Abstract
FG syndrome is a rare X-linked multiple congenital anomaly-cognitive impairment disorder caused by the p.R961W mutation in the MED12 gene. We identified all known patients with this mutation to delineate their clinical phenotype and devise a clinical algorithm to facilitate molecular diagnosis. We ascertained 23 males with the p.R961W mutation in MED12 from 9 previously reported FG syndrome families and 1 new family. Six patients are reviewed in detail. These 23 patients were compared with 48 MED12 mutation-negative patients, who had the clinical diagnosis of FG syndrome. Traits that best discriminated between these two groups were chosen to develop an algorithm with high sensitivity and specificity for the p.R961W MED12 mutation. FG syndrome has a recognizable dysmorphic phenotype with a high incidence of congenital anomalies. A family history of X-linked mental retardation, deceased male infants, and/or multiple fetal losses was documented in all families. The algorithm identifies the p.R961W MED12 mutation-positive group with 100% sensitivity and 90% spec-ificity. The clinical phenotype of FG syndrome defines a recognizable pattern of X-linked multiple congenital anomalies and cognitive impairment. This algorithm can assist the clinician in selecting the patients for testing who are most likely to have the recurrent p.R961W MED12 mutation.
Keywords: FG syndrome, Opitz-Kaveggia syndrome, MED12, p.R961W, X-linked mental retardation, multiple congenital anomalies, algorithm
In 1974,Opitz and Kaveggia described three brothers and their two male first cousins,who had mental retardation,multiple congenital anomalies, a distinctive personality, congenital hypotonia, and a disproportionately large head and designated this X-linked condition FG syndrome.1 Although Opitz and Kaveggia noted that multiple congenital anomalies were seen in FG syndrome, and mentioned it in the title of their report, with time macrocephaly, hypotonia and constipation became the basis for the diagnosis of FG syndrome in many individuals. Because these features are common in individuals with many forms of mental retardation, FG syndrome was included in the differential diagnosis of many patients, both male and female, with syndromic and nonsyndromic mental retardation. As more patients considered to have FG syndrome were reported and studied by cytogenetic and molecular genetic analysis, heterogeneity became apparent. A number of X chromosome loci were designated “FG syndrome” loci. In 2007, Risheg et al. identified a recurrent mutation, p.R961W, in the MED12 gene in 10 individuals from 6 families with FG syndrome including the only surviving affected man and his obligate carrier mother from the original report of FG syndrome.2 Since then, many individuals with a clinical diagnosis of FG syndrome have been reexamined and tested for the p.R961W MED12 mutation. In only a small percentage (<3%) of such cases has the clinical diagnosis of FG syndrome been confirmed by molecular testing. In 2009, Lyons et al. reported several new diagnoses in a MED12 mutation-negative group of patients who had been given a clinical diagnosis of FG syndrome, based on clinical examination, chromosome analyses, mutation screening, and microarray comparative genomic hybridization.3
MATERIALS AND METHODS
In this report, we review all p.R961W mutation confirmed patients with FG syndrome in the literature and describe one new family (Family 1). To date, 23 male patients have been identified from 10 families (Fig. 1) with the recurrent p.R961W mutation in the MED12 gene. In each family, the diagnosis of FG syndrome was made first on clinical grounds and confirmed later with molecular analysis. We examined new or archived clinical information on the surviving affected male patients and two of the deceased affected male patients from the nine previously reported families and one new family to describe their phenotype and document previously underappreciated findings. Clinical information on some members of Families 2–10 has been reported previously.1,3–10 Graham et al.9 reported the behavioral features exhibited by many of these male patients with FG syndrome.
Fig. 1.
Pedigrees of 10 families with FG syndrome (see Figures, Supplemental Digital Content 1 and 2, http://links.lww.com/GIM/A87 and http://links.lww.com/GIM/A88.). Family 1 has not been reported previously. Family 2, III-1 was included in the report of Briault et al.7 Family 3, IV-1 was reported by Lyons et al.3 Family 4, III-2, III-4, III-5, III-9, and III-10 (the original FG family) were reported first by Opitz and Kaveggia.1 Family 4, III-11 was first reported by Riccardi et al.5 as patient I-6. Family 5, II-1, II-3, and II-10 were reported by Keller et al.4 Family 6, III-3 was reported by McCardle and Wilson6 as J.B. Family 7 was reported by Graham et al.8 as Family 1. Family 8 was reported by Graham et al.8 as Family 3. Family 9 was reported by Graham et al.9 and by Opitz et al.10 as Family 3, Patient 4. Family 10 was reported by Graham et al.9
For this report, 1 or more of the authors personally examined 13 of these 23 individuals, including at least 1 member of each of the 10 families, using a standardized examination protocol: Family 1: IV-2, Family 2: II-3, III-1, Family 3: IV-1, Family 4: III-10, Family 5: II-1, II-10, Family 6: III-3, Family 7: III-1, Family 8: II-5, III-1, Family 9: III-4, and Family 10: III-5. Detailed clinical information is presented for 4 of these 13 patients and 2 of their deceased relatives. All individuals were photographed, and early photographs were reviewed when available. Figure 2 demonstrates Family 1 photographs, all other families are presented in Figures, Supplemental Digital Content 1 and 2, http://links.lww.com/GIM/A87 and http://links.lww.com/GIM/A88.
Fig. 2.
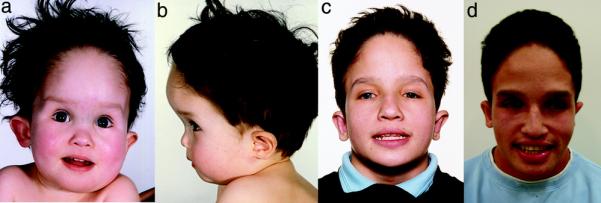
a–d, Family 1, Patient IV-2, at age 2 years 3 months, 11 years 10 months, and 18 years.
We also examined and photographed patients who were clinically diagnosed with FG syndrome but did not have a MED12 mutation, using the same standardized examination protocol, to determine a set of core features that distinguish FG syndrome from other mental retardation disorders. By comparing the features in the p.R961W mutation-positive group with those in the p.R961W mutation-negative patients, we identified discriminating features that separate these two groups.
In each of these 10 families, DNA from at least 1 individual was studied for a defect in the MED12 gene using targeted mutation analysis and/or complete gene sequencing using methods reported by Risheg et al.2 The same mutation, p.R961W was identified in 13 affected male patients from these 10 families. No other mutations were identified in the MED12 gene in these samples. Molecular testing was performed at the Molecular Diagnostic Laboratory at the Greenwood Genetic Center in Greenwood, South Carolina (Families 2–10) and in the laboratory of Dr. Lucy Raymond in Cambridge, United Kingdom (Family 1).
To develop and test the algorithm that we report here, we examined 48 MED12 mutation-negative patients in two groups. The first group of 29 (22 male patients) MED12 mutation-negative patients previously diagnosed with FG syndrome were recruited from the FG syndrome support group in the United States and reported by Lyons et al.3 They were examined by several authors (R.D.C., J.G., M.L., C.E.S., R.E.S.) in April 2007 at Greenwood, South Carolina. The clinical features of this US group were analyzed to determine which features to include in the algorithm. The second group was recruited from the patient series reported by Battaglia et al.11 in Italy. This second group of 19 clinically diagnosed Italian patients with FG (13 male patients), also negative for the MED12 mutation, were examined by several authors (A.B., R.D.C., J.G.) in June 2007 at Pisa, Italy, using the standardized examination protocol used by Lyons et al. Clinical data from the second group were used to test the algorithm and confirm its specificity.
RESULTS
The clinical features for the patients from these 10 families are summarized in Table, Supplemental Digital Content 3, http://links.lww.com/GIM/A86. Previously unreported patients are described later in detail as is the patient reported briefly by Lyons et al.3 and his deceased brother. Patient histories of these six affected male patients are summarized later.
The algorithm correctly identified the 13 mutation-positive patients we examined (for whom all algorithm data were available) with a sensitivity of 100%. The other 10 patients did not have enough data points to analyze the algorithm (see Table, Supplemental Digital Content 3, http://links.lww.com/GIM/A86). All 13 patients are males with some degree of cognitive impairment, and all have a family history consistent with X-linkage, without any affected female patients. Nine patients met all six criteria in the algorithm (two lacked congenital anomalies but had an affected relative with an anomaly); the remaining four patients met five criteria: one lacked small ears, three lacked absolute or relative macrocephaly. These features were seen most often in affected male patients: characteristic facies (13 of 13), an affable personality (13 of 13), a congenital anomaly (11 of 13) or an affected relative with a congenital anomaly (2 of 13), and at least 1 of these 3 traits: infantile hypotonia, feeding difficulties, and constipation (13 of 13). Twelve had small ears that measured below the 10th percentile (the other one had simple ears). Ten had macrocephaly (five absolute, five relative).
Of the 29 MED12 mutation-negative US patients whose features were used to develop the algorithm, 3 patients would be selected for testing using the algorithm and 26 patients would be correctly identified as unaffected, for a specificity of 3 of 29 or 90%.
The Italian patients had these characteristics: 13 of 19 were male patients, 5 of 19 had a positive family history consistent with X-linkage (including 1 with second cousin parents and 2 affected sons), 18 of 19 were mentally retarded, 3 of 19 had an affable personality, 17 of 19 had infantile hypotonia, constipation or early feeding problem, 15 of 18 had macrocephaly (1 unmeasured due to lack of cooperation), 5 of 19 had a congenital anomaly in the patient or affected relative (abnormal corpus callosum in 1 of 19), 0 of 17 had small ears, and 0 of 19 had characteristic facies.
When we tested the algorithm in the 19 mutation-negative Italian patients, none were selected for testing, for a specificity of 100%. Of these 19 Italian mutation-negative patients, 9 were mentally retarded male patients with a negative family history. Of these 9, 7 had hypotonia, constipation, or feeding problems, 7 had macrocephaly, 2 had congenital anomalies, 1 had an affable personality, and none had characteristic facies or small ears. Of the 19 patients, only 3 were mentally retarded male patients with a positive family history. Of these three patients, one male patient was excluded, because he had a mentally retarded sister. The remaining two patients had hypotonia, constipation, feeding problems, and macrocephaly, and one had an affable personality. None of the patients had congenital anomalies, small ears, or characteristic facies.
Using the exclusion criteria alone, 8 of 19 Italian mutation-negative patients were eliminated: 6 female patients, 1 mentally retarded male patient with a positive family history of an affected sister and 1 male patient who was not mentally retarded.
Using the algorithm, 16 tests, selected from all 13 mutation-positive and 3 of the 48 mutation-negative patients, would have been performed to identify all 13 mutation-positive patients. Without the algorithm, 61 tests (from all patients: 13 mutation-positive and 48 mutation-negative patients) would have been performed to identify the same 13 mutation-positive patients. The algorithm reduces the number of tests by 74% that must be performed to identify all mutation-positive patients.
PATIENT SUMMARIES
Additional clinical information is available online in Supplemental Digital Content 1 and 2, http://links.lww.com/GIM/A87 and http://links.lww.com/GIM/A88.
Family 1, IV-2
This 19-year-old British male patient was clinically diagnosed with FG syndrome in 1991 as a toddler. His findings have not been reported previously. His birth weight was 2.6 kg at term. He was hypotonic and required tube feeding initially. He did not pass meconium for 4 days and remained constipated and hypotonic. He had surgery for pyloric stenosis and a partial colonic resection for megacolon. He has had severe constipation throughout his life. Brain magnetic resonance imaging (MRI) revealed the absence of the corpus callosum and empty pituitary sella. Ophthalmologic assessment demonstrated optic nerve hypoplasia. His cardiac status is still under investigation with a possible atrial septal defect. The results of chromosome analysis were normal.
His development was significantly delayed. He achieved sitting balance at 27 months and first walked unaided at 6 years. Speech was less delayed than other areas, and he was very verbal before he could walk. He spoke in sentences and engaged in conversation with an engaging chatty personality. He had a good memory and reasonable self-help skills with prompting.
On physical examination, his adult height was 163 cm (10th percentile) and his head circumference was 60.5 cm (>97th percentile). He had hypertelorism, a high forehead, upswept frontal hair, and small cup-shaped ears (for which he has had surgery), wide mouth, full lips, broad palate, and broad neck. He had a depressed xiphisternum and limited extension and supination at the elbow. He had a broad-based and flat-footed gait. His thumbs, considered broad in childhood, appeared normal.
Family 2 (K9367), III-1 and II-3
The patient, III-1, now 17 years old, is Patient 3 in the report of Briault et al.7 His birth at 35 weeks’ gestation was complicated by asphyxia and intraventricular hemorrhage. He was hypotonic at birth. He had macrocephaly and craniosynostosis. Pyloric stenosis was corrected surgically at 3 months of age. He required gastrostomy feeding until 18 months of age. He had gastroesophageal reflux but did not have constipation or anal anomalies. An atrial septal defect resolved by the age of 4 years. Febrile seizures, from the age of 3 years, were followed by afebrile seizures. He has had no seizures since 5 years of age. An MRI of the brain showed agenesis of the corpus callosum and enlarged ventricles.
His dysmorphic features include short stature (160 cm), slender body habitus, macrocephaly, frontal upsweep of hair and other “cowlicks,” left strabismus, open mouth, drooling, two fused teeth, crowded teeth and delayed exfoliation of teeth, narrow high palate, small low set ears, and narrow auditory canals. His thumbs and great toes are wide and flat.
Early developmental milestones were delayed. He is happy, outgoing, and social and enjoys interacting with others. His speech is mildly dysarthric. He eats well without choking at the age of 17 years. Psychoeducational evaluation at the age of 11 years revealed reading and math scores of 40 (Wechsler Individual Achievement Test-II),12 an expressive language score of 67 (Expressive One-Word Picture Vocabulary Test),13 and a receptive language score of 44 (Peabody Picture Vocabulary Test-III),14 all of which are at or below the 1st percentile.
The maternal uncle of the above patient, II-3, also has FG syndrome. His clinical information has not been reported previously. This man, now 43 years old, lives in a sheltered residential community. He was delivered at term with a normal birth weight of 3.6 kg. Atelectasis delayed his discharge home until 5 days of age. He had difficulty in breastfeeding because of hypotonia. Strabismus of his left eye, inguinal hernia and an undescended testicle required surgical interventions. Mitral valve prolapse did not require treatment. Myoclonic seizures, diagnosed at 37 years of age, are worse with stress, fatigue, or cold. An MRI of the brain at that time showed an abnormal cerebellum, which was also found in his mother. Also he has dysgenesis of the corpus callosum. His IQ is <50. His behavior is gregarious and attention seeking with obsessive-compulsive tendencies. He has “cocktail party” conversational skills.
His dysmorphic features include short stature (165 cm), macrocephaly (62.2 cm, >98th percentile), frontal upsweep, high forehead, dolicocephaly, strabismus, narrow ear canals, narrow high palate, crowded teeth, low set ears, sacral dimple, flat feet, wide flat thumbs, and great toes. His early hypotonia has resolved.
Family 3 (K9411), IV-2
This boy was examined at the age of 7½ years.3 Vaginal vertex delivery required forceps because of a large head. Dysmorphic features prompted chromosome analysis. He required nasogastric tube feeding. At 5 weeks of age, congestive heart failure led to surgical correction of an ASD and VSD. Plagiocephaly was successfully treated with a helmet at 6 months. Fundoplication and gastrostomy were needed at 14 months for gastroesophageal reflux and aspiration pneumonia. Chronic constipation, noted in infancy, improved after he started walking and drinking more water.
His first words were at 12 months, and he walked at 37 months. His personality was exceedingly friendly, happy, and affectionate, and he constantly sought attention and affirmation. He was anxious and he perseverated. Using the Stanford-Binet Intelligence Scale (4th edition),15 he attained the following scores: Verbal Reasoning, 98; Abstract/Visual Reasoning, 72; Quantitative Reasoning, 100; Short-Term Memory, 75; and Composite IQ, 84. For comparison, his mother's scores were Verbal Reasoning, 101; Abstract/Visual Reasoning, 114; Quantitative Reasoning, 90; Short-Term Memory, 86; and Composite IQ, 97.
At the age of 7½ years, he had a slender build and tactile defensiveness. His head was dolicocephalic. Hair whorls were at the vertex and anteriorly in the right frontal area, with a frontal upsweep of scalp hair and a high anterior hairline. His face was long, narrow, and mildly myopathic with an open mouth and mild malar flattening. His fingers were long and slender with squared tips, distal tapering, and mild clinodactyly of the index fingers. His thumbs were broad and flat distally. The great toenails were broad. The distal edge of the nail plates adhered to the very distal tips of the fingers and toes. There were fetal pads on the fingers and toes. Supination of the elbows was limited to 135°. The muscle tone was low. His gait was unusual with a bouncing stride.
His brother, IV-1, who died at 1 hour of age in 2001, was diagnosed retrospectively from photographs and his history. Polycystic kidneys and absent amniotic fluid were noted on fetal ultrasound examination. He was born prematurely in breech presentation. He had an atrioventricular canal defect, tall narrow head, small ears, micrognathia, and syndactyly of the fingers.
Family 6 (K9073), II-4
In 1993, McCardle and Wilson6 reported FG syndrome in their patient, J.B. (designated here as III-3 in Family 6). The maternal uncle of the proband, II-4, was born in 1965 and died at 10 months of age. Clinical information and photographs were obtained from his two admissions to The Children's Hospital Medical Center in Boston. He was born at 42 weeks’ gestation after an induced labor. His appearance at the age of 9 days is shown in Figure, Supplemental Digital Content 2, http://links.lww.com/GIM/A88. Dysmorphic features included patent posterior fontanelle, excess peri-orbital tissue causing “slit-like eyes” small, low-set ears, stenotic auditory canals, small chin, prominent occiput, Grade II harsh systolic cardiac murmur, left cryptorchidism, diastasis recti, single umbilical artery, single transverse palmar creases, and hypotonia. Head circumference was normal. An electroencephalogram showed epileptiform activity with asynchronous changes suggesting “lack of major commissural tracts.” Because he was dysmorphic, a geneticist examined him and ordered chromosome analysis, which was normal. There was a systolic murmur, a large heart and increased pulmonary flow on chest x-ray. A cardiac catheterization for cardiac failure showed primary pulmonary hypertension. Cardiac fluoroscopy showed “transposition with septal defect.” He improved with digoxin. He was discharged at 5 weeks of age.
At age 7 months, he was readmitted for “developmental retardation.” He was floppy and dysmorphic. His ears were described as “low-set, small, and pointed.” He had cardiomegaly on chest x-ray. His discharge diagnoses were “multiple congenital anomalies, cerebral dysgenesis, congenital heart disease with pulmonary artery hypertension, and coarctation of the aorta.”
The family history at that time was pertinent for a maternal uncle (I-3) and a cousin with mental retardation. (The exact relationship of the cousin is unknown and therefore was not recorded on the pedigree here.)
DISCUSSION
In this report, we delineate the clinical features and natural history of FG syndrome by reexamining the surviving affected male patient from the original report of FG syndrome and reviewing affected male patients from nine other families who share the p.R961W MED12 mutation.2 We have focused on these 10 families, the only families, published or unpublished, known to these authors to have the recurrent p.R961W MED12 mutation, to separate them from the other heterogeneous group of individuals diagnosed with FG syndrome but lacking a documented mutation in this gene. These 23 affected male patients, in whom the clinical diagnosis of FG syndrome has been confirmed with molecular testing, offered us the chance to define the phenotype of this rare X-linked syndrome.
The algorithm outlined in Table 1 was developed to help identify all patients with FG syndrome with the fewest number of molecular tests, hence both sensitivity and specificity are high. Using the algorithm, the great majority of mutation- negative patients will be eliminated before testing, saving the cost of unnecessary molecular analysis. The algorithm incorporates several lessons learned from our analysis. A positive family history consistent with X-linkage is a discriminating characteristic that selects for mutation-positive patients and eliminates mutation-negative patients. The presence of an X-linked family history is not mandatory. However, all 10 families described here are either multiplex or have significant reproductive losses. Therefore, a patient with a negative family history has to meet more stringent criteria. Congenital anomalies are given more weight as an independent criterion in the diagnosis of this disorder. The anomalies in FG syndrome are more likely to affect the corpus callosum than any other organ. Therefore, head imaging studies may be helpful in evaluating patients suspected to have FG syndrome. The facial features are distinctive, especially the narrow, long face, tall forehead, puffy eyelids, and open mouth. Small, simple prominent ears are a separate criterion, because this is an uncommon finding in the mutation-negative group and almost universal in those with a MED12 mutation. The affable personality is very common in the mutation-positive group and uncommon in the mutation-negative group, whereas anxiety, which is present in both groups, is less discriminatory. Exclusion criteria include female sex, lack of intellectual disability, and autosomal patterns of inheritance. The diagnosis of FG syndrome should be considered only in male patients who have some degree of intellectual disability because there have been no convincing reports of FG syndrome in either heterozygous female carriers or in male patients whose intelligence is similar to their unaffected relatives. Although hypotonia, constipation, and feeding problems that require medical intervention in the early years are consistent features in the mutation-positive group, they are not weighted separately because they almost invariably occur together. Whether alone or together, they discriminate poorly between the mutation-positive and mutation-negative groups. Fewer criteria are needed for the diagnosis in the newborn period because neither the characteristic personality nor mental retardation would be evident until an older age.
Table 1.
Algorithm for clinical criteria for molecular testing for FG syndrome
| When the family history is compatible with an X-linked trait as defined below, testing is indicated when four criteria are met in males under age 5 (excluding affable personality) or when five criteria are met in males older than 5 yr (including affable personality). Without an X-linked family history, testing is indicated when five criteria are met in a male under age 5 (excluding affable personality) or when six criteria are met in a male older than 5 yr. | |
|---|---|
| Family History: | X-linked pattern of inheritance requires development delay (younger than 3 yr) or cognitive impairment (older than 3 yr) in the male proband and at least one other male related through the proband's maternal lineage without affected females or male-to-male transmission |
| If only one male is cognitively impaired in the family, an X-linked pattern of inheritance can be presumed with the additional finding of multiple miscarriages, or family history of stillborn male(s), or early male deaths in the proband's sibship or maternal lineage (born to his sister, maternal aunt, maternal grandmother, etc.) | |
| Required: | 1. Male sex |
| 2. Developmental delay (younger than 3 yr) or Cognitive impairment/Intellectual disability (older than 3 yr). | |
| Note: IQ may be in the borderline to low normal range if IQs of parents and unaffected siblings are in the average or above average range | |
| Clinical Criteria: | 1.Small ears–10th percentile or less |
| 2.Characteristic facies: narrow tall head (dolicocephaly), tall forehead, frontal upsweep, long narrow face, puffy eyelids, open mouth | |
| 3. Congenital anomaly(ies) of corpus callosum, anus, heart, or skeleton in the proband or in an affected male relative from the maternal lineage | |
| 4. Affable, eager-to-please personality with or without anxiety (evident by age 5 yr) | |
| 5. Macrocephaly (>98th percentile) or relative (head circumference percentile greater than the percentile for height) | |
| 6. Early hypotonia, constipation or feeding problems severe enough to require medical intervention. | |
| Exclusion criteria: | 1. Female sex |
| 2. Normal intelligence with IQ similar to parents and unaffected siblings. | |
| 3. Affected females in the pedigree | |
| 4. Male-to-male inheritance in the pedigree | |
In Figure 2 and Figures, Supplemental Digital Content 1 and 2, http://links.lww.com/GIM/A87 and http://links.lww.com/GIM/A88, photographs of male patients with FG syndrome from infancy through adulthood demonstrate that affected male patients with the p.R961W mutation share a consistent, distinctive, and recognizable pattern of dysmorphic features that defines the clinical phenotype of FG syndrome. Their clinicians noted the dysmorphic appearance of these affected male patients when they were infants, before their cognitive impairment was evident. The facial gestalt may be easier to recognize in early childhood. The key elements of the clinical phenotype are absolute or relative macrocephaly with a long narrow face; tall forehead; dolicocephalic head shape; an open mouth; small, simple, low-set, prominent ears; and puffy eyelids. Of these, the small ears are the most discriminating facial feature that separates those with the MED12 p.R961W mutation from those without it.
Congenital hypotonia and poor gastrointestinal function, including feeding difficulties that required medical or occupational/ physical therapy, poor oral motor function, decreased gastric motility, reflux, and chronic constipation, are characteristic of FG syndrome and were present in all affected individuals. However, these features were also common in the mutation-negative group and as such, they do not help establish the diagnosis of FG syndrome in the absence of other more distinctive features.
This review should help redefine FG syndrome as a multiple congenital anomaly syndrome. There should be no doubt that congenital anomalies are common in FG syndrome because one or more of the affected individuals in each of the 10 families had at least one congenital anomaly. Of the 23 individuals reviewed in Table, Supplemental Digital Content 3, http://links.lww.com/GIM/A86, 20 had multiple congenital anomalies. Congenital anomalies were uncommon in the mutation-negative group.
A wide range of anomalies is seen in FG syndrome (see Table, Supplemental Digital Content 3, http://links.lww.com/GIM/A86). The most characteristic anomalies were agenesis or hypoplasia of the corpus callosum (13 of 13), anal fistula, stenosis and atresia (11 of 19), and congenital cardiac anomalies (11 of 18). Macrocephaly has always been considered part of FG syndrome, but it is not universally present in these affected male patients. Absolute macrocephaly (head circumference greater than the 98th percentile) was confirmed in fewer than half of patients (7 of 18) as was relative macrocephaly (head circumference percentile greater than height percentile). Eye anomalies were reported in 10 patients: strabismus/exotropia in 3 patients; optic nerve hypoplasia in 2; coloboma in 2; phthisis bulbi, nystagmus, retinal detachment, and cataract in 1 patient each. This justifies a formal ophthalmologic assessment when FG syndrome is being considered. Limited supination of the elbow was reported in six patients and may prove to be a useful sign in the older child evaluated in the outpatient clinic. Megacolon, pyloric stenosis, renal cysts and stones, cryptorchidism, skeletal anomalies including joint contractures, hip dysplasia, pectus deformities, vertebral and rib anomalies, syndactyly or oligodactyly of the fingers, and ocular anomalies were frequent. Craniosynostosis was found in two patients.
FG syndrome may be best considered as a multiple congenital anomaly syndrome, relatively few of which are X-linked. In this context, the differential diagnosis becomes more manageable, and FG syndrome should be more readily identified. When considered from this perspective, FG syndrome may be more easily distinguished from the many X-linked disorders without congenital anomalies that cause a rather nonspecific picture of congenital hypotonia and mental retardation as reviewed by Rogers et al.16 FG syndrome can be life threatening. In these 10 families, early deaths and multiple miscarriages were common (Fig. 1). This history should be sought when the diagnosis is considered. However, after the first year of life, mortality does not seem to be significantly increased. Hypotonia improves or resolves with age. Three of these male patients are doing well in their 5th and 6th decades.
A family history of multiple affected male patients in an X-linked pattern of segregation is present in 8 of the 10 families. The two families (Families 1 and 10) with a single affected individual reported multiple miscarriages in both and a stillborn male baby, whose death was attributed to a nuchal cord in one. There have been no de novo patients with a confirmed MED12 mutation. Further studies are needed in the maternal grandparents to determine the origin of the mutations.
Graham et al.9 delineated the behavioral phenotype in 8 of these 10 families. They confirm the previously documented friendly, loquacious, eager-to-please personality with concurrent anxiety and need for sameness. Some individuals can be aggressive, impulsive, and/or obsessive-compulsive. The degree of intellectual disability is variable from borderline to severe. All these individuals have cognitive disability. In most patients, IQ scores were below 70 or equivalent. Only the proband in Family 3 functions in the low normal range but lower than his family members. None of the patients had a level of intellectual functioning comparable with their unaffected siblings and parents.
Heterozygous females do not share the same phenotype seen in male patients affected with FG syndrome. There are no mentally retarded females in these 10 families. All mothers in our series are intellectually normal and all are heterozygous for the p.R961W mutation in MED12. Although these mothers were obligate heterozygotes, they generally had no recognizable clinical features of the disorder. A frontal upsweep of hair was reported in the mother in Family 1, and constipation was noted in the mother in Family 9. The fact that carrier females are clinically unaffected is consistent with the observation that most of our patients have a positive family history over several generations. If carrier females expressed the disorder, their reproductive fitness might be diminished leading to fewer familial cases.
Although the 10 families reported here produced 30 live-born affected male patients, 1 or more affected individuals died in childhood in 8 of these 10 families. The FG syndrome pheno-type, summarized in Table, Supplemental Digital Content 3 (http://links.lww.com/GIM/A86), is derived primarily from the survivors in these families. Only two deceased male infants have been included in this review (Family 3, IV-2, Family 6, II-4). Our retrospective analysis of the deceased male patients was limited by the fact that many of them were undiagnosed at the time of death, and their clinical findings were not well documented. Our study could be subject to ascertainment bias because congenital anomalies and other life-threatening problems may be less likely in the group of affected male patients who survive and more likely in those who die. One way to avoid this type of ascertainment bias is to exclude the probands and define the phenotype based on the findings in the nonproband affected members of the family. However, in a disorder with high lethality, it is impractical to exclude the probands, as too few survivors remain. In only 4 of the 10 families reviewed here were there more than 1 affected survivor. Although our findings probably describe the milder end of the FG phenotype, this is the phenotype that will be typically encountered in a child with FG syndrome who presents for diagnosis.
At this time, the diagnosis of FG syndrome may be limited to those patients with the MED12 p.R961W mutation; however, as more information becomes known about the effects of other mutations in the MED12 gene, this may change. Another mutation in the MED12 gene is responsible for Lujan-Fryns syndrome.17 It is reasonable to assume that other mutations will be found in the MED12 gene that may cause Lujan-Fryns syndrome, FG syndrome, and a spectrum of related conditions, similar to the recurrent mutations in FGFR3 responsible for a family of skeletal dysplasias: achondroplasia, hypochondroplasia, thanatophoric dysplasia, and variants of these disorders. For the time being, when the clinical diagnosis of FG syndrome is suspected, we recommend first testing for the recurrent mutation, followed by gene sequencing if targeted mutation analysis is negative.
FG syndrome should now be separated from the heterogeneous group of disorders that were previously called by this name. We discourage the use of other terms, including “the FG syndromes” or numbered subtypes (e.g., FG syndrome type 1, FGS2), to describe different phenotypes formerly called FG syndrome. The MED12-negative patients may share some of the less specific features of FG syndrome, but they lack the characteristic phenotype described herein and, as a group, they are clinically and molecularly heterogeneous. These patients are better considered as individuals with intellectual disability of unknown cause at this time.
With only 10 mutation-positive families, FG syndrome is a rare but distinctive cause of intellectual disability and congenital anomalies in male patients. By reexamining all known patients who share this MED12 mutation, we have delineated the clinical features of FG syndrome that discriminate between FG syndrome and other conditions that have been mistaken for it. The algorithm we propose is cost effective because it selects all patients who have FG syndrome for testing, and eliminates most unaffected patients from further testing. Most patients who will benefit from its use will be those who do not have FG syndrome as they will avoid the cost and delays that come with unnecessary testing.
The clinical and molecular definition of FG syndrome provides an important opportunity to redefine and better study the many types of mental retardation that had been grouped under this diagnosis. Now that the diagnosis of FG syndrome is being reconsidered in MED12 mutation-negative patients, many of the disorders that were defined only by the nonspecific features that they shared with FG syndrome (FG2, FG3, FG4, etc.), are being redefined as unique syndromes.18–21 By defining the phenotype and establishing the criteria for molecular testing, we hope that FG syndrome will be more readily identified, at less cost, and that other disorders that had been misidentified as FG syndrome in the past may now be recognized as clinically and molecularly distinct conditions.
ACKNOWLEDGMENTS
The research was supported in part by grants from Action Medical Research, NIHR (to F.L.R.); the South Carolina Department of Disabilities and Special Needs and NIH Grant HD26202 (to C.E.S.); Share's Childhood Disability Center, the Steven Spielberg Pediatric Research Center, the NIH/NICHD Program Project Grant HD22657, and the Medical Genetics NIH/NIGMS Training Program Grant 5-T32-GM08243 (to J.M.G.); and US DHHS, HRSA, Division of Genetic Services, Grant 6 U22MC03959-04-03 (to J.B.M.).
We thank the many families who have participated in this research to delineate the FG syndrome, their support group, The FG Syndrome Family Alliance, and Cindy Skinner, R.N., Dr. Richard Simensen, Dr. Mira Irons, and others, who provided essential evaluations, medical records, and otherwise facilitated this effort.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.geneticsinmedicine.org).
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Opitz JM, Kaveggia EG. Studies of malformation syndromes of man 33: the FG syndrome. An X-linked recessive syndrome of multiple congenital anomalies and mental retardation. Z Kinderheilkd. 1974;117:1–18. doi: 10.1007/BF00439020. [DOI] [PubMed] [Google Scholar]
- 2.Risheg H, Graham JM, Jr, Clark RD, et al. A recurrent mutation in MED12 leading to R961W causes Opitz-Kaveggia syndrome. Nat Genet. 2007;39:451–453. doi: 10.1038/ng1992. [DOI] [PubMed] [Google Scholar]
- 3.Lyons MJ, Graham JM, Jr, Neri G, et al. Clinical experience in the evaluation of 30 patients with a prior diagnosis of FG syndrome. J Med Genet. 2009;46:9–13. doi: 10.1136/jmg.2008.060509. [DOI] [PubMed] [Google Scholar]
- 4.Keller MA, Jones KL, Nyhan WL, Francke U, Dixson B. A new syndrome of mental deficiency with craniofacial, limb and anal abnormalities. J Pediatr. 1976;88:589 –591. doi: 10.1016/s0022-3476(76)80012-1. [DOI] [PubMed] [Google Scholar]
- 5.Riccardi VM, Hassler E, Lubinsky MS. The FG syndrome: further characterization, report of a third family, and of a sporadic case. Am J Med Genet. 1977;1:47–58. doi: 10.1002/ajmg.1320010106. [DOI] [PubMed] [Google Scholar]
- 6.McCardle P, Wilson B. Language and development in FG syndrome with callosal agenesis. J Commun Disord. 1993;26:83–100. doi: 10.1016/0021-9924(93)90002-r. [DOI] [PubMed] [Google Scholar]
- 7.Briault S, Hill R, Shrimpton A, et al. A gene for FG syndrome maps in the Xq12–q21.31 region. Am J Med Genet. 1997;73:87–90. [PubMed] [Google Scholar]
- 8.Graham JM, Jr, Tackels D, Dibbern K, et al. FG syndrome: report of three new families with linkage to Xq12–22.1. Am J Med Genet. 1998;80:145–156. [PubMed] [Google Scholar]
- 9.Graham JM, Jr, Visootsak J, Huddleston L, et al. Behavior of 10 patients with FG syndrome (Opitz-Kaveggia syndrome) and the recurrent p.R961W mutation in the MED12 gene. Am J Med Genet. 2008;146:3011–3017. doi: 10.1002/ajmg.a.32553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opitz JM, Smith JF, Santoro L. The FG syndromes (Online Mendelian Inheritance in Man 305450): perspective in 2008. Adv Pediatr. 2008;55:123–170. doi: 10.1016/j.yapd.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia A, Chines C, Carey JC. The FG syndrome: report of a large Italian series. Am J Med Genet. 2006;140:2075–2079. doi: 10.1002/ajmg.a.31302. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler D. WIAT-II: wechsler individual achievement test. 2nd ed. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 13.Dunn LM, Dunn LM. PPVT-III: peabody picture vocabulary test. 3rd ed. American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- 14.Brownell R. EOWPVT: expressive one-word picture vocabulary test. Western Psychological Services; Los Angeles, CA: 2000. [Google Scholar]
- 15.Thorndike RL, Hagen EP, Sattler JM. The Stanford-Binet Intelligence Scale. 4th ed. Riverside Publishing Company; Chicago, IL: 1986. [Google Scholar]
- 16.Rogers RC, Stevenson RE, Simensen RJ, Holden KR, Schwartz CE. Finding new etiologies of mental retardation and hypotonia: X marks the spot. Dev Med Child Neurol. 2008;50:104–111. doi: 10.1111/j.1469-8749.2007.02022.x. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz CE, Tarpey PS, Lubs HA, et al. The original Lujan syndrome family has a novel missense mutation (p.N1007S) in the MED12 gene. J Med Genet. 2007;44:472–477. doi: 10.1136/jmg.2006.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz CE, Stevenson RE. FG syndrome: return to 1974?. Presented at: 29th David W. Smith Workshop on Malformations and Morphogenesis; Mont-Tremblant, Quebec, Canada. August 8–13, 2008. [Google Scholar]
- 19.Piluso G, D'Amico F, Saccone V, et al. A missense mutation in CASK causes FG syndrome in an Italian family. Am J Hum Genet. 2009;84:162–177. doi: 10.1016/j.ajhg.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unger S, Mainberger A, Spitz C, et al. Filamin A mutation is one cause of FG syndrome. Am J Med Genet. 2007;143:1876–1879. doi: 10.1002/ajmg.a.31751. [DOI] [PubMed] [Google Scholar]
- 21.Tarpey PS, Raymond FL, Nguyen LS, et al. Mutations in UPF3B, a member of the nonsense-mediated mRNA decay complex, cause syndromic and nonsyndromic mental retardation. Nat Genet. 2007;39:1127–1133. doi: 10.1038/ng2100. [DOI] [PMC free article] [PubMed] [Google Scholar]



