Abstract
Long-acting glucagon-like peptide-1 receptor (GLP-1R) agonists have both glucose- and weight-lowering effects. The brain is poised to mediate both of these actions since GLP-1Rs are present in key areas known to control weight and glucose. Although some research has been performed on the effects of exendin-4 in the brain, little data exists on the central effects of liraglutide, a long-acting GLP-1R agonist with much closer structural homology to native GLP-1. In lean, Long-Evans rats, we found that direct intra-third cerebroventricular (i3vt) administration of 0.26 nmol liraglutide caused a 50% reduction in food intake. However, exendin-4 produced the same reduction in food intake with 10-fold greater potency (0.02 nmol). These data are supported by similar c-Fos immunoreactivity in the hypothalamic paraventricular nuclei by exendin-4 as compared to liraglutide despite differing doses. The anorectic effects of both drugs were blocked with i3vt pre-treatment of a GLP-1R competitive antagonist, exendin(9-39), indicating that both drugs required the GLP-1R for their effects. Exendin-4, and not liraglutide, caused hyperglycemia when given i3vt prior to an oral glucose tolerance test, although liraglutide did not lower glucose. Thus, these data show that GLP-1R agonists have differing anorectic potencies in the CNS, which may account for some of their clinical differences. Additionally, we show here that the glucose lowering properties of acute administration of GLP-1R agonists are not accounted for by their central effects.
Keywords: GLP-1R, liraglutide, brain, exendin-4
1. Introduction
Glucagon-like peptide-1 (GLP-1) is an incretin, secreted from intestinal L-cells, that improves postprandial glucose homeostasis. Through a G-protein coupled receptor (GLP-1R), GLP-1 improves glucose homeostasis by increasing insulin secretion, decreasing gastric emptying, and decreasing glucagon secretion [3, 17]. However, GLP-1 also reduces appetite [3] and GLP-1R analogs cause weight loss [5]. The CNS is vital in weight regulation [19] and expresses GLP-1Rs in key areas important for food intake [15]. Additionally, GLP-1 is synthesized in neurons that project to the same brain areas important for regulation of food intake [20]. Thus, the brain is poised to mediate the weight lowering effects of GLP-1/GLP-1R analogs.
While data suggest that central GLP-1R signaling may not have a physiologic role in weight regulation, these receptors are important for the weight lowering effects of long-acting GLP-1R agonists [18]. Since GLP-1 is rapidly degraded, long-acting GLP-1 agonists have been created as anti-diabetic therapies. Interestingly, of all anti-diabetic drugs, only the long-acting GLP-1 agonists such as exendin-4 and liraglutide are able to cause significant weight loss [10] even when compared to DPP-IV inhibitors which increase the levels of endogenous GLP-1 but do not produce weight loss. It is important to note that while liraglutide is over 90% structurally homologous to native GLP-1, exendin-4 is only 50% homologous. Previously, our lab has shown i3vt administration of exendin-4 is 100-fold more potent than GLP-1 in reducing food intake [2]. Since liraglutide is much closer in structure to GLP-1 than exendin-4, we hypothesized that the liraglutide’s direct CNS actions would be closer to those of GLP-1. Given that exendin-4 is clinically given at a smaller dose than liraglutide, it is important to determine why these clinical differences exist in order to more effectively exploit these differences for future therapies. Thus, we directly administered both exendin-4 and liraglutide into the CNS to determine if the clinical differences observed between these drugs are related to their effects in the CNS.
2. Methods
2.1. Animals
Adult, male Long-Evans rats (Harlan, Indianapolis, IN) were singly housed at the University of Cincinnati Laboratory Animal Medical Services Facility at the Metabolic Disease Institute on a 12 hour light/dark cycle with ad libitum access to water and standard rodent chow (Harlan Teklad #7012, Indianapolis, IN) unless otherwise specified. All studies were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
2.2. Surgeries
Cannulas (22-ga, 11 mm, Plastics One, Roanoke, VA) were surgically implanted into the third cerebral ventricle (i3vt) under ketamine anesthesia using stereotaxic (David Kopf Instruments, Tujunga, CA) coordinates (2.2 mm posterior to bregma, 7.8 mm ventral to dura, directly on midline) as determined by the atlas of Paxinos and Watson [6, 11]. Rats were allowed to recover to their presurgery body weight prior to any studies. Cannula placement was verified by a water intake of ≥ 5 mL, 1 hour after a 5 ng/1 μL injection of angiotensin II (American Peptide, Sunnyvale, CA) into the i3vt. Only animals with a positive angiotensin test were included in analyses.
2.3. Peptides
Exendin-4 was obtained from American Peptides (Sunnyvale, CA). Liraglutide was a generous gift from Novo Nordisk (Maaloev, Denmark). Exendin (9-39) was synthesized by 21st Century Peptide (Marlboro, MA). All peptides were dissolved in saline.
2.4. Food Intake Studies
For all food intake studies, rats were matched for body-weight and fasted 4 hours prior to the dark cycle onset. For all i3vt studies, 2 μl GLP-1 agonists were injected 45 minutes prior to dark cycle onset. I3vt liraglutide dose response studies were conducted with liraglutide 0.02, 0.08, 0.26, and 1.33 nmol. The high dose of 1.33 nmol was chosen because it was the highest effective dose on a log scale that did not cause barrel-rolling. Initially, a dose of 2.6 nmol was used instead of 1.33 nmol. However this caused barrel-rolling in the first cohort. Therefore a second experiment was conducted with a 1.33 nmol dose replacing the 2.6 nmol dose. The results of the two cohorts were pooled with the 2.6 nmol dose results excluded. These studies were performed 2 weeks apart. Food intake comparisons between GLP-1 agonists were conducted on the same cohort 2 weeks later using liraglutide 0.26 nmol and exendin-4 0.02 and 0.26 nmol i3vt. For GLP-1 receptor antagonism studies, a new cohort of animals was given 1 μL exendin(9-39) (29 nmol) or saline i3vt 30 minutes prior to 2 μL liraglutide (0.26 nmol) or exendin-4 (0.02 nmol). This dose of exendin(9-39) was chosen so that it was at least in 100-fold excess of our drugs and was previously shown to block the effects of 0.1 μg (~0.02 nmol) exendin-4 [2]. A cross-over study was performed 2 weeks later. For all food intake studies, food hoppers were manually measured at baseline, 1, 2, 4, 24, and 48 hours after lights out. Spillage from hoppers was taken into account. Body weights were measured at baseline, 24, and 48 hours for all studies except the antagonism food intake studies where body weights were only measured at baseline. Molecular weight of liraglutide = 3751.2 g/mol, exendin-4 = 4186.6 g/mol, and exendin(9-39) = 3369.8 g/mol.
2.5. c-Fos Immunohistochemistry
Ad lib fed rats, 8 weeks after food-intake studies, were injected with 2 μL i3vt liraglutide (0.26 nmol; n=2), exendin-4 (0.02 nmol; n=2), or saline (n=2). 2 hours later, rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with 0.1M PBS containing 0.5% sodium nitrite followed by 4% paraformaldehyde. Brains were postfixed at 4°C overnight in 4% paraformaldehyde/PBS and then stored in 30% sucrose/PBS + 0.01% sodium azide at 4°C. Serial coronal sections were collected at 35 μm and stored in cryopreservative at −20°C. Sections were then rinsed in 0.1M PBS and incubated in 1% sodium borohydride/PBS for 30 min. They were rinsed again in 0.1M PBS, incubated in 1% H2O2/PBS for 10 min, and then rinsed with PBS again. Sections were then blocked with 0.1M PBS/4% normal goat serum/0.4% Triton-X-100 for 1 hour and incubated overnight with 1:2500 dilution of rabbit anti-cFos (sc52; Santa Cruz Biotechnology, Santa Cruz, CA). The next day, sections were rinsed with PBS, incubated with biotinylated goat anti-rabbit IgG diluted 1:300 in PBS/4% normal goat serum/0.4% Triton-X-100 for 1 hour, and rinsed again with 0.1M PBS. Sections were then incubated in 1:600 diluted ABC/PBS solution (PK6100; Vector Laboratories, Burlingame, CA) for 1 hour. After 0.1M PBS rinses, sections were incubated for 10 minutes in a solution containing DAB (D5905, Sigma Aldrich), Nickel sulfate (N73-100; Fisher Scientific), H2O2, and PBS. They were rinsed with PBS and then placed in 0.3% gelatin. Sections were mounted on slides, air dried overnight, and coversliped.
For quantification of c-Fos immunoreactivity in the hypothalamic arcuate (ARC) and paraventricular (PVN) nuclei, digital images of sections were photographed through a digital camera attached to a Zeiss microscope (Zeiss, Thornwood, NY). C-Fos immunoreactivity was quantified as optical density using the National Institute of Health program ImageJ (16). Three anatomically aligned sections from each animal were then counted and averaged for each region (ARC and PVN).
2.6. Oral Glucose Tolerance Test
Rats (new cohort) were fasted for 4 hours. 1 hour prior to dextrose administration, body-weight randomized rats were injected i3vt with 2 μL liraglutide (0.26 nmol), exendin-4 (0.02 nmol), or saline. At t=0 minutes, they were given 2.25 mL D25W by gavage (average 1.5 mg/kg). Glucose measurements were taken at −60, 0, 5, 15, 30, 45, 60, and 120 minutes relative to glucose administration. Blood was collected by tail bleeding at −60, 0, 5, 15, and 30 minutes and placed immediately on ice. The blood was centrifuged and the plasma was collected and stored at −80°C until further analysis. Insulin levels were determined with a Rat Insulin ELISA (Crystal Chem, Downers Grove, IL).
2.7. Statistical Analysis
Results are presented as mean ± SE. Results are analyzed by a one-way or two-way ANOVA as appropriate with Tukey’s post-hoc analysis where appropriate. The level of significance was set as p < 0.05. Data were analyzed GraphPad Prism version 5.01.
3. Results
3.1. Comparison of the anorectic effects of liraglutide vs. exendin-4 i3vt
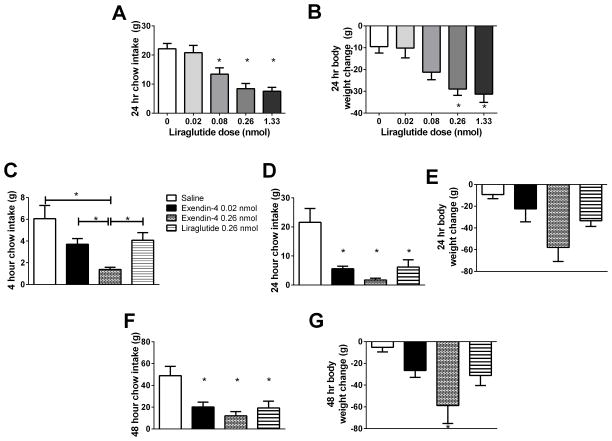
I3vt liraglutide significantly decreased 24 hour food intake at 0.08 nmol (13.41 ± 2.18 g, n=14, p=0.008), 0.26 nmol (8.43 ± 1.80 g, n=12, p<0.001), and 1.33 nmol (7.56 ± 1.32 g, n=8, p<0.001) compared to saline (22.14 ± 1.82 g, n=12) (Fig. 1A). Body weight changes at 24 hours were also significantly decreased at 0.26 nmol (−28.95 ± 2.90 g, p=0.003) and 1.33 nmol (−31.28 ± 3.79 g, p=0.003) compared to saline (−9.47 ± 2.94 g) (Fig. 1B). However 4 hours after i3vt administration, 0.26 nmol of exendin-4 i3vt reduced food intake (1.39 ± 0.21 g, n=7) compared to both saline (6.06 ± 1.21 g, n=3, p<0.001) and an equimolar dose of liraglutide (4.07 ± 0.70 g, n=5, p=0.014) (Fig. 1C). Comparable anorectic effects were observed between i3vt 0.02 nmol of exendin-4 (3.70 ± 1.21 g, n=7) and 0.26 nmol of liraglutide (p=0.963, Fig. 1C), suggesting that when injected i3vt, exendin-4 is ten-fold more potent than liraglutide. All drug treatments caused decreased food intake at 24 and 48 hours compared to saline (see supplemental results), although no differences were observed between treatments (Fig. 1D, F). Body weight changes mirrored food intake differences, although only i3vt exendin-4 0.26 nmol treatment caused significantly more weight loss at 48 hours (−58.79 ±16.49 g) than saline-treated animals (−5.26 ± 4.17 g, p=0.031; Fig. 1E, G).
Figure 1.
Direct i3vt exenatide-4 is a more potent anorexic agent than liraglutide. A. I3vt liraglutide decreased 24 hour food intake at 0.08, 0.26, and 1.33 but not at 0.02 nmol when compared to saline. B. Liraglutide i3vt caused decreased body weight at 0.26 and 1.33 nmol compared to saline. C. Exendin-4 0.26 nmol reduced food intake more than liraglutide 0.26 nmol. Exendin-4 0.02 nmol caused comparable food intake reduction to liraglutide 0.26 nmol, which were both significantly different from saline. D. At 24 hours, all GLP-1R agonists caused decreased food intake compared to saline. There were no differences between the drugs. E. No significant differences in body weight occurred between the drugs at 24 hours. F. At 48 hours, all drugs still caused decreased cumulative food intake relative to saline. G. At 48 hours, animals treated with exendin-4 0.26 nmol had significantly reduced body weights compared to saline. * p < 0.05 compared to saline unless noted with a bar (p-values noted in text).
3.2. GLP-1 receptor antagonism
We next sought to determine if the anorectic effects of GLP-1 receptor agonists are driven by action at the GLP-1 receptor and therefore can be reversed by a competitive antagonist. Based on the previous study, we chose to use doses of GLP-1 agonists that had the same anorectic effects. At 24 hours, exendin-4 0.02 nmol and liraglutide 0.26 nmol caused similar decreases in food intake (6.83 ± 1.04 g, n=12 vs. 10.97 ± 1.87 g, n=12; p=0.420), when pretreated with saline. These were both significantly decreased from saline/saline treated animals (21.05 ± 3.77 g, n=10; p<0.001 for both drugs). However, pre-treatment with 29 nmol of the antagonist exendin(9-39) attenuated the anorectic effects of both exendin-4 0.02 nmol (15.49 ± 1.25 g, p=0.001 vs. saline/exendin-4) and liraglutide 0.26 nmol (18.76 ± 1.93 g, p=0.005 vs. saline/liraglutide) (Fig. 2). Thus, these data demonstrate that both exendin-4 and liraglutide require the GLP-1 receptor for anorectic effects.
Figure 2.
Competitive GLP-1R antagonist exendin(9-39) blocks anorectic effects of liraglutide and exendin-4. I3vt pretreatment with 29 nmol exendin(9-39) blocked the anorectic effect of 0.02 nmol of exendin-4 when compared to saline pretreatment. Pretreatment with exendin(9-39) also blocked the anorectic effect of 0.26 nmol of liraglutide when compared to saline pretreatment. Both saline-pretreated drugs caused similar reductions in food intake when compared to saline/saline treated animals. There was no difference in the saline-pretreated liraglutide and exendin-4 food intakes. There were also no differences in the exendin(9-39) treated animals compared to saline/saline treated animals nor compared to each other. * p < 0.05 compared to saline/saline treated animals; $ p < 0.05 compared to saline pre-treatment, same drug.
3.3. c-Fos Immunoreactivity
To determine if GLP-1 agonists activate neurons differently, we injected exendin-4 (0.02 nmol) and liraglutide (0.26 nmol) i3vt and assessed c-Fos immunoreactivity in the hypothalamus. At equianorectic doses, exendin-4 induced similar c-Fos immunoreactivity (40.06 ±0.84 positive nuclei/100,000 μm2) as liraglutide (39.97 ± 6.15 positive nuclei/100,000 μm2) in the paraventricular nucleus, and both induced more c-Fos reactivity than saline (12.38 ± 1.87 positive nuclei/100,000 μm2, p=0.022 vs. exendin-4, p=0.022 vs. liraglutide) (Fig. 3A). In the arcuate nucleus, there was again no difference in c-Fos immunoreactivity between the drugs (exendin-4 27.78 ± 1.01 vs. liraglutide 33.47 ± 3.38 positive nuclei/100,000 μm2) but significant increases compared to saline (11.38 ± 2.89 positive nuclei/100,000 μm2, p=0.038 vs exendin-4, p=0.016 vs. liraglutide; Fig. 3B).
Figure 3.
GLP-1R agonists cause increased c-FOS expression in key brain regions. A. c-FOS expression is increased in the paraventricular nucleus (PVN) of animals treated with 0.02 nmol of exendin-4 and 0.26 nmol of liraglutide compared to saline. B. I3vt treatment with 0.26 nmol of liraglutide and 0.02 nmol of exendin-4 increased c-FOS expression in the arcuate (ARC) compared to saline. * p < 0.05 compared to saline.
3.4. Long-acting GLP-1R agonists have different effects centrally on glucose tolerance
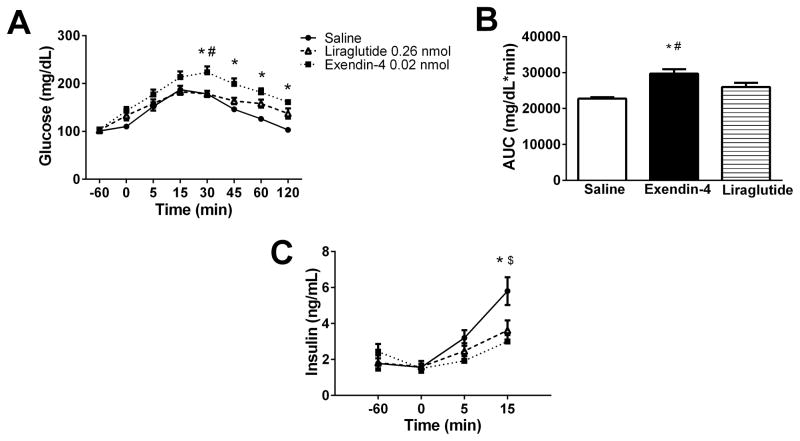
Exendin-4 is known to acutely cause hyperglycemia in rats [12] but it is unknown if liraglutide has this same effect. Consistent with previous reports, i3vt exendin-4 (0.02 nmol) caused sustained hyperglycemia compared to saline beginning 30 minutes after an oral glucose gavage (Fig. 4A). However, i3vt liraglutide (0.26 nmol) did not cause hyperglycemia (Fig. 4A). Overall, exendin-4 caused a greater glucose excursion, evidenced by a greater incremental AUC (29714 ± 1249, n=10) than both saline (22749 ± 385.8, n=9, p<0.001) and liraglutide (25998 ± 1162, n=10, p=0.042) (Fig. 4B). Interestingly, both i3vt liraglutide and exendin-4 had lower insulin levels at 15 minutes compared to saline (3.00 ± 1.14 ng/dL, p<0.001 and 3.62 ± 1.76 ng/dL, p=0.005 vs. 5.80 ± 2.31; Fig. 4C). These data demonstrate that acutely, neither exendin-4 nor liraglutide is able to improve glucose excursions when given centrally.
Figure 4.
GLP-1R agonists cause hyperglycemia when administered centrally. A. Oral glucose tolerance test after i3vt administration of equianorectic doses of exendin-4 (0.02 nmol) and liraglutide (0.26 nmol) compared to saline. Exendin-4 caused hyperglycemia compared to saline at 30, 45, 60, and 120 minutes and relative to liraglutide at 30 minutes. Liraglutide was not different compared to saline at any time point (p > 0.05). B. Area under the curve. I3vt treatment with 0.02 nmol of exendin-4 caused a larger incremental AUC compared to saline and to 0.26 nmol liraglutide. There was no difference between the AUC of liraglutide vs. saline treated animals. C. Insulin concentrations. Saline treated animals had higher insulin levels than animals treated with 0.26 nmol of liraglutide and 0.02 nmol of exendin-4 at 15 min. There was no difference between insulin levels at 15 minutes in liraglutide vs. exendin-4 treated animals. * p < 0.05 exendin-4 vs. saline; # p < 0.05 liraglutide vs. exendin-4; $ p < 0.05 liraglutide vs. saline.
4. Discussion
Exendin-4 and liraglutide are potent GLP-1 receptor agonists useful for both their glucose lowering effects and their ability to lower weight. Although often used interchangeably in clinical practice, the dosage of exendin-4 (10–20 μg/day subcutaneously) is much smaller than liraglutide (600–1800 μg/day subcutaneously). Given that long-acting GLP-1 receptor agonists are the only current anti-diabetic treatment to have a weight lowering effect, it is critical to fully understand the mechanism of action of these drugs. Our data demonstrate that when given directly into the third ventricle, exendin-4 is 10 times more potent than liraglutide at reducing food intake. Although our data do not have statistically significant differences in body weight between the treatment paradigms, the trend for increased potency of exendin-4 is also consistent across the 48 hours of observation. This may be secondary to a lack of power for the body weight analyses. Interestingly, we have previously shown that exendin-4 is 100-fold more potent than native GLP-1 [2]. Given that liraglutide is much closer in structure to GLP-1 than exendin-4 (approximately 94% vs. 41% homology), one can speculate that these differences in potency are secondary to their structure. Indeed, it has been previously shown that exendin-4 has a higher affinity for the GLP-1R than GLP-1 due to its structural differences [14] and in a recent study, exendin-4 was found to be approximately 10 times more potent than liraglutide at inducing GLP-1R internalization [13]. The differences in receptor activation may account for the potency differences seen both clinically and in these data [5].
GLP-1R are expressed in the paraventricular and arcuate nuclei of the hypothalamus, two regions clearly demonstrated to regulate food intake and body weight [19]. Both exendin-4 [8] and liraglutide [7] can cross the blood-brain-barrier. Previous data has shown that peripheral administration of exendin-4 activates both the PVN and arcuate and that this activation is through a combination of vagal and direct CNS action [1]. Consistent with these previous reports, our data shows that central administration of both liraglutide and exendin-4 increase neuronal activity in the PVN and arcuate nuclei. We have previously shown that direct administration of GLP-1 into the arcuate does not affect food intake while direct PVN administration decreases food intake [15]. The similar activation of c-Fos by exendin-4 and liraglutide is consistent with their anorectic effects, showing again that exendin-4 has a greater potency than liraglutide in the brain.
Consistent with previous data [12], we found acute central exendin-4 causes hyperglycemia within 30 minutes of an oral glucose load. Surprisingly, liraglutide was not different from saline at any time point. Given the profound glucose lowering effects of GLP-1R agonists peripherally, the lack of an effect centrally is striking. This is especially true in light of previous data showing improved glucose homeostasis after direct arcuate administration of GLP-1 and worsened glucose homeostasis after i3vt administration of a GLP-1R inhibitor [15]. However, we recently published data showing that peripheral liraglutide still has potent glucose lowering effects despite knockdown of the GLP-1R in the neurons of mice [18]. We do acknowledge that the overall impact of GLP-1 agonists in the brain are difficult to detect during a GTT secondary to their ability to activate stress responses as well as simultaneously suppressing hepatic glucose production while reducing peripheral glucose uptake. We specifically chose to evaluate these drugs in the presence of endogenous GLP-1 signaling through the use of an oral GTT in order to more closely mimic their use in humans. Our data suggest that direct CNS signaling by these long-acting agonists do not enhance, but rather seem to inhibit the beneficial glucose lowering and insulin-secreting effects of endogenous GLP-1R signaling.
Despite lacking a clear glucose-lowering effect of GLP-1R agonists by the brain, these drugs may have other metabolic effects. Previous studies show conflicting reports on the effects of GLP-1R activators/inhibitors on glucose homeostasis when given directly into the brain [4, 9, 15]. Most of these studies are done under clamp conditions and interestingly do not show differences in whole-body insulin sensitivity but do show specific effects on muscle or liver. Thus, i3vt liraglutide may have beneficial metabolic effects despite lacking a difference in absolute glucose values. It is also possible that long-acting GLP-1R agonists do not need central mechanisms for their glucose-lowering effects. This conclusion would be consistent with recent data from our group where the GLP-1R was selectively knocked down in the CNS [18]. In these mice, peripheral liraglutide administration had a greatly reduced ability to suppress food intake but a normal ability to improve glucose tolerance.
In conclusion, these data highlight differences in the central actions of long-acting GLP-1R agonists on food intake and glucose tolerance. Our data show that the effects of the GLP-1R agonists are dependent upon signaling through the GLP-1R. Structural variations in long-acting GLP-1R agonists are important in determining their anorectic properties, which are likely driven by action in the CNS. Additionally, our data show that direct central administration of long-acting GLP-1R agonists are not capable of decreasing glucose excursions and suggest these drugs may exert their glucose lowering abilities through direct peripheral mechanisms. Identifying the differences in central vs. peripheral GLP-1R activation by GLP-1R agonists holds promise for creating new therapies with more potent anorectic properties. Additionally, identifying the key GLP-1R populations involved in the anorectic vs. glucose-lowering abilities of long-acting GLP-1R agonists will be important for understanding current clinical therapies as well as for the design of new therapies for type 2 diabetes.
Supplementary Material
Highlights.
Long-acting GLP-1R agonists have differing anorectic potencies in the CNS
The different anorectic profiles of liraglutide vs. exendin-4 mirror clinical use
Anorectic differences in GLP-1R agonists correlate with c-fos immunoreactivity
Direct CNS administration of GLP-1R agonists do not lower glucose acutely
Acknowledgments
The authors would like to acknowledge Joyce Sorrell, Emily Orr, Heinz Hoppert, Bailing Li, Danielle Harper, and Maureen Fitzgerald for their superior technical assistance.
Funding.
1F32DK091077-01A1 to SS, Novo Nordisk to RJS and DAS, DK082480 to DAS, DK093848 to RJS.
Footnotes
Author Contributions: Stephanie Sisley – project lead, study design and execution, data analysis, and manuscript preparation; Kathleen Smith - data acquisition; Darleen Sandoval and Randy Seeley - experimental design, data analysis, and critical manuscript revision.
Financial Disclosures.
The authors declare the following competing financial interests: DAS receives research support from Ethicon Endo-Surgery, Novo Nordisk, and Boehringer-Ingelheim, serves as a consultant for Givaudan, and is on the scientific advisory board at Ethicon Endo-Surgery. RJS is a paid speaker for Novo Nordisk and Merck. RJS serves on scientific advisory boards or as a consultant for Novo Nordisk, Novartis, Angiochem, Zealand, Takeda, Eli Lilly, Boehringer-Ingelheim, Eisai, Givaudan and Forest Pharmaceuticals. RJS receives research support from Novo Nordisk, Ethicon Endo-Surgery, Ablaris, Boehringer-Ingelheim, and Zealand. RJS has equity in Zafgen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baraboi E-D, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1011–24. doi: 10.1152/ajpregu.00424.2010. [DOI] [PubMed] [Google Scholar]
- 2.Barrera JG, D’Alessio DA, Drucker DJ, Woods SC, Seeley RJ. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes. 2009;58:2820–7. doi: 10.2337/db09-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–9. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 4.Burmeister MA, Ferre T, Ayala JE, King EM, Holt RM, Ayala JE. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab. 2012;302:E334–43. doi: 10.1152/ajpendo.00409.2011. [DOI] [PubMed] [Google Scholar]
- 5.Buse JB, Nauck M, Forst T, Sheu WH-H, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M, Porter L, Schernthaner G. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–24. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 6.Chavez M, Seeley RJ, Woods SC. A comparison between effects of intraventricular insulin and intraperitoneal lithium chloride on three measures sensitive to emetic agents. Behav Neurosci. 1995;109:547–50. [PubMed] [Google Scholar]
- 7.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313–8. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 9.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Grémeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115:3554–63. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niswender K, Pi-Sunyer X, Buse J, Jensen KH, Toft AD, Russell-Jones D, Zinman B. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab. 2013;15:42–54. doi: 10.1111/j.1463-1326.2012.01673.x. [DOI] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. Compact 6t. London: Academic Press; 2009. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 12.Pérez-Tilve D, González-Matías L, Aulinger Ba, Alvarez-Crespo M, Gil-Lozano M, Alvarez E, Andrade-Olivie AM, Tschöp MH, D’Alessio Da, Mallo F. Exendin-4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. Am J Physiol Endocrinol Metab. 2010;298:E1088–96. doi: 10.1152/ajpendo.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roed SN, Wismann P, Underwood CR, Kulahin N, Iversen H, Cappelen KA, Schäffer L, Lehtonen J, Hecksher-Soerensen J, Secher A, Mathiesen JM, Bräuner-Osborne H, Whistler JL, Knudsen SM, Waldhoer M. Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol Cell Endocrinol. 2014;382:938–49. doi: 10.1016/j.mce.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Runge S, Schimmer S, Oschmann J, Schiødt CB, Knudsen SM, Jeppesen CB, Madsen K, Lau J, Thøgersen H, Rudolph R. Differential structural properties of GLP-1 and exendin-4 determine their relative affinity for the GLP-1 receptor N-terminal extracellular domain. Biochemistry. 2007;46:5830–40. doi: 10.1021/bi062309m. [DOI] [PubMed] [Google Scholar]
- 15.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scrocchi LA, Marshall BA, Cook SM, Brubaker PL, Drucker DJ. Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes. 1998;47:632–9. doi: 10.2337/diabetes.47.4.632. [DOI] [PubMed] [Google Scholar]
- 18.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest. 2014 Apr 24; doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sisley S, Sandoval D. Hypothalamic control of energy and glucose metabolism. Rev Endocr Metab Disord. 201;12:219–33. doi: 10.1007/s11154-011-9189-x. [DOI] [PubMed] [Google Scholar]
- 20.Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007;1149:118–26. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.