Abstract
Background
Cellular retinol binding-protein I (CRBPI) and cellular retinol binding-protein II (CRBPII) serve as intracellular retinoid chaperones that bind retinol and retinal with high affinity and facilitate substrate delivery to select enzymes that catalyze retinoic acid (RA) and retinyl ester biosynthesis. Recently, 9-cis-RA has been identified in vivo in the pancreas, where it contributes to regulating glucose-stimulated insulin secretion. In vitro, 9-cis-RA activates RXR (retinoid×receptors), which serve as therapeutic targets for treating cancer and metabolic diseases. Binding affinities and structure–function relationships have been well characterized for CRBPI and CRBPII with all-trans-retinoids, but not for 9-cis-retinoids. This study extended current knowledge by establishing binding affinities for CRBPI and CRBPII with 9-cis-retinoids.
Methods
We have determined apparent dissociation constants, , through monitoring binding of 9-cis-retinol, 9-cis-retinal, and 9-cis-RA with CRBPI and CRBPII by fluorescence spectroscopy, and analyzing the data with non-linear regression. We compared these data to the data we obtained for all-trans- and 13-cis-retinoids under identical conditions.
Results
CRBPI and CRBPII, respectively, bind 9-cis-retinol ( , 11 nM and 68 nM) and 9-cis-retinal ( , 8 nM and 5 nM) with high affinity. No significant 9-cis-RA binding was observed with CRBPI or CRBPII.
Conclusions
CRBPI and CRBPII bind 9-cis-retinol and 9-cis-retinal with high affinities, albeit with affinities somewhat lower than for all-trans-retinol and all-trans-retinal.
General significance
These data provide further insight into structure–binding relationships of cellular retinol binding-proteins and are consistent with a model of 9-cis-RA biosynthesis that involves chaperoned delivery of 9-cis-retinoids to enzymes that recognize retinoid binding-proteins.
Keywords: Cellular retinol binding-protein, Retinol, Retinal, Retinoic acid, Vitamin A, Retinoid binding affinities
1. Introduction
Specific binding-proteins, enzymes, and receptors control flux through retinoid pathways, and ultimately produce retinoic acids (RA), active forms of vitamin A that control numerous physiological processes [1–4]. RA occurs in several isomeric forms in vivo with different biological actions [5]. Two isomers activate type II nuclear receptors: all-trans-RA activates RA receptors and peroxisome proliferators-activated receptors δ/β, whereas 9-cis-RA activates RA receptors and RXR (retinoid×receptors) [5]. 9-cis-RA activation of RXR may exert additional biological effects through dimerization of RXR with an array of other type II nuclear receptors [6]. A body of literature describes all-trans-retinoid metabolism in vitro and in vivo, but gaps exist in understanding 9-cis-retinoid metabolism [7]. Interest in 9-cis-RA synthesis and function in vivo has been motivated by its pleiotropic effects on numerous biological pathways activated by RXR, and the potential therapeutic uses of RXR ligands, including for treating cancer and metabolic diseases [8,9]. Renewed interest in 9-cis-RA biosynthesis has been prompted by a recent report that 9-cis-RA occurs in vivo in the pancreas, where it contributes to regulating glucose-stimulated insulin secretion [10].
RA biosynthesis involves reversible and rate-limiting dehydrogenation of retinol into retinal, catalyzed by membrane-bound, short-chain retinol dehydrogenases/reductases, followed by irreversible dehydrogenation of retinal by soluble retinal dehydrogenases [1,7]. A number of these enzymes are active with 9-cis-retinoids [11,12]. Dietary 9-cis-β-carotene generates 9-cis-retinoids via cleavage into 9-cis-retinal and all-trans-retinal [13]. Liver of chow-fed wild-type mice contain 9-cis-retinol [14]. This indicates that the intestine, a major site of β-carotene cleavage, reduces 9-cis-retinal into 9-cis-retinol, similar to its reduction of all-trans-retinal into all-trans-retinol, and esterifies 9-cis-retinol for transport to liver, i.e. 9-cis-retinol is a naturally occurring retinoid in vertebrates. Physiologically, intracellular retinol occurs bound to CRBP (cellular retinol binding-protein) [15]. Thus, the 15 kDa cytosolic CRBP function as intracellular retinoid chaperones by binding retinol and retinal, but not RA, with high affinity [16]. CRBP assists in regulating retinoid homeostasis by protecting retinoids from non-specific oxidation, facilitating delivery of retinoids to specific enzymes, and regulating retinyl ester synthesis and hydrolysis [17]. CRBPI is widely expressed in the adult and is essential for retinol storage in the liver [18,19]. CRBPII is expressed mainly in the adult small intestine, where it functions in vitamin A absorption/uptake [20,21]. Expression patterns, conformational changes upon binding, affinities for retinoids, and interactions with enzymes and membranes unique to each binding-protein suggest distinct functions for CRBPI and CRBPII [22,23].
Affinity and structure–function relationships have been well characterized for CRBPI and CRBPII binding with all-trans-retinol and all-trans-retinal, but not for 9-cis-retinol or 9-cis-retinal [16,24–27]. Here we report affinities of CRBPI and CRBP II for 9-cis-retinoids, and compare the data to results with all-trans- and 13-cis-retinoids.
2. Materials and methods
2.1. Materials
Retinoids were purchased from Sigma-Aldrich (St. Louis, MO), except 9-cis-retinol, which was prepared as described by reduction of 9-cis-retinal with NaBH4, followed by identity verification using spectrophotometry and chromatography [13]. Retinoid solutions were prepared in ethanol on the day of use. Concentrations were verified spectrophotometrically.
2.2. CRBP preparation
CRBPI and CRBPII were expressed in E. Coli with the vectors pMONCRBP and pMONCRBPII, respectively, and were isolated and quantified as described [24,26]. CRBPI and CRBPII were purified further by fast protein liquid chromatography as described [23,28]. CRBPI/II concentrations were determined from absorbance at 280 nm using published ε values [23,28]. The A340/A280 ratios, used to assess purity, were at least 1.6 for FPLC-purified preparations. Stock and binding assay solutions of CRBPI and CRBPII were prepared in 20 mM KH2PO4, 100 mM KCl, pH=7.4 (buffer A). value determinations were performed with 150 nM CRBPI or CRBPII, except 13-cis-retinol, where 300 nM CRBPI and CRBPII were used. CRBPI and CRBPII have amino acid sequences that are highly conserved across species (>95% for CRBPI and ~91% for CRBPII), and share 56% amino acid sequence homology [15]. CRBPI/CRBPII tryptophan residues (responsible for the intrinsic protein fluorescence) are located at positions 9, 89, 107, and 110.
2.3. Fluorescence measurements
Data were generated with a SLM-8100 fluorometer with a 450-watt Xe lamp. Measurements were made at 25 °C. CRBPI and CRBPII were excited at 280 nm. Emission was monitored at 340 nm with spectral band passes of 4 nm. Retinoid solutions were added with a glass Hamilton syringe, gently mixed, and equilibrated for 5 min, before measuring the solution fluorescence. For titrations with retinol, increasing retinol fluorescence was monitored at 490 nm with excitation at 350 nm, as a function of added retinoid. Retinal and RA do not fluoresce; therefore, only CRBP fluorescence was monitored when evaluating these retinoids. The excitation shutter was closed between measurements and retinoid solutions (kept shielded from light) were added in the dark. Inner filter effects were negligible at the protein and retinoid concentrations used and were not corrected. Excitation and emission spectra were corrected using the appropriate blank. Blank contributions were <2% of total intensity. Data were analyzed by non-linear least square fit according to Eq. (1), where F is the observed fluorescence, Ff is the fluorescence of free protein, Fb is the fluorescence of bound protein, Pt is the total protein concentration, Rt is the total retinoid concentration, and is the apparent dissociation constant [27]. Data were fit to Eq. (1) using GraphPad Prism (version 5.0). Before adding retinoids, apo-CRBPI and apo-CRBPII exhibited no detectable emission at wavelengths >400 nm, indicating absence of retinol. Binding experiments were done at least three times.
| (1) |
2.4. Sephadex G-50 column
Sephadex G-50 (fine) was equilibrated in buffer A for several days and used to construct individual columns (~2–3″ long×½″ diameter) in disposable 10 cm3 syringes. Separate columns were used for each protein-substrate combination. Excitation spectra were collected for CRBP bound to retinol before and after passing through the Sephadex G-50 columns. Excitation spectra also were collected for an approximately equivalent amount of free retinol in buffer A.
3. Results and discussion
To determine if 9-cis-retinoids bind to CRBPI and/or CRBPII, we used a fluorescence method modified from Cogan et al. and Levin et al. to measure binding affinities [24,29]. Briefly, ligand binding quenches the intrinsic fluorescence of the apo-proteins by energy transfer from tryptophan residues. This causes a complimentary increase in retinol fluorescence that is red-shifted in comparison to free retinol. Data for binding of 9-cis-retinol, 9-cis-retinal, and 9-cis-RA, and for direct comparison, all-trans- and 13-cis-isomers to CRBPI and CRBPII were determined by calculating the apparent dissociation constant ( ) using non-linear least squares fit to Eq. (1) (Figs. 1 and 2) (Table 1). Recovered values were statistically equivalent, whether determined by quenching of protein fluorescence, or by increases in retinoid fluorescence.
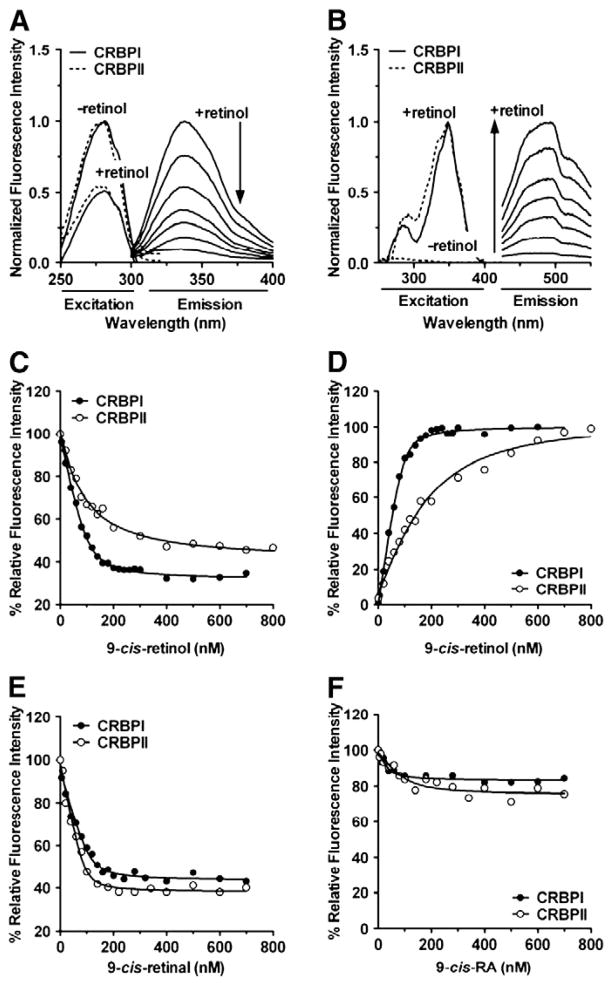
Fig. 1.
Binding data for 9-cis-retinoids with CRBPI and CRBPII. Normalized fluorescence excitation and emission were obtained in the absence or presence of retinol by monitoring either (A) CRBP protein or (B) retinol fluorescence. CRBPs were titrated with (C, D) 9-cis-retinol, (E) 9-cis-retinal, and (F) 9-cis-RA.
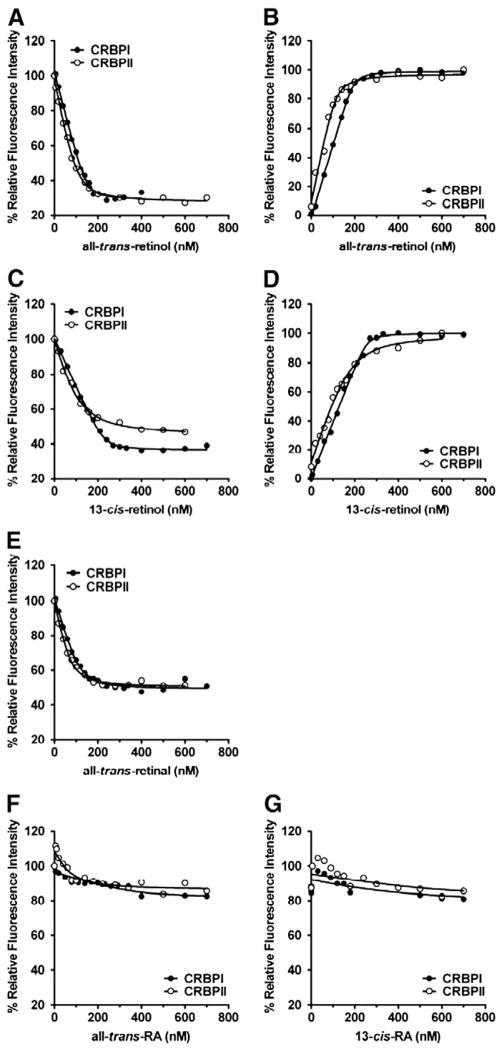
Fig. 2.
(A, C, E–G) Fluorescence quenching of intrinsic CRBPI/CRBPII tryptophan emission or (B, D) increases in retinol fluorescence emission upon binding increasing amounts of ligand. CRBPI or CRBPII were titrated with (A, B) all-trans-retinol, (C, D) 13-cis-retinol, (E) all-trans-retinal, (F) all-trans-RA and (G) 13-cis-RA (C, D).
Table 1.
Recovered binding affinities for 9-cis-, all-trans-, and 13-cis-retinoids. K′d values recovered for representative data by non-linear least squares fitting of the data (to Eq. (1)).
|
K′d (nM)
|
||
|---|---|---|
| Substrate | CRBPI | CRBPII |
| 9-cis-retinol | 11±2 | 68±7 |
| 9-cis-retinal | 8±4 | 5±3 |
| 9-cis-RA | n.b. | n.b. |
| all-trans-retinol | 3±2* | 10±3+ |
| all-trans-retinal | 9±4 | 11±4 |
| all-trans-RA | n.b. | n.b. |
| 13-cis-retinol | 3±1* | 30±12+ |
| 13-cis-retinal | – | – |
| 13-cis-RA | n.b. | n.b. |
n.b., no binding.
–, not measured.
The stoichiometry was 1:1 for all retinoids that exhibited binding.
P<0.05 as compared to CRBPI-9cROL.
P<0.05 as compared to CRBPII-9cROL.
Emission maxima for CRBPI and CRBPII are 340 nm, when excited at 280 nm (Fig. 1A, shown for 9-cis-retinol binding to CRBPI). Emission maxima for retinol bound to CRBPI or CRBPII are 490 nm when excited at 350 nm (Fig. 1B, shown for 9-cis-retinol binding to CRBPI). Free retinal and RA do not fluoresce under these experimental conditions, and therefore only protein quenching was monitored for these retinoids.
Protein quenching (Fig. 1C) and retinol fluorescence (Fig. 1D) are shown for 9-cis-retinol binding to CRBPI and CRBPII. The for 9-cis-retinol was 11 nM for CRBPI binding and 68 nM for CRBPII binding, indicating that CRBPI has ~6-fold stronger affinity for 9-cis-retinol compared to CRBPII. Both values are significantly below 9-cis-retinol concentrations in vivo, indicating that 9-cis-retinol would exist physiologically as does all-trans-retinol: bound to either CRBPI or CRBPII [13]. CRBPI exhibits ~3-fold lower affinity for 9-cis-retinol relative to all-trans-and 13-cis-retinol. CRBPII exhibits decreasing affinity for retinol ligands in the order: all-trans-retinol > 13-cis-retinol > 9-cis-retinol (Fig. 2A and D). Thus, CRBPI and CRBPII might foster somewhat different outcomes for 9-cis-retinol metabolism, based on their different affinities for this retinoid.
Fig. 1E shows binding of CRBPI or CRBPII to 9-cis-retinal by monitoring quenching of intrinsic tryptophan fluorescence. values are similar for CRBPI and CRBPII binding to 9-cis-retinal: 8 nM and 5 nM, respectively. Binding of 9-cis-retinal also is similar compared to all-trans-retinal for either CRBPI (9 nM) or CRBPII (11 nM) (Fig. 2E). 13-cis-retinal was not commercially available and its binding was not measured.
Neither 9-cis-RA (Fig. 1F) nor other RA isomers (all-trans- or 13-cis-) (Fig. 2F and G) exhibited significant binding to CRBPI or CRBPII, as expected from previous binding and structural analyses [15,17,24–27]. Instead, RA isomers are chaperoned by cellular retinoic acid binding-proteins, which do not bind retinal or retinol [1,5,16].
CRBPI and CRBPII show 1:1 stoichiometry for 9-cis-retinol and 9-cis-retinal binding, consistent with the stoichiometry for all-trans-retinol and all-trans-retinal [17,24–27]. Our values of CRBPI and CRBPII binding with all-trans-retinol agree well with previous reports (~10 nM), as do values for all-trans-retinal binding to CRBPI (~10–50 nM) and CRBPII (~10–90 nM) [21,24,25,27]. 13-cis-retinol binding affinity to CRBPII (30 nM) agrees with a previous determination (40 nM); however, CRBPI affinity for 13-cis-retinol was stronger (3 nM) than a previous report (40 nM) [25]. This discrepancy may result from recovery methods. Previous studies relied on the less accurate method of linearizing data, instead of using non-linear regression analysis. Also, previously, only all-trans-retinal binding was measured directly, whereas other retinal isomers were measured indirectly, by assessing their ability to compete with retinol [25]. Thus, this report is the first quantitative and direct measurement of CRBPI and CRBPII binding with 9-cis-retinal. 9-cis-retinol had not been assessed quantitatively to date, because binding to either CRBPI or CRBPII had not been observed. In contrast, we observed significant 9-cis-retinol binding to CRBPI and CRBPII (Fig. 1C and D).
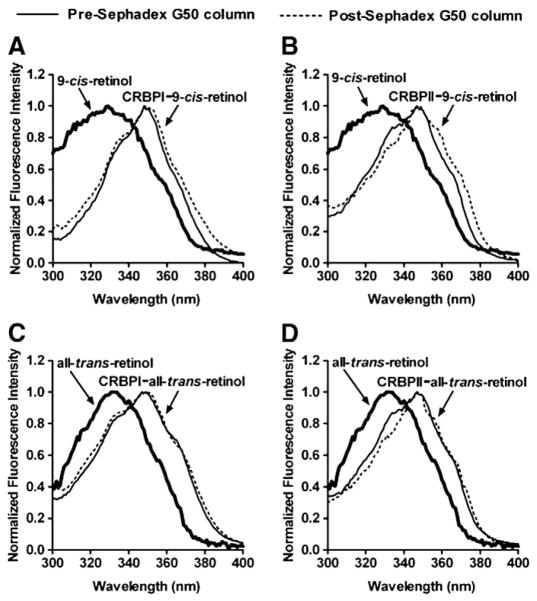
To address the possibility of non-specific binding, we eluted the complexes of CRBPI and CRBPII with 9-cis-retinol through Sephadex G-50 columns to separate free from bound ligand (Fig. 3). 9-cis-retinol eluted bound to CRBPI and CRBPII, similar to all-trans-retinol bound to CRBPI and CRBPII, indicating high-affinity complexes (Fig. 3). Ligand retention was verified by super-imposable excitation spectra characteristic of CRBP-bound retinol (pre- and post-Sephadex G-50 column), red-shifted compared to unbound 9-cis-retinol and all-trans-retinol in buffer.
Fig. 3.
Normalized excitation spectra of CRBPI (A, C) or CRBPII (B, D) bound to 9-cis-retinol (A,B) or all-trans-retinol (C, D) before and after passing through a Sephadex G-50 column to remove free and/or non-specifically bound substrate. Normalized excitation spectra for free 9-cis-retinol (A, B bold) and free all-trans-retinol (C, D bold) also are shown.
These data indicate that CRBPI has ~3-fold greater affinity for all-trans-retinol relative to CRBPII. Previous fluorimetric determinations derived values for both CRBPI and CRBPII of ~10 nM, whereas 19F NMR assessment of all-trans-retinol transfer between CRBPI and II estimated a value of CRBPI ~10–100-fold less than the value of CRBPII [27]. Our results for 9-cis-retinol binding to CRBPI (11 nM) and CRBPII (68 nM) indicate a differential interaction, analogous to all-trans-retinol. Previous 19F NMR studies with all-trans-retinal suggested similar values for both CRBPI and CRBPII [26], which we corroborate here. Our recovered values for 9-cis-retinal with CRBPI (8 nM) and CRBPII (5 nM) indicate behavior similar to all-trans-retinal.
The differences in fluorometrically determined between this work and previous studies are likely attributable to our use of direct measurement with CRBP concentrations an order of magnitude lower than used in previous studies, and our use of non-linear regression analysis. It should be noted that fluorometric determination methods are somewhat limited by the need to use protein concentrations above values because of fluorometer sensitivity limitations, leading to stoichiometric binding. With stoichiometric binding, most ligand is bound: the concentration of unbound ligand remains very small until saturation. Practically, this can underestimate true affinities of tight-binding ligands; therefore, fluorometrically determined values are likely closer to an upper limit for tight binding retinoids [29]. The non-linear regression analysis used here eliminates a significant amount of the uncertainty introduced in previous determinations that linearized ligand-binding data. Thus, these direct mathematical fitting methods have significantly improved the accuracy of assessing retinoid binding affinities [30].
4. Conclusions
Our data show that 9-cis-retinol and 9-cis-retinal bind with high affinity to both CRBPI and CRBPII, major intracellular chaperones for retinol and retinal. These new results suggest the possibility that these retinoid binding-proteins contribute to 9-cis-RA biosynthesis in vivo by chaperoning 9-cis-retinoid delivery to retinoid-metabolizing enzymes. Different CRBPI and CRBPII binding affinities with 9-cis-retinoids are consistent with these two binding-proteins having unique affects on the metabolism of their ligands.
Acknowledgments
This work was supported by NIH Grants DK36870, DK46839, AG13566 (JLN) and an NIH Kirschstein Individual Fellowship F32 DK066924 (MAK). Thanks to Charles Krois and James Chithalen for assistance with the CRBPI/II preparation. We also thank Marc Levin for sharing the vectors pMONCRBP and pMONCRBPII.
References
- 1.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim Biophys Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 2.Giguere V. Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr Rev. 1994;15:61–79. doi: 10.1210/edrv-15-1-61. [DOI] [PubMed] [Google Scholar]
- 3.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 4.Rochette-Egly C, Germain P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs) Nucl Recept Signal. 2009;7:e005. doi: 10.1621/nrs.07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noy N. Ligand specificity of nuclear hormone receptors: sifting through promiscuity. Biochemistry. 2007;46:13461–13467. doi: 10.1021/bi7018699. [DOI] [PubMed] [Google Scholar]
- 6.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 7.Napoli JL. Enzymology and biogenesis of retinoic acid. In: Livrea MA, editor. Vitamin A and Retinoids: An Update of Biological Aspects and Clinical Applications. Bikhauser Verlag; Basel-Bosten-Berlin: 2000. pp. 17–28. [Google Scholar]
- 8.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 9.Singh Ahuja H, Liu S, Crombie DL, Boehm M, Leibowitz MD, Heyman RA, Depre C, Nagy L, Tontonoz P, Davies PJ. Differential effects of rexinoids and thiazolidinediones on metabolic gene expression in diabetic rodents. Mol Pharmacol. 2001;59:765–773. doi: 10.1124/mol.59.4.765. [DOI] [PubMed] [Google Scholar]
- 10.Kane MA, Folias AE, Pingitore A, Perri M, Obrochta K, Krois CR, Cione E, Ryu JY, Napoli JL. Identification of 9-cis-Retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion. Proc Natl Acad Sci USA. 2010;107:21884–21889. doi: 10.1073/pnas.1008859107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napoli JL. 17beta-Hydroxysteroid dehydrogenase type 9 and other short-chain dehydrogenases/reductases that catalyze retinoid, 17beta- and 3alpha-hydro-xysteroid metabolism. Mol Cell Endocrinol. 2001;171:103–109. doi: 10.1016/s0303-7207(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 12.Belyaeva OV, Kedishvili NY. Comparative genomic and phylogenetic analysis of short-chain dehydrogenases/reductases with dual retinol/sterol substrate specificity. Genomics. 2006;88:820–830. doi: 10.1016/j.ygeno.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Nagao A, Olson JA. Enzymatic formation of 9-cis, 13-cis, and all-trans retinals from isomers of beta-carotene. FASEB J. 1994;8:968–973. doi: 10.1096/fasebj.8.12.8088462. [DOI] [PubMed] [Google Scholar]
- 14.Kane MA, Folias AE, Napoli JL. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem. 2008;378:71–79. doi: 10.1016/j.ab.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison EH, Blaner WS, Goodman DS, Ross AC. Subcellular localization of retinoids, retinoid-binding proteins, and acyl-CoA: retinol acyltransferase in rat liver. J Lipid Res. 1987;28:973–981. [PubMed] [Google Scholar]
- 16.Li E. Structure and function of cytoplasmic retinoid binding proteins. Mol Cell Biochem. 1999;192:105–108. [PubMed] [Google Scholar]
- 17.Newcomer ME, Jamison RS, Ong DE. Structure and function of retinoid-binding proteins. Subcell Biochem. 1998;30:53–80. doi: 10.1007/978-1-4899-1789-8_3. [DOI] [PubMed] [Google Scholar]
- 18.Ghyselinck NB, Bavik C, Sapin V, Mark M, Bonnier D, Hindelang C, Dierich A, Nilsson CB, Hakansson H, Sauvant P, Azais-Braesco V, Frasson M, Picaud S, Chambon P. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napoli JL. A gene knockout corroborates the integral function of cellular retinol-binding protein in retinoid metabolism. Nutr Rev. 2000;58:230–236. doi: 10.1111/j.1753-4887.2000.tb01870.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XEL, Lu J, Tso P, Blaner WS, Levin MS, Li E. Increased neonatal mortality in mice lacking cellular retinol-binding protein II. J Biol Chem. 2002;277 doi: 10.1074/jbc.M205519200. [DOI] [PubMed] [Google Scholar]
- 21.Ong DE. Cellular transport and metabolism of vitamin A: roles of the cellular retinoid-binding proteins. Nutr Rev. 1994;52:S24–31. doi: 10.1111/j.1753-4887.1994.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Cistola DP, Li E. Two homologous rat cellular retinol-binding proteins differ in local conformational flexibility. J Mol Biol. 2003;330:799–812. doi: 10.1016/s0022-2836(03)00629-6. [DOI] [PubMed] [Google Scholar]
- 23.Jamison RS, Newcomer ME, Ong DE. Cellular retinoid-binding proteins: limited proteolysis reveals a conformational change upon ligand binding. Biochemistry. 1994;33:2873–2879. doi: 10.1021/bi00176a017. [DOI] [PubMed] [Google Scholar]
- 24.Levin MS, Locke B, Yang NC, Li E, Gordon JI. Comparison of the ligand binding properties of two homologous rat apocellular retinol-binding proteins expressed in Escherichia coli. J Biol Chem. 1988;263:17715–17723. [PubMed] [Google Scholar]
- 25.MacDonald PN, Ong DE. Binding specificities of cellular retinol-binding protein and cellular retinol-binding protein, type II. J Biol Chem. 1987;262:10550–10556. [PubMed] [Google Scholar]
- 26.Li E, Locke B, Yang NC, Ong DE, Gordon JI. Characterization of rat cellular retinol-binding protein II expressed in Escherichia coli. J Biol Chem. 1987;262:13773–13779. [PubMed] [Google Scholar]
- 27.Li E, Qian SJ, Winter NS, d’Avignon A, Levin MS, Gordon JI. Fluorine nuclear magnetic resonance analysis of the ligand binding properties of two homologous rat cellular retinol-binding proteins expressed in Escherichia coli. J Biol Chem. 1991;266:3622–3629. [PubMed] [Google Scholar]
- 28.Penzes P, Napoli JL. Holo-cellular retinol-binding protein: distinction of ligand-binding affinity from efficiency as substrate in retinal biosynthesis. Biochemistry. 1999;38:2088–2093. doi: 10.1021/bi982228t. [DOI] [PubMed] [Google Scholar]
- 29.Cogan U, Kopelman M, Mokady S, Shinitzky M. Binding affinities of retinol and related compounds to retinol binding proteins. Eur J Biochem. 1976;65:71–78. doi: 10.1111/j.1432-1033.1976.tb10390.x. [DOI] [PubMed] [Google Scholar]
- 30.Norris AW, Cheng L, Giguere V, Rosenberger M, Li E. Measurement of subnanomolar retinoic acid binding affinities for cellular retinoic acid binding proteins by fluorometric titration. Biochim Biophys Acta. 1994;1209:10–18. doi: 10.1016/0167-4838(94)90130-9. [DOI] [PubMed] [Google Scholar]