Abstract
Purpose
Urinary diversion (UD) has been used as a surgical option for treatment of some bladder diseases. We aimed to develop a UD model in the rat and examined the effects of UD on the bladder.
Materials and Methods
Female Sprague-Dwaly rats were distributed into age-matched control, sham UD, and UD groups. Each group was subsequently evaluated at 1 week, or 8 weeks after UD or sham procedures. UD was performed by surgical disconnection of the ureters from the bladder and implantation to uterine cervix. Conscious cystometry (CMG) was examined. The bladders were harvested for histological examination and quantification of smooth muscle, urothelium, and collagen. The histology of the vagina was observed. The expressions of muscarinic and purinergic receptors in bladder were examined.
Results
All the animals survived the UD procedures. The bladder weight decreased in the UD group. CMG showed decreased intercontractile intervals and voided volume in the UD compared to control and sham groups. Compliance was reduced in the UD rats. Smooth muscle and urothelium, as a percentage of the total cross sectional area in bladder, were decreased. Collagen increased in 1-week and 8-week UD rats compared with controls. Histological examination of the vaginal wall showed mild swelling in 2 rats. UD caused the reduction of M3 and P2X1 receptors expression, but no change of M2 and P2X2 receptors.
Conclusions
Creation of a UD model by ureterovaginostomy in rat is feasible. UD causes distinct functional and morphometric alterations of the bladder.
Keywords: Urinary diversion, Ureterovaginostomy, Cystometry, Histology, Animal model
INTRODUCTION
Urinary diversion (UD) has long been used as a surgical option for management of patients with a variety of conditions including bladder cancers, neurogenic bladder, congenital disorders, hemorrhagic cystitis, or by default when patients with end-stage renal failure, prior to renal transplantation, cease to have urinary output 1-4. The term ‘disuse atrophy’ has been used for bladders subject to UD. However, little is known about the impact of disuse of the bladder on its morphology and functional status, and therefore about implications of such changes on future potential use of the bladder. Previous studies have shown that UD results in the rapid atrophy of the bladder smooth muscle, and in a reduction in contractile function in fetal sheep, young rabbits, dog, or humans 5-9. However, there are few studies 10 to examine the natural history of the disused bladder in an adult small rodent such as the rat. In this study we introduce a new UD model created by ureterovaginostomy in rat, and further examine the temporal functional, histological, and molecular changes in the bladder in this model.
MATERIALS AND METHODS
Experimental animals and design
Female Sprague-Dawley rats matched by date of birth (10 weeks-old, Harlan), were used in this study. The animals were randomly allocated to three groups: age-matched control (n=20), sham UD (n=24), and UD (n=24). UD was performed by surgical ureterovaginostomy. Sham procedure included laparotomies and the identification of ureters. No surgery was performed in controls. Each group was subsequently evaluated at 1 week, or 8 weeks after UD. At designed time points, half of the animals were tested by conscious cystometry (CMG), and then were sacrificed. The bladder was removed for examination of muscarinic and purenergic receptors using immunoblotting. The remaining half animals were sacrificed directly and the bladder was removed at the level of the bladder neck, weighed, and sectioned at the equatorial midline. The bottom half of the bladder was fixed in 10% formalin for histological staining. The urethra and vagina were fixed in 10% formalin. Animal were sacrificed by injection of pentobarbital (200 mg/kg, ip). The experimental protocol was approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Urinary diversion
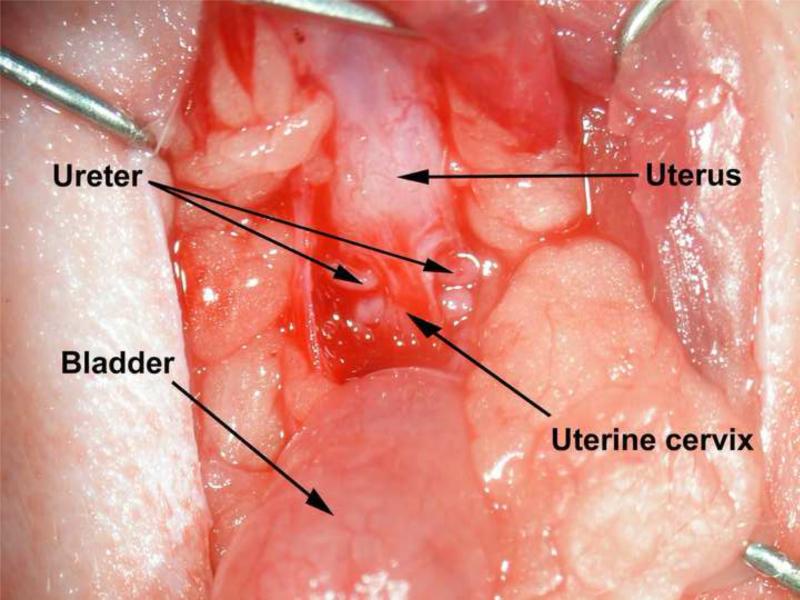
Animals were anesthetized by an intraperitoneal injection comprised of a mixture of ketamine (100mg/kg body weight) and xylazine (10mg/kg body weight). A lower ventral midline incision was made. The ureters were identified and ligated distally. Two small orifices were made using a drill (Dremel, Model 770, Racine, WI) in the uterus cervix and 1-2 mm ureter was brought through the orifice and was secured to the cervix with one suture close to the medial side (3 o’clock point) (Figure 1). The abdomen was closed. Antibiotics were administered for 72 hours. The vaginal cavity was checked every day in the first 2 weeks, and then once a week to make sure that no crystals had formed.
Figure 1.
Urinary diversion in rat. UD was performed by surgical disconnection of the ureters from the bladder and implantation to uterine cervix. The ureters were identified and ligated distally. Two small orifices were made using a drill in the uterus cervix and 1-2 mm ureter was brought through the orifice and was secured to the cervix with one suture close to the medial side (3 o’clock point). The abdomen was closed.
Suprapubic bladder catheter implantation and conscious CMG measurement
Catheter implantation was performed under anesthesia 11. The bladder was exposed and a circular purse string suture of 7-0 silk was placed on the bladder wall. A small incision was made in the bladder wall and the catheter (PE-50 tubing with a flared tip) was implanted; a purse string suture was tightened around the catheter. The catheter was tunneled subcutaneously and externalized at the back of the neck, out of reach of the animal. The distal end of the tubing was sealed, and the skin and abdominal incisions were closed separately.
Two days later, the CMG was performed as described before 11. Briefly, the bladder was filled via the catheter with 0.9% saline (5 ml/hour) while bladder pressure was recorded. The data on at least 5 representative micturition cycles were collected and means were calculated for analyzing the cystometric parameters including bladder capacity, peak detrusor leak pressure, and mean intercontraction interval. In addition, bladder compliance was calculated.
Histology
The bladder and the middle-vagina and urethra were processed, embedded, sectioned transversely (5 μm), and stained with Masson's trichrome. Cross-sections of the specimens were examined using light microscopy and photographed.
Image analysis
Mason's trichrome stained sections at the equatorial midline were analyzed with Image-Pro Plus 5.1 image analysis software (Media Cybernetics) 12. This software can distinguish regions stained with different colors and can accurately measure such areas. This color segmentation method was employed to determine the whole cross-section area and the tissue area that was stained “pink” (urothelium), “blue” (collagen), and “red” (smooth muscle). Therefore, three components were expressed as percentage of the total tissue area. In all cases, the processing of images was performed by one investigator, unaware of treatment group assignments.
Immunoblotting
Frozen bladder tissues were homogenized. Proteins were separated by SDS-PAGE. Equal amounts of protein extract (40 g) from 3 groups at same time points were distributed to the same gel to reduce any other non-treatment effects. The proteins were then transferred to nitrocellulose membranes, probed with a primary antibody, and then incubated with the secondary antibodies. The bands were visualized using enhanced chemiluminescence and HyBlot CL autoradiography film (Denville Scientific Inc, Metuchen, NJ). The primary antibodies used were mouse-anti-M2 (1:5000, Affinity BioReagents Inc, Golden, CO), rabbit-anti-M3 (1:200, Sigma-Aldrich, St Louis, MO), rabbit-anti-P2X1 (1:5000, Chemicon, Billerica, MA), and rabbit-anti-P2X2 (1:200, Alomone, Jerusalem, Israel). Membrane was also incubated with anti β-actin antibodies (A 5441, 1:5000, Sigma-Aldrich, St Louis, MO). Band intensities were evaluated using Scion Image Beta 4.02 software (Scion Corporation, Frederick, MD). The intensity of each target band was divided by that of the β-actin band of the same sample.
Statistical analysis
All data is expressed as the mean plus-or-minus standard error of the mean (SEM). Comparisons of general information, CMG measurements, and bladder tissue components among the control, sham and UD groups at the different time points were performed with the two-way ANOVA test. Comparisons of immunoblotting measurements among the control, sham and UD groups at the same time point were performed with the one-way ANOVA test. Bonferroni corrections were used to adjust for multiple pair-wise comparisons and a P <0.017 was established as statistically significant. Prism 4 (GraphPad, La Jolla) was used for analysis.
RESULTS
General characteristics
Urinary diversion by ureterovaginostomy was performed successfully (Figure 1). All the rats survived for 1 week or 8 weeks, and were healthy and active. The body weight was similar among three groups at the same time point. However, the bladder weights decreased in the 1-week UD group, and further in the 8-week UD group. The ratio of bladder weight / body weight had a similar trend with the change of bladder weight (Table 1).
Table 1.
General information of age-matched control, sham UD and UD rats.
| Time point | Group | Body wt | Bladder wt | Bladder/body wt |
|---|---|---|---|---|
| 1 week | Control | 240.7±0.88 | 91.0±2.65 | 0.38±0.01 |
| Sham | 247.5±1.98 | 88.3±2.01 | 0.36±0.01 | |
| UD | 242.7±1.51 | 66.0±2.15# | 0.27±0.01# | |
| 8 weeks | Control | 280.9±4.29* | 90.4±1.83 | 0.32±0.01 |
| Sham | 281.5±3.04* | 89.0±2.08 | 0.32±0.01 | |
| UD | 277.3±4.88* | 43.5±2.36#† | 0.16±0.01#† |
Values are expressed as the mean ± standard error of the mean.
Significantly different from corresponding value in 1-week control group (p < 0.01).
Significantly different from corresponding value in control and sham group (p < 0.01).
Significantly different from corresponding value in 1-week UD group (p < 0.01).
A potential complication was the formation of urine crystals in the vagina of some rats, particularly within the 1-2 week period after UD.
CMG measurement
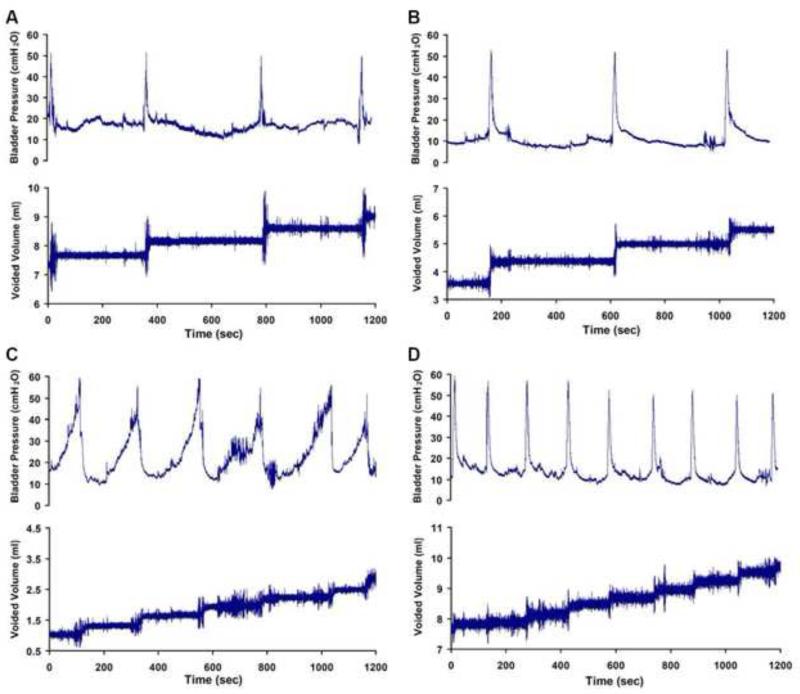
All rats showed a regular and periodic emptying of the bladder (Figure 2). One-week UD, and eight-week UD rats void more frequently than do the control and sham animals. Rats in the 1-week UD and 8-week UD groups showed decreased micturition interval, voided volume, and compliance levels during CMG than did those in the control and sham groups (Table 2). However, there were no significant differences in peak pressure among the three groups.
Figure 2.
Representive tracings of CMG of age-matched control (A), sham UD (B), 1-week UD (C) and 8-week UD rats (D).
Table 2.
Parameters of bladder activity during CMG in age-matched control, sham UD and UD rats.
| 1 week | 8 week | |||||
|---|---|---|---|---|---|---|
| Control | Sham | UD | Control | Sham | UD | |
| Peak P (cmH2O) | 58.96±9.06 | 60.23±17.86 | 52.10±1.80 | 59.18±4.92 | 63.67±3.11 | 58.45±9.75 |
| Interval (seconds) | 477.7±61.7 | 554.5±29.5 | 143.3±24.5* | 542.3±69.3 | 574.2±26.7 | 118.5±22.8*# |
| Voided V (ml) | 0.62±0.07 | 0.72±0.01 | 0.20±0.02* | 0.70±0.04 | 0.74±0.00 | 0.14±0.02* |
| Compliance (ml/cmH2O) | 0.43±0.052 | 0.39±0.057 | 0.01±0.002* | 0.36±0.081 | 0.40±0.07 | 0.07±0.010*# |
Values are expressed as the mean ± standard error of the mean. P, pressure; V, volume.
Significantly different from corresponding value in control and sham group (p < 0.01).
Significantly different from corresponding value in 1-week UD group (p < 0.01).
Histology
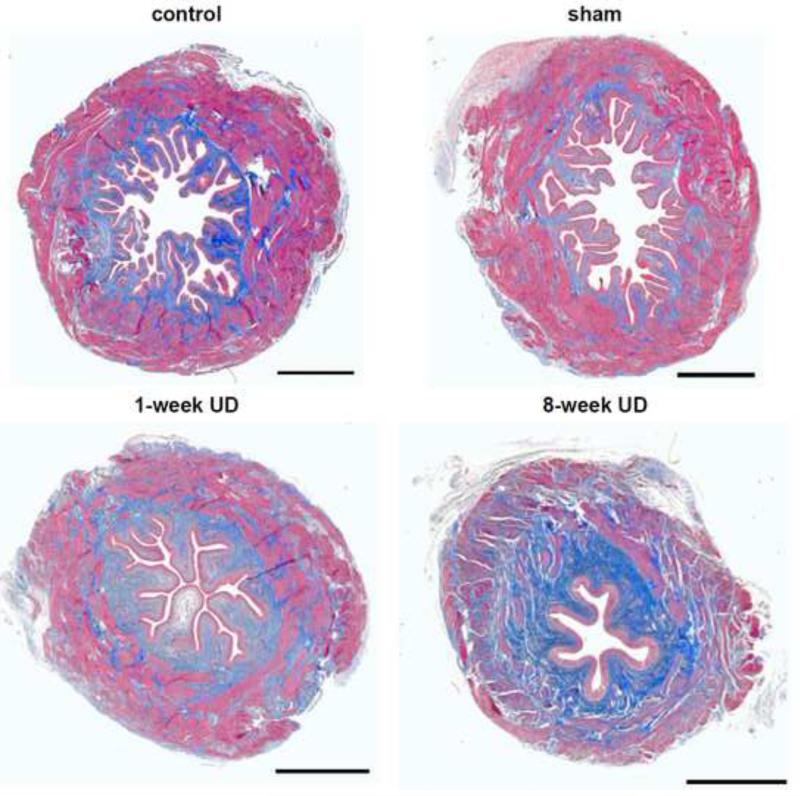
Histological examination of cross-sectioned areas of the bladder showed bladder atrophy in the 1-week UD and 8-week UD rats (Figure 3). The urothelium deep folds decreased in a time-dependent manner in UD rats. Three components (urothelium, detrusor and collagen) were expressed as a percentage of the total tissue area and quantified data were summarized in Table 3. The percentage of the urothelium area, located in the internal edge of the bladder lumen, was not significantly different among the three groups at 1 week after UD, but decreased at 8 weeks after UD compared with control and sham groups. The percentage of collagen area, localized in the lamina propria, and within and between the muscle bundles, increased, but smooth muscle area decreased in both the 1-week UD and 8-week UD groups. There were no significant differences between control and sham rats in any of the three tissue component areas at any of the time points (p>0.05).
Figure 3.
Representative images of Masson's trichrome staining of equatorial sections of urinary bladders from age-matched control, sham UD, 1-week UD and 8-week UD rats, showing smooth muscle (outer magenta), collagen (blue) and urothelium (inner light magenta). Scale bar, 1 mm.
Table 3.
Temporal changes of urothelium, collagen, and smooth muscle areas as percentage (%) of total tissue cross-sectional area in the bladder of age-matched control, sham UD and UD rats.
| Time point | Group | Percentage of Collagen | Percentage of muscle | Percentage of urothelium |
|---|---|---|---|---|
| 1 week | Control | 41.4±3.9 | 48.0±3.3 | 8.9±0.4 |
| Sham | 36.3±1.4 | 53.7±2.0 | 10.5±0.8 | |
| UD | 48.8±2.3* | 38.9±2.2* | 8.1±0.8 | |
| 8 weeks | Control | 42.3±0.7 | 47.3±0.8 | 11.8±1.2 |
| Sham | 39.8±0.6 | 46.9±1.0 | 10.1±0.5 | |
| UD | 50.9±1.6* | 38.7±2.0* | 5.2±0.7*# |
Values are expressed as the mean ± standard error of the mean.
significantly different from the corresponding value in age-matched control group (p <0.01).
Significantly different from corresponding value in 1-week UD group (p < 0.01).
Histological examination of the vaginal wall using light microscopy showed mild swelling in 2 rats, but no obvious abnormal in others (Figure 4).
Figure 4.
Representative images of Masson's trichrome staining of middle section of vagina and urethre from age-matched control, sham UD, 1-week UD and 8-week UD rats. Scale bar, 1 mm.
Immunoblotting
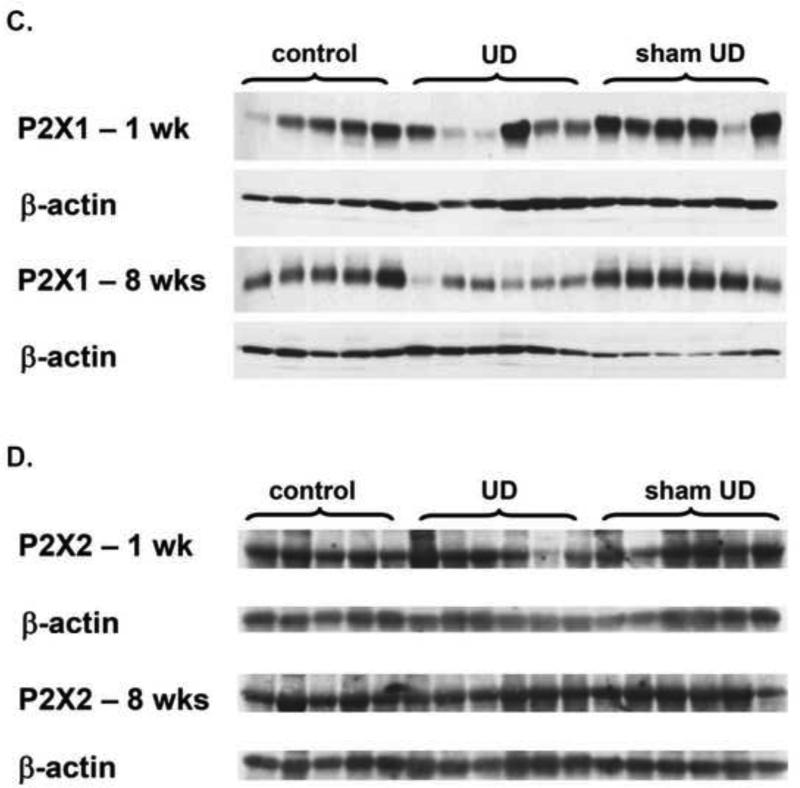
Figure 5 showed both muscarinic and purinergic receptor expression in the bladders in different groups. M3 receptors appeared as a doublet in immunoblotting. Quantified immunoblotting data were summarized in Table 4. The results revealed that no significant differences were found in M2 and P2X2 receptor proteins among the three groups. However, M3 and P2X1 receptor proteins were significantly decreased at 1 week and 8 weeks after UD compared with those of control and sham groups. Considering urinary diversion may reduce the tissue concentration of β-actin 13,14, the results of the current study may overestimate the expression of the mentioned receptor proteins in the UD group.
Figure 5.
Muscarinic receptor-2 (A), muscarinic receptor-3 (B), P2X1 (C), and P2X2 (D) receptor expression in the bladder of age-matched control, sham UD and UD rats. Each lane was from a single rat.
Table 4.
Muscarinic and purinergic receptor expression in the bladder of age-matched control, sham UD and UD rats.
| Time point | Group | M2 | M3 | P2X1 | P2X2 |
|---|---|---|---|---|---|
| 1 week | control | 0.63±0.04 | 0.55±0.05 | 1.04±0.09 | 0.60±0.05 |
| sham | 0.53±0.02 | 0.53±0.08 | 1.25±0.03 | 0.65±0.04 | |
| UD | 0.55±0.05 | 0.28±0.04* | 0.49±0.10* | 0.63±0.11 | |
| 8 weeks | control | 0.41±0.03 | 0.20±0.01 | 0.93±0.11 | 0.69±0.07 |
| sham | 0.37±0.03 | 0.20±0.03 | 1.27±0.11 | 0.63±0.03 | |
| UD | 0.44±0.03 | 0.10±0.02* | 0.51±0.07* | 0.74±0.06 |
Values are expressed as the mean ± standard error of the mean. The values represent the ratio of band densities to beta actin from the same sample.
Indicates a statistically significant difference compared to corresponding value in control and sham group in same time point (p < 0.01).
DISCUSSION
The function of the bladder includes storing urine and then expelling it periodically via coordinated and sustained contractions 15. However, if urine is diverted the bladder fails to fill and empty regularly, it becomes defunctionalized. The key questions in the mind of urologists become to what extent the bladder becomes defunctionalized and over what period of time? These questions are not infrequently encountered by urologists who use UD either as a temporary or permanent measure for management of conditions such as congenital anomalies or neurogenic bladders, or in the situation of the stoppage of urine production in patients with end-stage renal failure 1-4. To better answer this question, it is necessary to set up a UD model in a commonly used rodent such as the rat.
Here, we introduce a new UD model in rat. UD is performed by diverting the ureters to the cervix, which immediately drains the urine into the vagina (ureterovaginostomy). The epithelium of the vagina consists of keratinized squamous cells, similar to skin, with less permeability to urine compared to other choices for diversion such as colon or uterus. The main complication is the formation of urine crystals in the vagina, particularly in the first week after surgery. Knowing this, the vagina should be checked every day to make sure that no crystal has been formed, thereby blocking urine outflow. Another possible complication is blockage at the uteruo-ureteral anastomosis (which happened infrequently during our pilot study). As a result, we modified our technique by using only one suture to secure the ureters to the uterus, instead of the four sutures used previously. We did not find uterine blockage with this method. The third potential and mild complication was swelling of the epithelium of the vagina in some of UD rats. However, because we did not check the estrus cycle of the rats, we don't know whether the swelling was estrus cycle-related or methodology-related.
Urinary diversion affects the bladder function significantly. The obvious changes in CMG measurement are decreased intercontractile intervals and voided volume per micturition in the UD rats, compared to that in control and sham animals. In addition, the compliance was significantly reduced in the UD rat but there were no significant differences in peak micturition pressure. The altered bladder function is likely because of the histological and molecular changes.
Urinary diversion leads to significant bladder atrophy. The bladder weight of the UD rats was about two-thirds of the controls in the 1-week UD, and half of the controls in the 8-week UD. Histological examination showed thinning of the muscle layer and atrophied muscle bundles. The urothelium folding was decreased. There appeared to be connective tissue infiltration and dispersal of the smooth muscle elements. Although the percentage of smooth muscle was significantly reduced, the peak pressure was unaffected by UD in this study. The relationship between pressure and volume is determined by the law of LaPlace, which, for a sphere, states that P = 2T/r, where P is detrusor pressure, T is bladder wall tension, and r is the radius of the bladder. Therefore, intravesical pressure is dependent on both the bladder wall tension and bladder volume. Bladder wall tension is determined by the active and passive properties of its constituent parts including smooth muscle fibers, collagen, and elastic tissue 16. In UD bladders, both bladder wall tension (increased percentage of collagen and decreased percentage of detrusor muscle) and the radius of the bladder (decreased bladder capacity) were reduced, which may explain the lack of observed change in peak pressure.
Appropriate ratio of smooth muscle and connective tissues maintains the appropriate bladder compliance. The relatively reduced detrusor muscle and increased collagen percentage probably contributed to the reduced compliance. As we know, during physiologic bladder filling there is little or no increase in vesical pressure despite large increases in volume. This process or accommodation is due primarily to the passive elastic properties of the smooth muscle and connective tissue that comprise the bladder wall, and to inhibition of detrusor muscle activity 17. Appropriate bladder filling and voiding provides a balance in muscle and connective tissues. Present study showed disuse leads to a gradual percentage decrease in smooth muscle and urothelium, and increase in connective tissue. Muscle atrophy may result from depressed protein synthesis and/or enhanced protein degradation. The activation of the ubiquitin (Ub)–proteasome pathway is involved in accelerated proteolysis in muscle 18. Some studies showed the atrophied muscles show a two- to fourfold increase in levels of messenger RNA (mRNA) for polyubiquitin and certain proteasome subunits 19,20. In addition, skeletal muscle atrophy observed in diabetes, disuse, and cancer cachexia has been linked to the NF-κB pathway 21.
The mechanisms of bladder fibrosis in the UD animals might be related to fibroblast activation. Studies showed that the fibrotic process in dystrophic muscles is a very complex process and might involve several important growth factors such as transforming growth factor-beta1 (TGF-beta1), connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF), as well as various pathophysiological and biochemical pathways 22. Growth factors could stimulate adjacent fibroblasts to produce excessive collagen, or they could stimulate muscle fibers themselves to produce fibrosis-related proteins 22. Previous study has showed that the increased TGF-beta1 expression induced by chronic ischemia can leading to fibrosis in the bladder 23.
In addition to functional and morphometric changes, we examined the expression of muscarinic and purinergic receptors in bladder. Normal bladder contraction is mediated mainly through stimulation of muscarinic and purinergic receptors in the detrusor muscles 24-27. A previous study using immunoprecipitations methods showed that 3 days after ureteral diversion into the colon, the bladder was atrophied and both M2 and M3 receptors were decreased 28. In another study, P2X1 receptor did not change in hypertrophied bladder 10 days after outlet obstruction 29. The results of the present study are not fully consistent with the previous data form atrophied bladder or contrary with those from outlet obstruction-induced bladder hypertrophy. We found the expression of M3 and P2X1 receptors, which are mainly responsible for normal contraction, were reduced, but M2 and P2X2 were not changed in atrophied bladders after 1-week or 8-week UD. Interestingly, M3 receptors appeared as doublets in the western blots which may reflect post translational modification (eg, glycosylation or phosphorylation). The altered receptor density may partially contribute to the altered bladder function.
Our described UD model can be used for a variety of translational research scenarios related to clinical situations in which temporary or long-term disuse of the bladder is carried out to, a) evaluate the effects of long-term hemodialysis on the bladder of patients with end-stage renal disease before kidney transplantation; b) exclude the effects of urine in some special research projects related to bladder. We hope that our reported findings have added to insights regarding related translational research questions.
CONCLUSIONS
Creation of a UD model by ureterovaginostomy in the rat is feasible for testing the impact of disuse on the bladder. Urinary diversion causes distinct functional and morphometric alterations of the bladder.
ACKNOWLEDGMENT
To Anthony Kanai, Ph.D., for sharing his expertise of the in-vivo techniques.
Grant: The study was supported by NIH grant - KO8 DK02631 and Animal Models of Diabetic Complications Consortium (www.amdcc.org) grant DK61018-02S1
Key of Definitions for Abbreviations
- CMG
Cystometry
- UD
Urinary diversion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jayanthi VR, McLorie GA, Khoury AE, Churchill BM. The effect of temporary cutaneous diversion on ultimate bladder function. J Urol. 1995;154:889. doi: 10.1097/00005392-199508000-00155. [DOI] [PubMed] [Google Scholar]
- 2.Alexander F, Kay R. Cloacal anomalies: role of vesicostomy. J Pediatr Surg. 1994;29:74. doi: 10.1016/0022-3468(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 3.Krahn CG, Johnson HW. Cutaneous vesicostomy in the young child: indications and results. Urology. 1993;41:558. doi: 10.1016/0090-4295(93)90104-i. [DOI] [PubMed] [Google Scholar]
- 4.Ushigome H, Sakai K, Suzuki T, Nobori S, Yoshizawa A, Akioka K, et al. Kidney transplantation for patients on long-term hemodialysis. Transplant Proc. 2008;40:2297. doi: 10.1016/j.transproceed.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto S, Kogan BA, Levin RM, Howard PS, Macarak EJ. Response of the fetal sheep bladder to urinary diversion. J Urol. 2003;169:735. doi: 10.1097/01.ju.0000045680.05119.84. [DOI] [PubMed] [Google Scholar]
- 6.Lipski BA, Yoshino K, Yao LY, Carr MC, Mitchell ME. A unique new model to study the effects of urinary diversion in the developing rabbit bladder. J Urol. 1998;160:1454. [PubMed] [Google Scholar]
- 7.Jayanthi VR, McLorie GA, Khoury AE, Churchill BM. The effect of temporary cutaneous diversion on ultimate bladder function. J Urol. 1995;154:889. doi: 10.1097/00005392-199508000-00155. [DOI] [PubMed] [Google Scholar]
- 8.Wei W, Howard PS, Kogan B, Macarak EJ. Altered extracellular matrix expression in the diverted fetal sheep bladder. J Urol. 2007;178:1104. doi: 10.1016/j.juro.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Chun AL, Ruzich JV, Wein AJ, Levin RM. Functional and pharmacological effects of ureteral diversion. J Urol. 1989;141:403. doi: 10.1016/s0022-5347(17)40783-x. [DOI] [PubMed] [Google Scholar]
- 10.Kruse MN, Bennett B, de Groat WC. Effect of urinary diversion on the recovery of micturition reflexes after spinal cord injury in the rat. J Urol. 1994;151:1088. doi: 10.1016/s0022-5347(17)35189-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Huang X, Liu G, Moore C, Bena J, Damaser MS, et al. Diabetes slows the recovery from urinary incontinence due to simulated childbirth in female rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R950. doi: 10.1152/ajpregu.00686.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G, Daneshgari F. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R837. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- 13.Braverman AS, Ruggieri MR., Sr. Muscarinic receptor transcript and protein density in hypertrophied and atrophied rat urinary bladder. Neurourol Urodyn. 2006;25:55. doi: 10.1002/nau.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malmqvist U, Arner A, Uvelius B. Contractile and cytoskeletal proteins in smooth muscle during hypertrophy and its reversal. Am J Physiol. 1991;260:C1085. doi: 10.1152/ajpcell.1991.260.5.C1085. [DOI] [PubMed] [Google Scholar]
- 15.Levin RM, Whitbeck C, Borow A, Burden O, Leggett RE. Effectiveness of vaginally administered oxybutynin on rabbit bladder function. Urology. 2003;61:1273. doi: 10.1016/s0090-4295(02)02577-3. [DOI] [PubMed] [Google Scholar]
- 16.Blaivas JG, Heritz DM. Physiological principles for surgical correction of detrusor dysfunction. J Endourol. 1996;10:213. doi: 10.1089/end.1996.10.213. [DOI] [PubMed] [Google Scholar]
- 17.Harris RL, Cundiff GW, Theofrastous JP, Bump RC. Bladder compliance in neurologically intact women. Neurourol Urodyn. 1996;15:483. doi: 10.1002/(SICI)1520-6777(1996)15:5<483::AID-NAU5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Jagoe RT, Goldberg AL. What do we really know about the ubiquitin proteasome pathway in muscle atrophy? Curr Opin Clin Nutr Metab Care. 2001;4:183. doi: 10.1097/00075197-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 20.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, et al. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 21.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 22.Sun G, Haginoya K, Wu Y, Chiba Y, Nakanishi T, Onuma A, et al. Connective tissue growth factor is overexpressed in muscles of human muscular dystrophy. J Neurol Sci. 2008;267:48. doi: 10.1016/j.jns.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Azadzoi KM, Tarcan T, Siroky MB, Krane RJ. Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J Urol. 1999;161:1626. [PubMed] [Google Scholar]
- 24.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995;273:959. [PMC free article] [PubMed] [Google Scholar]
- 25.Longhurst PA, Leggett RE, Briscoe JA. Characterization of the functional muscarinic receptors in the rat urinary bladder. Br J Pharmacol. 1995;116:2279. doi: 10.1111/j.1476-5381.1995.tb15065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 27.O'Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, Rymer JM, et al. A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J Urol. 2001;165:1730. [PubMed] [Google Scholar]
- 28.Braverman AS, Ruggieri MR., Sr. Hypertrophy changes the muscarinic receptor subtype mediating bladder contraction from M3 toward M2. Am J Physiol Regul Integr Comp Physiol. 2003;285:R701. doi: 10.1152/ajpregu.00009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott RS, Uvelius B, Arner A. Changes in intracellular calcium concentration and P2X1 receptor expression in hypertrophic rat urinary bladder smooth muscle. Neurourol Urodyn. 2004;23:361. doi: 10.1002/nau.20047. [DOI] [PubMed] [Google Scholar]