021-9290(88)90172-8. [DOI] [PubMed] [Google Scholar]
- 6.Reihsner R, Menzel EJ, Mallinger R, Millesi H. Biomechanical properties of elastase treated palmar aponeuroses. Connect Tissue Res. 1991;26:77–86. doi: 10.3109/03008209109152165. [DOI] [PubMed] [Google Scholar]
- 7.Muramoto K, Ramachandran J, Hall J, Hui A, Stern R. A rapid sensitive assay for the quantitation of elastin. Connect Tissue Res. 1984;12:307–317. doi: 10.3109/03008208409013693. [DOI] [PubMed] [Google Scholar]
- 8.Uitto J. Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. J Invest Dermatol. 1979;72:1–10. doi: 10.1111/1523-1747.ep12530093. [DOI] [PubMed] [Google Scholar]
- 9.Vered M, Burstein Y, Gertler A. Digestion of elastin by porcine pancreatic elastase i and elastase ii. Int J Pept Protein Res. 1985;25:76–84. doi: 10.1111/j.1399-3011.1985.tb02148.x. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald SE, Moore JE, Jr, Rachev A, Kane TP, Meister JJ. Experimental investigation of the distribution of residual strains in the artery wall. J Biomech Eng. 1997;119:438–444. doi: 10.1115/1.2798291. [DOI] [PubMed] [Google Scholar]
- 11.Millesi H, Reihsner R, Hamilton G, Mallinger R, Menzel EJ. Biomechanical properties of normal tendons, normal palmar aponeuroses, and tissues from patients with dupuytren’s disease subjected to elastase and chondroitinase treatment. Clin Biomech (Bristol, Avon) 1995;10:29–35. doi: 10.1016/0268-0033(95)90434-b. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa H, Mikawa Y, Watanabe R. Elastin in the human posterior longitudinal ligament and spinal dura. A histologic and biochemical study. Spine (Phila Pa 1976) 1994;19:2164–2169. doi: 10.1097/00007632-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Hansen KA, Weiss JA, Barton JK. Recruitment of tendon crimp with applied tensile strain. J Biomech Eng. 2002;124:72–77. doi: 10.1115/1.1427698. [DOI] [PubMed] [Google Scholar]
- 14.Lanir Y. A microstructure model for the rheology of mammalian tendon. J Biomech Eng. 1980;102:332–339. doi: 10.1115/1.3138231. [DOI] [PubMed] [Google Scholar]
- 15.Mikawa Y, Hamagami H, Shikata J, Yamamuro T. Elastin in the human intervertebral disk. A histological and biochemical study comparing it with elastin in the human yellow ligament. Arch Orthop Trauma Surg. 1986;105:343–349. doi: 10.1007/BF00449940. [DOI] [PubMed] [Google Scholar]
- 16.Lujan TJ, Underwood CJ, Henninger HB, Thompson BM, Weiss JA. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J Orthop Res. 2007;25:894–903. doi: 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- 17.Lujan TJ, Lake SP, Plaizier TA, Ellis BJ, Weiss JA. Simultaneous measurement of three-dimensional joint kinematics and ligament strains with optical methods. J Biomech Eng. 2005;127:193–197. doi: 10.1115/1.1835365. [DOI] [PubMed] [Google Scholar]
- 18.Provenzano PP, Heisey D, Hayashi K, Lakes R, Vanderby R., Jr Subfailure damage in ligament: A structural and cellular evaluation. J Appl Physiol. 2002;92:362–371. doi: 10.1152/jappl.2002.92.1.362. [DOI] [PubMed] [Google Scholar]
- 19.Bonifasi-Lista C, Lake SP, Small MS, Weiss JA. Viscoelastic properties of the human medial collateral ligament under longitudinal, transverse and shear loading. J Orthop Res. 2005;23:67–76. doi: 10.1016/j.orthres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Quapp KM, Weiss JA. Material characterization of human medial collateral ligament. J Biomech Eng. 1998;120:757–763. doi: 10.1115/1.2834890. [DOI] [PubMed] [Google Scholar]
- 21.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Tensile properties and fiber alignment of human supraspinatus tendon in the transverse direction demonstrate inhomogeneity, nonlinearity, and regional isotropy. J Biomech. 2010;43:727–732. doi: 10.1016/j.jbiomech.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cloyd JM, Elliott DM. Elastin content correlates with human disc degeneration in the anulus fibrosus and nucleus pulposus. Spine (Phila Pa 1976) 2007;32:1826–1831. doi: 10.1097/BRS.0b013e3181132a9d. [DOI] [PubMed] [Google Scholar]
- 23.Steigman SA, Oh JT, Almendinger N, Javid P, LaVan D, Fauza D. Structural and biomechanical characteristics of the diaphragmatic tendon in infancy and childhood: An initial analysis. J Pediatr Surg. 2010;45:1455–1458. doi: 10.1016/j.jpedsurg.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Hall DA, Czerkawski JW. The reaction between elastase and elastic tissue. 4. Soluble elastins. Biochem J. 1961;80:121–128. doi: 10.1042/bj0800121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood GC. The reconstitution of elastin from a soluble protein derived from ligamentum nuchae. Biochem J. 1958;69:539–544. doi: 10.1042/bj0690539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes AJ, Lord MS, Smith SM, Smith MM, Whitelock JM, Weiss AS, Melrose J. Colocalization in vivo and association in vitro of perlecan and elastin. Histochem Cell Biol. 2011;136:437–454. doi: 10.1007/s00418-011-0854-7. [DOI] [PubMed] [Google Scholar]
- 27.Pasquali Ronchetti I, Alessandrini A, Baccarani Contri M, Fornieri C, Mori G, Quaglino D, Jr, Valdre U. Study of elastic fiber organization by scanning force microscopy. Matrix Biol. 1998;17:75–83. doi: 10.1016/s0945-053x(98)90126-3. [DOI] [PubMed] [Google Scholar]
- 28.Smith KD, Vaughan-Thomas A, Spiller DG, Innes JF, Clegg PD, Comerford EJ. The organisation of elastin and fibrillins 1 and 2 in the cruciate ligament complex. J Anat. 2011;218:600–607. doi: 10.1111/j.1469-7580.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J, Tirlapur U, Fairbank J, Handford P, Roberts S, Winlove CP, Cui Z, Urban J. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat. 2007;210:460–471. doi: 10.1111/j.1469-7580.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etherington DJ. Collagen degradation. Ann Rheum Dis. 1977;36:14–17. [Google Scholar]
- 31.Polewski MD, Johnson KA, Foster M, Millan JL, Terkeltaub R. Inorganic pyrophosphatase induces type i collagen in osteoblasts. Bone. 2010;46:81–90. doi: 10.1016/j.bone.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]