Abstract
Background
Cardiac resynchronization therapy (CRT) is effective in reducing clinical events in systolic heart failure patients with a wide QRS. Previous retrospective studies suggest only patients with QRS prolongation due to a left bundle-branch block (LBBB) benefit from CRT. Our objective was to examine this by performing a meta-analysis of all randomized controlled trials of CRT.
Methods
Systematic searches of MEDLINE and the Food and Drug Administration official website were conducted for randomized controlled CRT trials. Trials reporting adverse clinical events (eg, all-cause mortality, heart failure hospitalizations) according to QRS morphology were included in the meta-analysis.
Results
Four randomized trials totaling 5,356 patients met the inclusion criteria. In patients with LBBB at baseline, there was a highly significant reduction in composite adverse clinical events with CRT (RR = 0.64 [95% CI (0.52–0.77)], P = .00001). However no such benefit was observed for patients with non-LBBB conduction abnormalities (RR = 0.97 [95% CI (0.82–1.15)], P = .75). When examined separately, there was no benefit in patients with right-bundle branch block (RR = 0.91 [95% CI (0.69–1.20)], P = .49) or non-specific intraventricular conduction delay (RR = 1.19 [95% CI (0.87–1.63)], P = .28). There was no heterogeneity among the clinical trials with regards to the lack of benefit in non-LBBB patients (I2 = 0%). When directly compared, the difference in effect of CRT between LBBB versus non-LBBB patients was highly statistically significant (P = .0001 by heterogeneity analysis).
Conclusions
While CRT was very effective in reducing clinical events in patients with LBBB, it did not reduce such events in patients with wide QRS due to other conduction abnormalities.
Heart failure is a growing epidemic, estimated to effect 6 million people with more than 400,000 new cases diagnosed each year and annual deaths approaching 200,000 in the US alone.1 In addition to optimal medical therapy, cardiac resynchronization therapy (CRT) has been shown to reduce morbidity and mortality in even mildly symptomatic heart failure patients.2,3 Initial guidelines recommended CRT in systolic heart failure patients with New York Heart Association (NYHA) III or IV symptoms and baseline QRS duration >120 milliseconds regardless of the type of conduction abnormality.4–7 In contrast, the Food and Drug Administration (FDA) recently approved CRT for patients with NYHA I or II and a wide QRS complex, but specifically due to left bundle branch block (LBBB).8 Given the radically different left ventricular (LV) activation sequences in different conduction abnormalities, the response to biventricular pacing is likely to vary substantially. However, the effect of this important treatment modality on clinical events in patients with different types of conduction abnormalities has not been systematically examined across the large-scale randomized-controlled trials of CRT. Therefore, our objective was to evaluate the effect of CRT on clinical events with regards to different types of baseline conduction abnormalities using data from randomized controlled trials.
Methods
Literature search
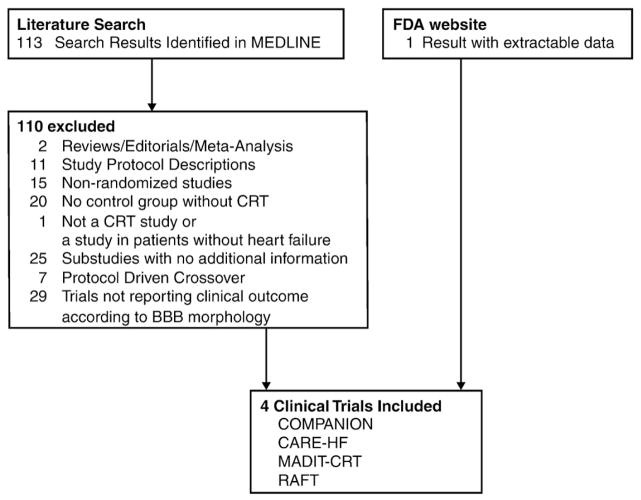
A systematic search of MEDLINE through January 2011 was performed to identify all published randomized controlled trials of CRT that reported clinical outcomes (including death and heart failure hospitalizations) according to bundle-branch block morphology. MEDLINE search terms of (heart failure and cardiac resynchronization therapy) limited to randomized controlled trials returned 113 articles. Additional search of the FDA website returned an additional trial with extractable data. The complete results of the literature search are demonstrated in Figure 1.
Figure 1.
Flowchart of cardiac resynchronization trials included.
Study selection
Trials which reported clinical outcomes (ie, death or hospitalization for heart failure and other causes of hospitalizations in some cases) of subgroups stratified by QRS morphology were included. All of the 114 articles identified from the literature search were reviewed for such information. Studies were excluded if they were not randomized, did not have a non-CRT control group, did not report clinical outcomes of interest such as death and hospitalization, or did not report clinical outcomes with relation to different types of QRS abnormalities.
Data extraction
Information on the inclusion criteria, study intervention and control, duration of follow-up, definitions of clinical end-points, drop-out and cross-over rates, and baseline patient characteristics were extracted for each trial independently by two of the investigators (IS and MH). Subsequently, data on the hazard ratios and confidence intervals for the subgroups according to QRS morphology were extracted. In cases where the actual hazard ratios were not specifically stated, this data was extracted from the published forest plots, when available, using electronic calipers.
Statistical analysis
Statistical heterogeneity was tested by Cochran’s Q statistic and reported as I2. Fixed effect models were used unless there was evidence of heterogeneity (I2 > 40%), where random effects models were used. The difference in the meta-analytic hazard ratio in patients with LBBB vs. non-LBBB conduction abnormality was assessed with heterogeneity analysis. Sensitivity analyses were performed using the one-study out method to assess whether any single study was primarily responsible for the main findings. Additional sensitivity analyses were performed for studies with and without background implantable cardiac defibrillator (ICD) therapy in both treatment arms. Data was analyzed using Comprehensive Meta-Analysis software version 2.2.048 (Biostat Inc, Engelwood, NJ). Funnel plots were generated according to BBB morphology to examine the possibility of publication bias. The Begg Rank Correlation method was also employed for this purpose. Meta-analytic power calculations were performed according to published formulas.9
No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Search results
The results of the literature search are shown in Figure 1. A total of 4 randomized controlled trials enrolling 5,356 patients (3,009 CRT, 2,347 controls) met the inclusion criteria for this meta-analysis.10–14 The PRISMA guideline for reporting meta-analyses is presented in the online Appendix A. A list of excluded trials and the reasons for exclusion are presented in the online Appendix B.
Study characteristics
Trial characteristics are presented in Table I. The COMPANION10 trial had 3 arms (medical therapy vs CRT only vs CRT with ICD). For this analysis, data from only the medical therapy vs. CRT only arms were included. In COMPANION10 and CARE-HF11 the control group did not receive a CRT device and hence these trials were not blinded. Only 3.8% of patients received an ICD in the CARE-HF trial. Patients in MADIT-CRT13 were randomized to receive CRT with ICD or ICD only. All patients in RAFT14 underwent concomitant ICD implantation regardless of their randomization arm and were randomized only after successful device implantation. In all trials, patients continued to receive medical therapy regardless of their treatment allocation. The RAFT,14 CARE-HF,11 and MADIT-CRT13,16 trials reported subgroup analysis regarding QRS morphology stratified into 3 groups (LBBB, right-bundle branch block [RBBB], and interventricular conduction delay [IVCD]) while the COMPANION10 trial reported the QRS morphology in 2 groups (LBBB and non-LBBB). The composite clinical end-point reported in subgroup analysis according to QRS morphology varied slightly across the trials but always included all-cause mortality and heart failure hospitalization. All trials had a blinded end-points committee.
Table I.
Study characteristics
| Study | Year | Sponsor | Inclusion Criteria
|
Study Intervention | Control | Average Follow-up Duration (months) | Subgroups reported according to BBB morphology | Composite End-point reported for QRS Subgroup Analysis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| NYHA Class | EF (%) | QRS duration (milliseconds) | ||||||||
| COMPANION10 | 2004 | Guidant | 3–4 | ≤35 | ≥120 | CRT + medical therapy* (n = 617) | Medical therapy (n = 308) | 16.2 (CRT), 11.9 (medical therapy) | LBBB = 641 Other = 283 |
All cause mortality or hospitalization |
| CARE-HF11,15 | 2005 | Medtronic | 3–4 | ≤35 | ≥120† | CRT + medical therapy (n = 409) | Medical therapy (n = 404) | 29.4 | RBBB = 35 LBBB = 730 NIVCD = 10 |
All cause mortality or hospitalization for major cardiovascular event including heart failure hospitalization |
| MADIT- CRT12,13,16 | 2009 | Boston Scientific | 1–2 | ≤30 | ≥130 | CRT + ICD (n = 1089) | ICD only (n = 731) | 28.8 | LBBB = 1281 RBBB = 228 IVCD = 308 |
All cause mortality or heart failure event (heart failure hospitalization or outpatient intravenous diuretic therapy) |
| RAFT14 | 2010 | Canadian Institutes of Health Research and Medtronic of Canada | 2–3 | ≤30 | ≥120 | CRT + ICD (n = 894) | ICD only (n = 904) | 40 | RBBB = 161 LBBB = 1295 NIVCD = 207 |
All cause mortality or hospitalization for heart failure |
The COMPANION trial had three arms (CRT, combined CRT and implantable-cardioverter defibrillator, medical therapy). For the purpose of this meta-analysis, only the CRT and the medical therapy arms were included.
In CARE-HF patients with a QRS interval of 120 to 149 milliseconds were required to meet two of three additional echocardiographic criteria for dyssynchrony: (1) an aortic pre-ejection delay of more than 140 milliseconds (2) an interventricular mechanical delay of more than 40 milliseconds (3) delayed activation of the posterolateral left ventricular wall.
All 4 trials were analyzed using the intention to treat principle. In COMPANION10 prior to reaching the primary endpoint, 13% of patients in the medical therapy group withdrew and 2% in the CRT group withdrew. For the CARE-HF11 trial, dropout rates were not presented and in the medical therapy group, a CRT or CRT-ICD device was implanted in 66 patients. In MADIT-CRT,13 1% did not receive a device in the CRT-ICD arm and 2.6% did not receive a device in the ICD only arm. In this trial, 12.4% of patients assigned to ICD only were switched to a CRT-ICD device before study end, whereas in the CRT arm 7.5% crossed over to ICD only. In RAFT, 5 patients in the ICD only group did not receive a device and in the ICD-CRT groups 6 patients did not undergo device implantation. Five patients in the ICD group withdrew or were lost to follow-up and 10 patients in the ICD-CRT group withdrew or were lost to follow-up. Five patients in the ICD group and 7 patients in the ICD-CRT group underwent heart transplantation prior to reaching the primary outcome. In the ICD group, 36 patients crossed over and received CRT prior to the primary outcome and in the ICD-CRT group, 53 patients did not receive CRT.
The baseline characteristics of patients enrolled in the trials are presented in Table II. Within each arm of the included trials, there were no statistically significant differences concerning age, gender, ejection fraction, functional class, QRS duration, or medication use.
Table II.
Patient characteristics
| Study Acronym | Mean or median age (years) | Male | Non-ischemic heart failure | Diabetes | Mean or median Baseline EF | Conduction anomaly
|
Mean or median QRS duration at baseline (milliseconds) | Treatment at baseline
|
ICD therapy during trials
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LBBB | RBBB | ACE inhibitor or angiotensin receptor blocker | Beta-Blocker | Spironolactone | CRT arm | Control arm | |||||||
| COMPANION10 | 67 | 67% | 45% | 42% | 22% | 69% | 11% | 160 | 89% | 67% | 54% | 0% | 0% |
| CARE-HF11,15 | 67 | 73% | 62% | NA | 25% | 90% | 5% | 160 | 95% | 72% | 56% | 2% | 5.7% |
| MADIT-CRT12,13 | 65 | 75% | 45% | 30% | 24% | 71% | 13% | 158 | 96% | 93% | 32% | 99% | 97.4% |
| RAFT14 | 66 | 83% | 33% | 34% | 23% | 72% | 9% | 157 | 97% | 90% | 42% | 99% | 99% |
Quantitative data synthesis
No evidence of publication bias was detected with the Begg Rank Correlation method (P ≥ .50). Funnel plots examining publication bias according to the QRS morphology are presented in the online Appendix C.
The impact of CRT on clinical events in patients with LBBB at baseline is shown in Figure 2. There was a statistically significant reduction in the risk for composite clinical events in each individual trial. On meta-analysis, patients randomized to CRT with LBBB had a highly statistically significant 36% risk reduction in composite clinical events (I2 = 72.7%, RR = 0.64 [95% CI (0.52–0.77)]). On the contrary, there was no statistically significant benefit for patients with baseline RBBB or IVCD in any of the individual trials regardless of the NYHA functional class criteria for enrollment (Figure 3). On meta-analysis, there was no significant benefit of CRT for reduction in composite clinical events in patients with non-LBBB conduction abnormality (n = 1,233, I2 = 0%, RR = 0.97 [95% CI (0.82–1.15)]). When examined separately, patients with RBBB did not experience a reduction in adverse clinical events with CRT (total n = 424, I2 = 0%, RR = 0.91 [95% CI (0.69–1.20)], P = .49). In patients with IVCD there was no reduction in clinical events with CRT as well (total n = 525, I2 = 0%, RR = 1.19 [95% CI (0.87–1.63)], P = .28) by fixed effect model. There was greater than 90% power to detect a 25% risk reduction in patients with non-LBBB conduction abnormality. When directly compared using heterogeneity analysis, the difference in effect of CRT on clinical events between patients with LBBB versus non-LBBB was highly statistically significantly (P = .0001).
Figure 2.
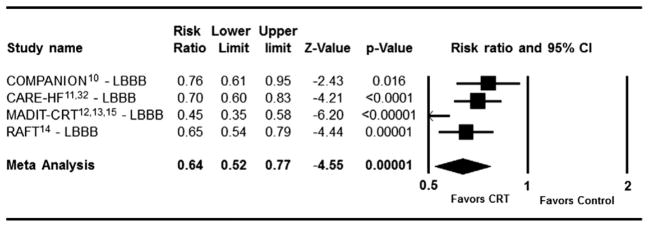
Effect of Cardiac Resynchronization Therapy on Composite Clinical Events in patients with LBBB (total n = 3,949, I2 = 72.7%, random effects model).
Figure 3.
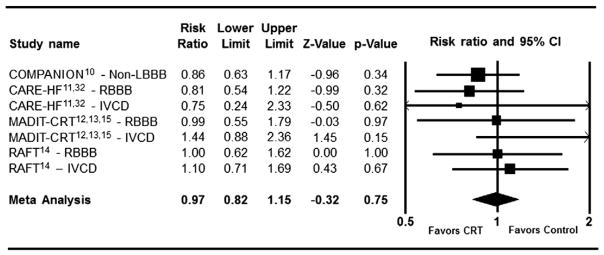
Effect of Cardiac Resynchronization Therapy on Composite Clinical Events in patients with non-LBBB morphology (total n = 1,232, I2 = 0%, fixed effect model).
Sensitivity analysis
The findings of the meta-analysis remained robust to sensitivity analysis. When analysis was limited to trials with nearly universal use of ICD in both arms of trials (MADIT-CRT, RAFT), statistically significant benefit was seen only in patients with LBBB (P < .001) and not in those with non-LBBB (P = .32) (Table III). When the analysis was limited to trials without background ICD devices (CARE-HF, COMPANION) again the benefit of CRT was observed only in patients with LBBB (P = .000001) and not in those with non-LBBB conduction abnormality (P = .15). Additional sensitivity analyses were performed by sequentially excluding each single trial. In each sensitivity analysis performed using the one-study out method, there remained a statistically significant difference in the impact of CRT on clinical events according to QRS morphology, demonstrating that the observed differences were not predominated by one single study.
Table III.
Sensitivity analyses
| I2 | Model | Risk Ratio and 95% CI | P-value | LBBB vs. non-LBBB | |
|---|---|---|---|---|---|
| With Background ICD | |||||
| LBBB | 80.8% | Random effect | 0.55 (0.38–0.78) | <.001 | <0.00001 |
| Non-LBBB | 0 % | Fixed effect | 1.13 (0.89–1.45) | .32 | |
| No Background ICD | |||||
| LBBB | 0 % | Fixed effect | 0.72 (0.63–0.82) | .000001 | 0.29 |
| Non-LBBB | 0 % | Fixed effect | 0.84 (0.66–1.07) | .15 | |
| One-study out | |||||
| COMPANION | |||||
| LBBB | 77.1 % | Random effect | 0.60 (0.47–0.76) | <.0001 | <0.0001 |
| Non-LBBB | 0 % | Fixed effect | 1.03 (0.84–1.26) | .79 | |
| CARE-HF | |||||
| LBBB | 79.3% | Random effect | 0.61 (1.46–0.81) | <.001 | <0.0001 |
| Non-LBBB | 0 % | Fixed effect | 0.94 (0.77–1.15) | .84 | |
| RAFT | |||||
| LBBB | 81.8% | Random effect | 0.63 (0.47–0.84) | .001 | 0.002 |
| Non-LBBB | 5.6% | Fixed effect | 0.94 (0.77–1.15) | .56 | |
| MADIT-CRT | |||||
| LBBB | 0 % | Fixed effect | 0.70 (0.63–0.78) | <.00000001 | 0.02 |
| Non-LBBB | 0 % | Fixed effect | 0.91 (0.75–1.10) | .34 | |
Discussion
Our results show that in patients with a LBBB, CRT is very effective in reducing adverse events with a relative risk reduction of 36% (P = .00001). Conversely, no benefit was observed in patients with other types of conduction abnormalities and a QRS duration >120 milliseconds. The narrow confidence interval for patients with QRS prolongation not due to LBBB rules out even a 20% relative risk reduction for these patients (P = .75).
The majority of studies evaluating CRT in patients with non-LBBB conduction abnormalities have been retrospective. Rickard et al observed that patients with RBBB and IVCD who received CRT derived less cardiac remodeling and less symptomatic improvement when compared to patients with LBBB.17 Egoavil et al followed up 61 patients with RBBB randomly assigned to CRT or no CRT and found no improvement in aerobic capacity (measured by maximal oxygen consumption) after 6 months.18 Adelstein et al and Wokhlu et al demonstrated that RBBB patients receiving CRT had a higher mortality rate and increased progression of heart failure (to transplant or assist device implantation); these studies also supported the finding that RBBB patients also derived less improvement in LV function and less reverse remodeling.19,20 The largest retrospective study on the importance of QRS morphology in CRT was performed by Bilchick et al evaluating 14,946 patients via the Medicare device registry, which again showed that patients with RBBB who received CRT had a higher mortality and hospitalization rates when compared to patients with LBBB.21 However, in these retrospective studies, without a control group not receiving CRT one cannot identify whether the poorer outcomes in non-LBBB patients is due to CRT inefficacy or due to excess baseline risk in heart failure patients with non-LBBB conduction abnormalities.22 Since there have been no large-scale randomized trials devoted to heart failure patients with non-LBBB conduction abnormalities, it was suggested that an urgent meta-analysis of non-LBBB subgroups of CRT trials is needed.23 Our meta-analysis included a total of 1,233 patients with non-LBBB conduction abnormalities randomly assigned to CRT or no-CRT, which is comparable in sample size to the major individual CRT trials such as the COMPANION trial (total n = 925) or CARE-HF (total n = 813). In our meta-analysis there was no trend for reduction in clinical events in patients with non-LBBB conduction abnormalities despite adequate power. In contrast there was a highly significant benefit in patients with LBBB.
Heart failure with RBBB is physiologically different from that with LBBB. In LBBB, the septum contracts first against a non-activated free LV wall followed by LV free wall contraction when the septum is already relaxed, leading to dysynchronous LV contraction. However in RBBB, it is the right ventricle that contracts asynchronously with mostly normal LV activation. While initial work by Fantoni et al. showed LV activation time (LVAT) in RBBB was prolonged, the number of RBBB patients studied was very small (n = 6) and several subsequent studies have not supported this finding.18,24,25 More recently Varma performed measurements of LVAT in a larger cohort of heart failure patients with RBBB and LBBB and in normal controls.26 This study showed that LVAT is only minimally increased in RBBB, but significantly increased in LBBB with a difference of 38 milliseconds between the LBBB and RBBB patients. Therefore, it is conceivable that biventricular pacing in an attempt to synchronize contraction of the LV does not improve outcomes in patients with RBBB. To the best of our knowledge change in LVAT in the setting of non-specific IVCD has never been examined systematically, although IVCD appears to be the second most common conduction abnormality after LBBB in patients with LV systolic dysfunction. However, the findings of this meta-analysis suggest that there is also no clinical event reduction with CRT in this group of patients as well; even a 15% reduction in clinical events is ruled out by the narrow confidence interval for IVCD.
Our study has certain limitations. While the total number of non-LBBB patients in our meta-analysis is comparable the sample sizes to other large scale CRT trials and provide adequate power, our study may be underpowered for RBBB alone as evidenced by a relatively wide 95% confidence interval (0.69–1.20) for this meta-analytic hazard ratio. Analysis of data from the other trials that did not report outcomes specifically for patients with RBBB (eg, COMPANION and MIRACLE) can further clarify the role of CRT in this group of patients. The composite outcome varied slightly amongst the included the trials, but always included total mortality and hospitalizations for heart failure. Despite the differences, the point estimate for risk reduction with CRT was always lower in the LBBB subgroups of all trials. Furthermore, there was no statistically significant reduction in any of the composite outcomes in the non-LBBB subgroup of any trial, indicating that variations in the main outcome variable did not influence the differential response of the 2 subgroups. Also, while practice guidelines strongly recommend optimizing medical therapy prior to device implantation, it is unknown if target dosing of these medications were achieved in all of the included trials and whether the interactions between QRS morphology and CRT response would be altered according to drug dosage. In addition, it is unknown whether different pacing programming strategies or alternative lead placement may be beneficial for patients with non-LBBB conduction abnormalities. For example, canine studies have suggested that RV-only pacing may be beneficial in the setting of RBBB.23 Theoretically, resynchronization of the right ventricle with multiple right ventricular leads or alternative right ventricular/LV lead positions may improve outcomes in these patients. It is also possible that device programming with right ventricular pacing preceding LV pacing may be beneficial in some patients. Finally, it is unknown whether echocardiographic measures of dyssynchrony can help identify subsets of non-LBBB that could benefit from CRT.27,28
Finally in addition to its morphology, the duration of the QRS complex is another key factor in determining efficacy of CRT. In the major randomized controlled trials and their meta-analysis, only patients with QRS >150 milliseconds benefited from CRT.10,13,14,29,30 In this context, Strauss et al have recently proposed stricter criteria with longer QRS durations for diagnosing LBBB including a wider QRS (>140 for men and >130 for women) for the current era of device therapies for heart failure.31 On the other hand, a small single-center study suggests that heart failure symptoms may improve with CRT even in the absence of QRS prolongation and LBBB.32 Unfortunately, since we did not have access to individual patient level data we were not able to examine the possible interactions between QRS morphology and its duration for determining CRT efficacy. An individual patient level analysis of major randomized trials can provide insight into these possible interactions and guide policy making regarding implantation of CRT devices.
In conclusion, while CRT was very effective in reducing clinical events in patients with LBBB and systolic heart failure, it did not reduce such events in patients with non-LBBB conduction abnormalities. These findings have important clinical implications for selection of patients for this important treatment modality.
Acknowledgments
Financial and material support: None.
Appendix A. PRISMA Checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (eg, Web address), and, if available, provide registration information including registration number. | NA |
| Eligibility criteria | 6 | Specify study characteristics (eg, PICOS, length of follow-up) and report characteristics (eg, years considered, language, publication status) used as criteria for eligibility, giving rationale. | 3 |
| Information sources | 7 | Describe all information sources (eg, databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 3,4 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 4 |
| Data collection process | 10 | Describe method of data extraction from reports (eg, piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought (eg, PICOS, funding sources) and any assumptions and simplifications made. | 4 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 5 |
| Summary measures | 13 | State the principal summary measures (eg, risk ratio, difference in means). | 5 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (eg, I2) for each meta-analysis. | 5 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (eg, publication bias, selective reporting within studies). | 5 |
| Additional analyses | 16 | Describe methods of additional analyses (eg, sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 5 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 20 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (eg, study size, PICOS, follow-up period) and provide the citations. | 5–7,25,26 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 7, Suppl.A |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 21–24 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 21–24 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 7 |
| Additional analysis | 23 | Give results of additional analyses, if done (eg, sensitivity or subgroup analyses, meta-regression [see Item 16]). | 8,27 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (eg, healthcare providers, users, and policy makers). | 8 |
| Limitations | 25 | Discuss limitations at study and outcome level (eg, risk of bias), and at review-level (eg, incomplete retrieval of identified research, reporting bias). | 10,11 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 11 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (eg, supply of data); role of funders for the systematic review. | 12 |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097
For more information, visit: www.prisma-statement.org.
Appendix B. Excluded Trials
| Study Name | Reference | Reasons for exclusion |
|---|---|---|
| Primary results from the Smart Delay determined AV optimazation: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. | Ellenbogen KA, et al. Circulation 2010 Dec 21:122(25)2660–2668 | No control group without CRT |
| A randomized double-blinded comparison of biverntricular versus left ventricular stimulation for cardiac resynchonirzaion therapy: the Biventricular versus Left Univentricular Pacing with ICD Back-up in Heart Failure Patients (B-LEFT HF) Trial | Boriani G, et al.. Am Heart J 2010, Jun:159(6):1052–1058 | No control group without CRT |
| Benefit of cardiac resynchronization in elderly patients: results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync Randomized Clinical Evaluation (MIRACLE-ICD) trials | Kron J, et al.. J Interv Card Electrophysiol 2009:Aug 25(2):91–96 | Not reporting clinical outcome of interest according to BBB morphology |
| Results of the Prospective Minnesota Study of ECHO/TDI in Cardiac Resynchronization Therapy (PROMISE-CRT) study | Bank AJ, et al.. J Card Fail, 2009: Jun 15(5):401–409 | Non-Randomized |
| Randomized trial of cardiac resychronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms (REVERSE) | Linde C, et al.. J Am Coll Cardiol 2008: Dec 2:52(23): 1834–1843 | Not reporting clinical outcome of interest according to BBB morphology |
| Cardiac resynchronization therapy in heart failure with narrow QRS complexes (RETHINQ) | Beshai JF, et al. NEJM 2007 Dec 13:357(24)2461–2471 | Not reporting clinical outcome of interest according to BBB morphology |
| Cardiac resynchronization therapy in patients with heart failure and atrial fibrillation: importance of new-onset atrial fibrillation and total atrial conduction time | Buck S, et al. Eurospace 2008 May: 10(5):558–565 | No control group without CRT |
| A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure | Leclercg C, et al. J Am Coll Cardiol 2008 Apr 15:51(15):1455–1462 | No control group without CRT |
| Time course of effects of cardiac resynchronization therapy in chronic heart failure: benefits in patients with preserved exercise capacity. | Piepoli MF, et al. Pacing Clin Electrophysiol 2008 Jun:31(6): 701–708 | Not reporting clinical outcome of interest according to BBB morphology |
| Reduced ventricular volumes and improved systolic function with cardiac resynchronization therapy: a randomized trial comparing simultaneous biventricular pacing, sequential biventricular pacing, and left ventricular pacing | Rao RK, et al. Circulation 2007 Apr 24:115(16):2136–2144 | No control group without CRT |
| Randomized comparison of simultaneous biventricular stimulation versus optimezed interventricular delay in cardiac resynchronization therapy. The Reynchronization for the HemodYnamic Treatment for Heart Failure Management II implantable cardiover defibrillator (RHTYHM II ICD) study | Boriani G, et al. Am Heart J 2006 May: 151(5):1080–1088 | Not reporting clinical outcome of interest according to BBB morphology |
| Long-term retention of cardiac resynchronization therapy | Knight BP, et al. J Am Coll Cardiol 2004 Jul 7:44(1)72–77 | Not reporting clinical outcome of interest according to BBB morphology |
| Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial | Young JB, et al. JAMA 2003 May28:289(20):2685–2694 | Not reporting clinical outcome of interest according to BBB morphology |
| Effects of Multisite Biventricular Pacing in Patients with Heart Failure and Intraventricular Conduction Delay (MUSTIC) | Cazeau S, et al. NEJM 2001 March 22:344:873–80 | Protocol Driven Crossover |
| Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). | Sutton MG, et al. Circulation 2006;113(2):266–272. | Not reporting clinical outcome of interest according to BBB morphology |
| Cardiac resynchronization in chronic heart failure (MIRACLE) | Abraham W, et al. NEJM 2002:346:1845–1853 | Not reporting clinical outcome of interest according to BBB morphology |
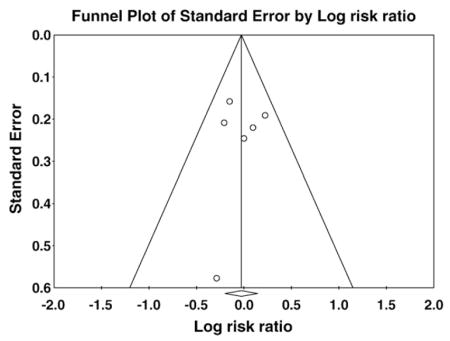
Appendix C. Funnel Plots
Funnel plots examining publication bias for left bundle branch block and non-left bundle branch block groups. The symmetric shape of each funnel plot shows a lack of relationship between treatment effect and study size, indicating that a publication bias is absent.
Panel A: Left bundle branch block
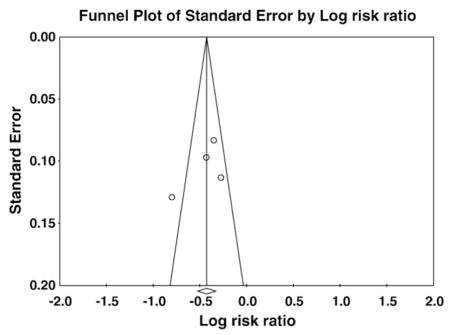
Panel B: Non–left bundle-branch block
Footnotes
Contributors:
Study concept and design - Sipahi, Fang.
Literature search - Sipahi, Chou, Hyden.
Data collection - Sipahi, Hyden.
Statistical analysis – Sipahi, Rowland.
Analysis and interpretation of data - Sipahi, Simon, Fang.
Drafting of the manuscript - Sipahi, Chou, Hyden.
Critical revision of the manuscript - Sipahi, Chou, Hyden, Rowland, Simon, Fang.
Study supervision - Sipahi, Simon, Fang.
Other Contributors: None.
Potential Financial Conflicts of Interest:
Dr. Sipahi: None.
Dr. Chou: None.
Dr. Hyden: None.
Dr. Rowland: None.
Dr. Simon serves on advisory boards for Cordis/Johnson & Johnson and Medtronic Vascular. He has received lecture honoraria from Cordis/Johnson & Johnson.
Dr. Fang: None.
References
- 1.Rodeheffer R, Redfield M. Heart failure: diagnosis and evaluation. In: Murphy JG, Lloyd MA, editors. Mayo Clinic Cardiology. 3. Rochester (Minn): Mayo Clinic Scientific Press; 2007. pp. 1101–12. [Google Scholar]
- 2.Bradley DJ, Bradley EA, Baughman KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730–40. doi: 10.1001/jama.289.6.730. [DOI] [PubMed] [Google Scholar]
- 3.McAlister FA, Ezekowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297:2502–14. doi: 10.1001/jama.297.22.2502. [DOI] [PubMed] [Google Scholar]
- 4.Executive summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:10–38. doi: 10.1016/j.cardfail.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 7.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–89. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 8.FDA. Food and Drug Administration Circulatory System Devices Panel Meeting: expanded indications for cardiac resynchronization therapy defibrillators based on MADIT-CRT Study. 2010 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM204607.pdf.
- 9.Borenstein M, Hedges LV, Higgins JPT, et al. Power Analysis for Meta-Analysis. Introduction to meta-analysis. Chichester, UK: John Wiley & Sons; 2009. pp. 257–75. [Google Scholar]
- 10.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 11.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 12.Moss A. MADIT-CRT: sponsor’s executive summary. Food and Drug Administration; 2010. [Google Scholar]
- 13.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 14.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 15.Gervais R, Leclercq C, Shankar A, et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail. 2009;11:699–705. doi: 10.1093/eurjhf/hfp074. [DOI] [PubMed] [Google Scholar]
- 16.Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–72. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 17.Rickard J, Kumbhani DJ, Gorodeski EZ, et al. Cardiac resynchronization therapy in non-left bundle branch block morphologies. Pacing Clin Electrophysiol. 2010;33:590–5. doi: 10.1111/j.1540-8159.2009.02649.x. [DOI] [PubMed] [Google Scholar]
- 18.Egoavil CA, Ho RT, Greenspon AJ, et al. Cardiac resynchronization therapy in patients with right bundle branch block: analysis of pooled data from the MIRACLE and Contak CD trials. Heart Rhythm. 2005;2:611–5. doi: 10.1016/j.hrthm.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Adelstein EC, Saba S. Usefulness of baseline electrocardiographic QRS complex pattern to predict response to cardiac resynchronization. Am J Cardiol. 2009;103:238–42. doi: 10.1016/j.amjcard.2008.08.069. [DOI] [PubMed] [Google Scholar]
- 20.Wokhlu A, Rea RF, Asirvatham SJ, et al. Upgrade and de novo cardiac resynchronization therapy: impact of paced or intrinsic QRS morphology on outcomes and survival. Heart Rhythm. 2009;6:1439–47. doi: 10.1016/j.hrthm.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Bilchick KC, Kamath S, DiMarco JP, et al. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation. 2010;122:2022–30. doi: 10.1161/CIRCULATIONAHA.110.956011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaszala K, Ellenbogen KA. When right may not be right: right bundle-branch block and response to cardiac resynchronization therapy. Circulation. 2010;122:1999–2001. doi: 10.1161/CIRCULATIONAHA.110.986943. [DOI] [PubMed] [Google Scholar]
- 23.Nery PB, Ha AC, Keren A, et al. Cardiac resynchronization therapy in patients with left ventricular systolic dysfunction and right bundle branch block: a systematic review. Heart Rhythm. 2011;8:1083–7. doi: 10.1016/j.hrthm.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Byrne MJ, Helm RH, Daya S, et al. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J Am Coll Cardiol. 2007;50:1484–90. doi: 10.1016/j.jacc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Garrigue S, Reuter S, Labeque JN, et al. Usefulness of biventricular pacing in patients with congestive heart failure and right bundle branch block. Am J Cardiol. 2001;88:1436–41. A8. doi: 10.1016/s0002-9149(01)02131-2. [DOI] [PubMed] [Google Scholar]
- 26.Varma N. Left ventricular conduction delays and relation to QRS configuration in patients with left ventricular dysfunction. Am J Cardiol. 2009;103:1578–85. doi: 10.1016/j.amjcard.2009.01.379. [DOI] [PubMed] [Google Scholar]
- 27.Fox DJ, Fitzpatrick AP, Davidson NC. Optimisation of cardiac resynchronisation therapy: addressing the problem of “non-responders”. Heart. 2005;91:1000–2. doi: 10.1136/hrt.2004.043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu CM, Lin H, Zhang Q, et al. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleland JG, Ghosh J, Freemantle N. Can cardiac-resynchronization therapy reduce mortality in patients suffering from advanced chronic heart failure? Nat Clin Pract Cardiovasc Med. 2004;1:10–1. doi: 10.1038/ncpcardio0005. [DOI] [PubMed] [Google Scholar]
- 30.Sipahi I, Carrigan TP, Rowland DY, et al. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med. 2011;171:1454–62. doi: 10.1001/archinternmed.2011.247. [DOI] [PubMed] [Google Scholar]
- 31.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–34. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Foley PW, Patel K, Irwin N, et al. Cardiac resynchronisation therapy in patients with heart failure and a normal QRS duration: the RESPOND study. Heart. 2011;97:1041–7. doi: 10.1136/hrt.2010.208355. [DOI] [PubMed] [Google Scholar]