Abstract
Background/Aims
The use of proton pump inhibitors or misoprostol is known to prevent the gastrointestinal complications of nonsteroidal anti-inflammatory drugs (NSAIDs). Rebamipide is known to increase the mucosal generation of prostaglandins and to eliminate free oxygen radicals, thus enhancing the protective function of the gastric mucosa. However, it is unknown whether rebamipide plays a role in preventing NSAID-induced gastropathy. The aim of this study was to determine the effectiveness of rebamipide compared to misoprostol in preventing NSAID-induced gastrointestinal complications in patients requiring continuous NSAID treatment.
Methods
We studied 479 patients who required continuous NSAID treatment. The patients were randomly assigned to groups that received 100 mg of rebamipide three times per day or 200 μg of misoprostol three times per day for 12 weeks. The primary endpoint of the analysis was the occurrence rate of gastric ulcers, as determined by endoscopy after 12 weeks of therapy.
Results
Of the 479 patients in the study, 242 received rebamipide, and 237 received misoprostol. Ultimately, 44 patients (18.6%) withdrew from the misoprostol group and 25 patients (10.3%) withdrew from the rebamipide group. There was a significant difference in withdrawal rate between the two groups (p=0.0103). The per protocol analysis set was not valid because of the dropout rate of the misoprostol group; thus, the intention to treat (ITT) analysis set is the main set for the efficacy analysis in this study. After 12 weeks, the occurrence rate of gastric ulcers was similar in the rebamipide and misoprostol groups (20.3% vs 21.9%, p=0.6497) according to ITT analysis. In addition, the therapeutic failure rate was similar in the rebamipide and misoprostol groups (13.6% vs 13.1%, p=0.8580). The total severity score of the gastrointestinal symptoms was significantly lower in the rebamipide group than in the misoprostol group (p=0.0002). The amount of antacid used was significantly lower in the rebamipide group than in the misoprostol group (p=0.0258).
Conclusions
Rebamipide can prevent gastric ulcers when used with NSAIDs and can decrease the gastrointestinal symptoms associated with NSAID administration. When the possibility of poor compliance and the potential adverse effects of misoprostol are considered, rebamipide appears to be a clinically effective and safe alternative.
Keywords: Anti-inflammatory agents, non-steroidal, Rheumatic diseases, Complications, Rebamipide, Misoprostol
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely prescribed for several conditions, including rheumatoid arthritis, osteoarthritis, and musculoskeletal injuries.1 The administration of NSAIDs, however, can cause gastrointestinal complications, such as bleeding, ulceration, perforation, and obstruction. The factors that increase the risk of NSAID-induced gastrointestinal complications include age over 60 years, concomitant use of systemic corticosteroids, or anticoagulants, and a history of peptic ulcer.2–6 NSAID-induced gastrointestinal complications are caused by various mechanisms, such as abnormalities in prostaglandin-dependent gastric mucosal protection caused by decreased gastric mucosal prostaglandins.7
Cyclooxygenase 2 (COX-2) inhibitors, which are known to be safer than other NSAIDs, are used to reduce NSAID-induced gastrointestinal side effects. Many doubts still exist, however, about the clinical safety of COX-2 inhibitors, as illustrated by the removal of the COX-2 inhibitor rofecoxib from the market.8 Cotherapy with misoprostol or proton pump inhibitors (PPIs) is another way to prevent NSAID-induced gastrointestinal complications; however, misoprostol itself can cause side effects, such as abdominal pain, diarrhea, and dyspepsia, which can decrease medication compliance.1 Long-term PPI administration is also problematic because issues such as osteoporosis, aspiration pneumonia, and atrophic gastritis may result.9
Rebamipide is an antiulcer drug that protects gastric epithelial cells, improves gastric defense mechanisms by increasing gastric mucus, increases prostaglandin production, and reduces free oxygen radicals.10–13 In healthy volunteers, rebamipide is effective at preventing the gastric injury caused by the administration of indomethacin. Although the preventive effects of rebamipide on NSAID-induced gastropathy are equivalent to those of misoprostol, rebamipide has been reported to cause fewer side effects (e.g., lower incidences of diarrhea, lower abdominal pain, and abdominal distension).14,15 The present study evaluated the efficacy and safety of rebamipide for preventing gastrointestinal complications due to NSAIDs by comparing it with misoprostol in a randomized, multicenter, double-blind study of patients with a high risk of NSAIDs complications.
MATERIALS AND METHODS
1. Patients
The present study was conducted in patients who presented at the Yeouido St. Mary’s Hospital of The Catholic University of Korea College of Medicine and 16 other hospitals from January 2008 to March 2010. The inclusion criteria were patients over the age of 19 years who had rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and other joint diseases that require continuous administration of NSAIDs for more than 12 weeks. Patients with a modified Lanza score below 3 in an upper gastrointestinal endoscopy who did not have current gastrointestinal symptoms and who had at least one of the following ulcer risk factors were selected for the study population: history of peptic ulcer identified through an upper gastrointestinal endoscopy, over the age of 60 years, and concomitant therapy with systemic corticosteroids (taking more than 5 mg/day of prednisolone or the equivalent dose of steroid).16 The following exclusion criteria were used: patients with a history of gastrointestinal surgery (except appendectomy); patients with abnormal bleeding tendency (abnormalities in platelets or blood clotting factors); patients with active gastroduodenal ulcers, gastroesophageal reflux disease, gastroesophageal varices, Barrett’s esophagus, esophageal strictures, or malignant tumors in an upper gastrointestinal endoscopy; patients diagnosed with malabsorption within the last 3 months; patients with a history of cerebrovascular disease, coronary artery disease, diabetes mellitus, pancreatitis, chronic kidney disease, chronic liver disease, or malignant tumors; patients who were using medications that affect gastrointestinal motility, such as sucralfate, H2-receptor antagonists, misoprostol, and prokinetics, within a week of participating in the study; patients who were using aspirin or anticoagulants; patients who were using a PPI within 2 weeks of participating in the study; pregnant or nursing women; and women of childbearing age who were not using a medically confirmed method of contraception.
Before the clinical trial was conducted, the protocols, informed consent forms, and all other related matters were approved by the Korea Food and Drug Administration and the Institutional Review Board of the corresponding institution that was conducting the clinical trial.
2. Methods
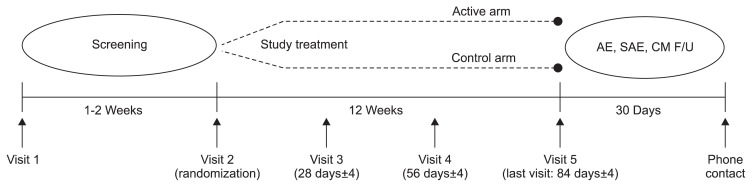
This study was as a double-blind, double-dummy, randomized, multicenter, parallel comparison clinical trial. This study consisted of a screening period for 1 or 2 weeks before enrollment, treatment period for 12 weeks, and safety follow-up period for the 30 days after last investigational product (IP) administration (Fig. 1). The subjects who agreed to participate and met the inclusion criteria were randomly assigned to either the treatment (rebamipide) or control (misoprostol) group. The treatment group received 100 mg of rebamipide three times a day, and the control group received 200 μg of misoprostol three times a day for 12 weeks. As for NSAIDs, after stopping NSAIDs taken currently for 1 to 2 weeks, one of medications in the following, which fits a subject, was administered for 12 weeks from the randomization day: 100 mg of aceclofenac twice a day, 15 mg of meloxicam once a day, or 1,000 mg of nabumetone once a day (Table 1). An upper gastrointestinal endoscopy was performed during screening and 12 weeks after the IP administration to evaluate the efficacy. To evaluate safety, vital signs and blood chemistry were assessed during every visit from screening period to the last day of IP administration. The subjects with major protocol deviation (e.g., less than 80% IP compliance and not taking NSAIDs for 5 consecutive days) were excluded from the per protocol (PP) analysis set.
Fig. 1.
Study design.
AE, adverse event; SAE, serious adverse event; CM, concomitant medication; F/U, follow-up.
Table 1.
Nonsteroidal Anti-Inflammatory Drugs That Were Prescribed in the PRESENT Study (Intention to Treat Population)
| Prescribed NSAIDs | Misoprostol (n=237) | Rebamipide (n=242) | Total (n=479) | p-value* |
|---|---|---|---|---|
| Aceclofenac (100 mg) | 75 (31.7) | 60 (24.8) | 135 (28.2) | 0.2489 |
| Meloxicam (7.5 mg) | 117 (49.4) | 132 (54.6) | 249 (52.0) | |
| Nabumetone (500 mg) | 45 (19.0) | 50 (20.7) | 95 (19.8) |
Data are presented as number (%).
NSAID, nonsteroidal anti-inflammatory drug.
Chi-square test.
Taking the following concomitant medications which can affect the clinical symptoms or efficacy evaluation was prohibited: anticholinergic drugs, H2-receptor antagonists, PPIs, sucralfate, prokinetics, antacids (except aluminum hydroxide gel, which was allowed as a rescue medication), prostaglandin drugs other than misoprostol, other drugs affecting gastric acid secretion and gastrointestinal motility, anticancer drugs, anticoagulants, and NSAIDs other than those provided by the sponsor.
The primary efficacy endpoint was the occurrence rate of gastric ulcer confirmed on gastrointestinal endoscopy that was performed 12 weeks after the IP administration. An ulcer was defined as an excavated mucosal layer with a diameter of greater than 3 mm. The occurrence rate of gastric ulcer was determined with the following equation: (the number of subjects with gastric ulcer/the total number of subjects in the analysis set)×100. The therapeutic failure rate, the severity of gastrointestinal symptoms, and the amount of antacid used were evaluated as secondary efficacy endpoints. Therapeutic failure was defined as developing a gastric ulcer or withdrawal due to intractable gastrointestinal symptoms, which might be major side effect of misoprostol.
The gastrointestinal symptoms consisted of seven items: heartburn, abdominal distention, nausea, vomiting, upper abdominal pain, lower abdominal pain, and diarrhea. To compare the total severity score of gastrointestinal symptoms between the two groups, each item was evaluated as no symptoms (0 points), mild symptoms (1 point), moderate symptoms (2 points), or severe symptoms (3 points). The amount of antacid used (the total number of tablets taken) in the two groups during the clinical trial was compared.
3. Statistics
From the results of previous clinical studies of misoprostol (the control drug), the occurrence rate of gastric ulcer was assumed to be 3.7%. The clinically acceptable margin (δ) for noninferiority trial was assumed to be 6.9%1,17 because 6.9% is half of 13.8%, which is the difference between the occurrence rate of gastric ulcer of placebo (17.5%) and misoprostol (3.7%). Assuming a significance level (a) of 0.025 in a one-sided test, a statistical power (1-b) of 90%, and a 1:1 treatment to control group ratio, the number of subjects (n) required for each group was 158. Assuming a dropout rate of 20%, the total number of subjects was set to 396 (198 in each group).
The efficacy variables included the occurrence rate of gastric ulcer, the frequency and rate of the therapeutic failure. For the occurrence rate of gastric ulcer and the therapeutic failure rate, frequency and percentage of the occurrence of gastric ulcer and the therapeutic failure were obtained, and the comparison of their rate between groups were performed using chi-square test. A repeated measures analysis of variance was used to compare the severity of gastrointestinal symptoms between the two groups. A two-sample t-test or Wilcoxon rank sum test was used to compare amount of antacid consumption between the two groups.
For the percentage of the subjects who experienced adverse events (AEs) more than once, a 95% confidence interval was obtained and the difference between groups was compared using chi-square test or Fisher exact test. SAS version 9.1 (SAS Institute, Cary, NC, USA) was used to perform the statistical analysis.
RESULTS
1. Patient population
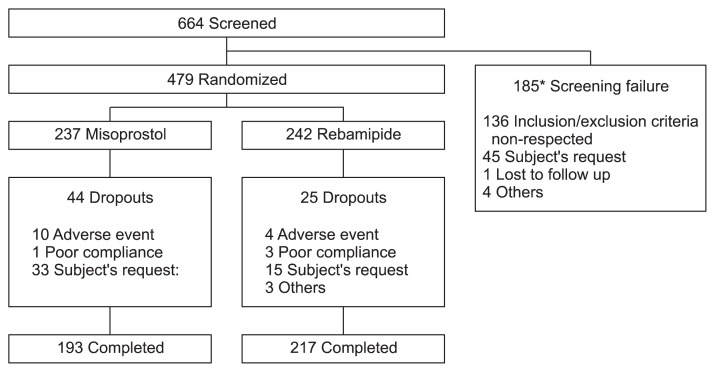
A total of 664 patients from 17 sites received a screening test. Among the 664 patients, 185 were excluded, and 479 were determined to meet the inclusion criteria. Of the 479 patients, 237 were randomly assigned to the control (misoprostol) group, and 242 were assigned to the treatment (rebamipide) group. Four hundred seventy-eight subjects received the IP at least once (one subject in the misoprostol group did not), and 410 subjects completed the clinical trial: 193 subjects in the misoprostol group and 217 subjects in the rebamipide group (Fig. 2). Sixty-nine of the randomly assigned subjects withdrew from the trial: 44 subjects in the misoprostol group (18.6%) and 25 subjects in the rebamipide group (10.3%). The withdrawal rate was significantly greater in the misoprostol group compared with the rebamipide group (p=0.0103 in a chi-square test). The patients provided the following reasons for withdrawal: 14 subjects withdrew because of AE (10 in the misoprostol group and four in the rebamipide group), four subjects withdrew because of poor protocol compliance (one in the misoprostol group and three in the rebamipide group), and 48 subjects withdrew because of the withdrawal due to gastrointestinal pain (33 in the misoprostol group and 15 in the rebamipide group) (Table 2). Therefore, the major analysis set for the efficacy in this study is the intention to treat (ITT) analysis set because the PP analysis set is decided as less valid than ITT analysis set due to significant difference in the withdrawal rate in between the two groups. There were no significant differences between the groups regarding age, sex, smoking history, ulcer risk factors, or modified Lanza scores from the endoscopy. The prevalence of ankylosing spondylitis, however, was significantly greater in the misoprostol group (p=0.0358) (Table 3).
Fig. 2.
Subject dispositions. *One subject had multiple reasons for screening failure (inclusion/exclusion criteria were not respected, and others).
Table 2.
Withdrawal Rates in the PRESENT Study (Intention to Treat Population)
| Reason for dropping out | Misoprostol (n=237) | Rebamipide (n=242) | Total (n=479) | p-value* |
|---|---|---|---|---|
| AEs | 10 | 4 | 14 | |
| Poor compliance with the protocol | 1 | 3 | 4 | |
| Consent withdrawal† | 33 | 15 | 48 | |
| Other | 0 | 3 | 3 | |
| Total, no. (%) | 44 (18.6) | 25 (10.3) | 69 (14.4) | 0.0103 |
AE, adverse event.
Chi-squared test;
Intractable gastrointestinal symptoms and insufficient pain control.
Table 3.
Demographic Characteristics of the Study Patients
| Characteristic | Misoprostol (n=237) | Rebamipide (n=242) | Total (n=479) | p-value |
|---|---|---|---|---|
| Age, yr | 55.8±12.2 | 55.8±12.2 | 55.8±12.2 | 0.9640* |
| Sex | ||||
| Male | 44 (18.6) | 42 (17.4) | 86 (18.0) | 0.8119† |
| Female | 193 (81.4) | 200 (82.6) | 393 (82.1) | |
| Smokers‡ | 36 (15.2) | 42 (17.4) | 78 (16.3) | 0.5383† |
| Risk factors | ||||
| Peptic ulcer history | 27 (11.4) | 21 (8.7) | 48 (10.0) | 0.3627† |
| ≥60 Years of age | 124 (52.3) | 118 (48.8) | 242 (50.5) | 0.4652† |
| Concomitant use of systemic corticosteroids | 132 (55.7) | 153 (63.2) | 285 (59.5) | 0.0951† |
| Concomitant disease | ||||
| Rheumatoid arthritis | 149 (62.9) | 160 (66.1) | 309 (64.5) | 0.5040† |
| Osteoarthritis | 79 (33.3) | 79 (32.6) | 158 (33.0) | 0.9226† |
| Ankylosing spondylitis | 14 (5.9) | 5 (2.1) | 19 (4.0) | 0.0358† |
| Other§ | 43 (18.1) | 40 (16.5) | 83 (17.3) | 0.7174† |
| Modified Lanza score (from the screening endoscopy) | ||||
| Grade 0 | 124 (52.3) | 132 (54.6) | 256 (53.4) | 0.1636† |
| Grade 1 | 48 (20.3) | 55 (22.7) | 103 (21.5) | |
| Grade 2 | 40 (16.9) | 24 (9.9) | 64 (13.4) | |
| Grade 3 | 25 (10.6) | 30 (12.4) | 55 (11.5) | |
| Grade 4 | 0 | 0 | 0 | |
| Grade 5 | 0 | 1 (0.4)|| | 1 (0.2) |
Data are presented as mean±SD or number (%).
Two-sample t-test;
Fisher exact test;
Smokers were defined as individuals who reported any tobacco use;
Osteoporosis, systemic lupus erythematous, and spinal stenosis;
Major deviation from the inclusion criteria.
2. Treatment efficacy
1) Primary efficacy endpoint
The primary efficacy endpoint was the occurrence rate of gastric ulcer from the upper gastrointestinal endoscopy (i.e., the percentage of subjects in each analysis set with a confirmed gastric ulcer). The subject who missed the gastrointestinal endoscopy evaluation in 12 weeks of IP administration due to the withdrawal was regarded as the subject with gastric ulcer (therapeutic failure) in the ITT analysis set. The occurrence rate of gastric ulcer did not significantly differ in the ITT analysis set: 21.9% (52/237 subjects) for the misoprostol group and 20.3% (49/242 subjects) for the rebamipide group (p=0.6497) (Table 4).
Table 4.
Occurrence Rate of Gastric Ulcer at 12 Weeks
| Population | Ulcer prevalence | p-value* | 95% two-sided CI | |
|---|---|---|---|---|
|
| ||||
| Misoprostol (n=237) | Rebamipide (n=242) | |||
| ITT† | 52 (21.9) | 49 (20.3) | 0.6497 | −9.00–5.61 |
Data are presented as number (%).
CI, confidence interval; ITT, intention to treat.
Chi-squared test;
Missing endoscopy results at 12 weeks were considered to be ulcers.
2) Secondary efficacy endpoints
The secondary efficacy endpoints were therapeutic failure rate, the severity of gastrointestinal symptoms, and the amount of antacid used. The therapeutic failure rates did not significantly differ between the two groups: 13.1% (31/237 subjects) for the misoprostol group and 13.6% (33/242 subjects) for the rebamipide group (p=0.8580) (Table 5).
Table 5.
Therapeutic Failure Rates in the Misoprostol and Rebamipide Groups
Data are presented as number (%).
Chi-squared test;
Therapeutic failures include gastric ulcers and patients dropping out because of intractable gastrointestinal symptoms.
The total severity score of gastrointestinal symptom in the misoprostol group were 0.33±0.59 before IP administration, 0.80±1.14 at 4 weeks, 0.67±1.19 at 8 weeks, and 0.67±1.24 at 12 weeks. The total severity scores in the rebamipide group were generally lower than those in the misoprostol group: 0.24±0.54 before IP administration, 0.44±0.92 at 4 weeks, 0.36±0.78 at 8 weeks, and 0.44±1.05 at 12 weeks. In the repeated measures analysis of variance, the severity of gastrointestinal symptoms was significantly lower in the rebamipide group compared with the misoprostol group (a significant group effect, p=0.0002) (Table 6).
Table 6.
Gastrointestinal Symptom Severity in the Misoprostol and Rebamipide Groups
| Gastrointestinal symptoms | Visit | Misoprostol | Rebamipide | p-value* | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. | Mean±SD | No. | Mean±SD | |||
| ITT (LOCF) | Baseline | 237 | 0.33±0.59 | 242 | 0.24±0.54 | 0.0002 |
| 4 wk | 218 | 0.80±1.14 | 236 | 0.44±0.92 | ||
| 8 wk | 218 | 0.67±1.19 | 236 | 0.36±0.78 | ||
| 12 wk | 218 | 0.67±1.24 | 236 | 0.44±1.05 | ||
ITT, intention to treat; LOCF, last observation carried forward.
Repeated-measures analysis of variance.
In the ITT analysis, the number of antacid tablet was 11.18±22.79 for the misoprostol group and 7.19±15.49 for the rebamipide group (p=0.0258) (Table 7).
Table 7.
Number of Subjects Who Consumed Antacid during the 12 Weeks of Nonsteroidal Anti-Inflammatory Drug Administration
| Misoprostol | Rebamipide | Total | p-value | |
|---|---|---|---|---|
| The no. of subjects in the ITT population | 237 | 242 | 479 | |
| Antacid consumption | 114 (48.1) | 96 (39.7) | 210 (43.8) | 0.0630* |
| The total no. of antacid tablets consumed per subject† | 11.18±22.79 | 7.19±15.49 | 9.16±19.53 | 0.0258‡ |
Data are presented as number (%) or mean±SD.
ITT, intention to treat.
Chi-squared test;
Subjects who did not take antacids were regarded as having taken 0 tablets;
Two-sample t-test.
3. Safety evaluation
The safety evaluation was conducted with the 478 subjects who were randomized and received IP at least once. One of subjects had excluded on the safety evaluation because the subject couldn’t take an IP. All the AEs which occurred in this study were classified into nontreatment emergent adverse event (non-TEAE) occurring before administration of the IP and from the day corresponding to five times the half-life after the last administration day until 30 days (after the last administration day); and exacerbation of pre-existing symptoms or AEs which the subject experienced initially after IP administration (TEAE). During the clinical trial period, 805 AEs occurred in 331 of the 478 subjects (69.3%): 166 misoprostol subjects (70.3%, 400 events) and 165 rebamipide subjects (68.2%, 405 events). There were 720 TEAEs in 319 subjects (66.7%), and the incidence of these events did not significantly differ between the groups: 161 subjects for misoprostol (68.2%, 373 events) and 158 subjects for rebamipide (65.3%, 347 events). However, the majority of the rebamipide group’s TEAEs were mild (misoprostol group, 76.1% [284/373 events]; rebamipide group, 85.0% [295/347 events]), and the proportion of moderate or severe TEAEs was higher in the misoprostol group (misoprostol group, 23.9% [89/373 events], rebamipide group, 15.0% [52/347 events]) (Table 8).
Table 8.
Treatment Emergent Adverse Events and Adverse Events in the Rebamipide and Misoprostol Groups
| TEAEs | AEs* | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Misoprostol (n=236) | Rebamipide (n=242) | Total (n=478) | Misoprostol (n=236) | Rebamipide (n=242) | Total (n=478) | |
| Total no., subjects (%) [events] | 161 (68.2) [373] | 158 (65.3) [347] | 319 (66.7) [720] | 166 (70.3) [400] | 165 (68.2) [405] | 331 (69.3) [805] |
| Exact 95% CI | 61.9–74.1 | 58.9–71.3 | 62.3–70.9 | 64.1–76.1 | 61.9–74.0 | 64.9–73.4 |
| p-value† | 0.4988 | 0.6215 | ||||
| No. of AEs | ||||||
| Mild | 284 (76.1) | 295 (85.0) | 579 (80.4) | 307 (76.8) | 347 (85.7) | 654 (81.2) |
| Moderate | 74 (19.8) | 45 (13.0) | 119 (16.5) | 78 (19.5) | 48 (11.9) | 126 (15.7) |
| Severe | 15 (4.0) | 7 (2.0) | 22 (3.1) | 15 (3.8) | 10 (2.5) | 25 (3.1) |
| Serious AEs, subjects (%) [events] | 3 (1.3) [3] | 7 (2.9) [9] | 10 (2.1) [12] | 3 (1.3) [3] | 10 (4.1) [13] | 13 (2.7) [16] |
| Exact 95% CI | 0.3–3.7 | 1.2–5.9 | 1.0–3.8 | 0.3–3.7 | 2.0–7.5 | 1.5–4.6 |
| p-value† | 0.3389 | 0.0882 | ||||
Data are presented as number (%).
TEAE, treatment emergent adverse event; AE, adverse event; CI, confidence interval.
All of the AEs (including the TEAEs) during the study;
Fisher exact test.
The most common adverse drug reaction (ADR) was gastrointestinal disorders: 64 subjects (27.1%) in the misoprostol group and 47 subjects (19.4%) in the rebamipide group; however, the difference was not statistically significant. Nausea was significantly more common in the misoprostol group (15 subjects, 6.4%) compared with the rebamipide group (four subjects, 1.7%) (p=0.0095) (Table 9).
Table 9.
Major Treatment Emergent Adverse Events Related to the Study Drug of Subjects in Either Treatment Group by System Organ Class and Preferred Term
| Misoprostol (n=236) | Rebamipide (n=242) | Total (n=478) | p-value* | |
|---|---|---|---|---|
| Total no. of TEAEs | 69 (29.2) [126] | 53 (21.9) [95] | 122 (25.5) [221] | 0.0746 |
| Gastrointestinal disorders | ||||
| Upper abdominal pain | 42 (17.8) [56] | 38 (15.7) [45] | 80 (16.7) [101] | 0.5432 |
| Abdominal distension | 12 (5.1) [12] | 8 (3.3) [9] | 20 (4.2) [21] | 0.3680 |
| Nausea | 15 (6.4) [19] | 4 (1.7) [4] | 19 (4.0) [23] | 0.0095 |
| Diarrhea | 10 (4.2) [11] | 5 (2.1) [10] | 15 (3.1) [21] | 0.1980 |
Data are presented as subjects (%) [events].
TEAE, treatment emergent adverse event.
Fisher exact test.
DISCUSSION
Approximately 10% to 60% of patients who take NSAIDs experience gastrointestinal symptoms (e.g., abdominal pain, heartburn, bloating, and indigestion). Interestingly, 10% to 20% of the rheumatoid arthritis patients who start NSAIDs discontinue them due to gastrointestinal symptoms.18 Thus, preventing gastrointestinal symptoms (in addition to preventing peptic ulcers) is an important treatment goal in patients taking NSAIDs. It is difficult to obtain an accurate incidence for NSAID-induced ulcers because many cases are asymptomatic. In an upper gastrointestinal endoscopy study of patients taking NSAIDs, gastric ulcers occurred in 10% to 40% of the patients within the first 3 months of NSAID use, and duodenal ulcers were reported in 4% to 15% of the cases.19
A PP analysis is not regarded as a valid in the current study due to the significantly greater number of patients in the misoprostol group who withdrew compared with the rebamipide group. In a previous lansoprazole study, the ITT results were also used as the primary analysis because the PP analysis was not valid due to significant difference in the withdrawal rate in between the two groups.19 In the ITT analysis of the current study, the occurrence rate of gastric ulcer in the misoprostol group (21.9%, 52/237 subjects) was not significantly different (p=0.6497) from the rate in the rebamipide group (20.3%, 49/242 subjects).
In a previous study about the efficacy of the PPI drug lansoprazole to prevent NSAID-induced gastric ulcers, the percentages of subjects without gastric ulcers after 12 weeks of medication were 51% for the placebo group, 93% for the misoprostol group (200 μg four times/day), 80% for the 15 mg lansoprazole group, and 82% for the 30 mg lansoprazole group. Although the lansoprazole groups had a significant (p<0.001) gastric ulcer protective effect compared with the placebo group, the protective effect was significantly smaller (p=0.01 and p=0.04 for the 15 and 30 mg groups, respectively) than the effect of misoprostol. However, the misoprostol group had several early trial withdrawals due to AEs. When these withdrawals were regarded as therapeutic failures, the successful treatment percentages were 69% for the misoprostol group, the 15 mg lansoprazole group, and the 30 mg lansoprazole group and 35% for the placebo group.19 Given the low medication compliance and high gastrointestinal-related AE rates for misoprostol, lansoprazole was judged to have high practical clinical value in patients using NSAIDs for extended periods; therefore, it was approved by the U.S. Food and Drug Administration in November 2011.
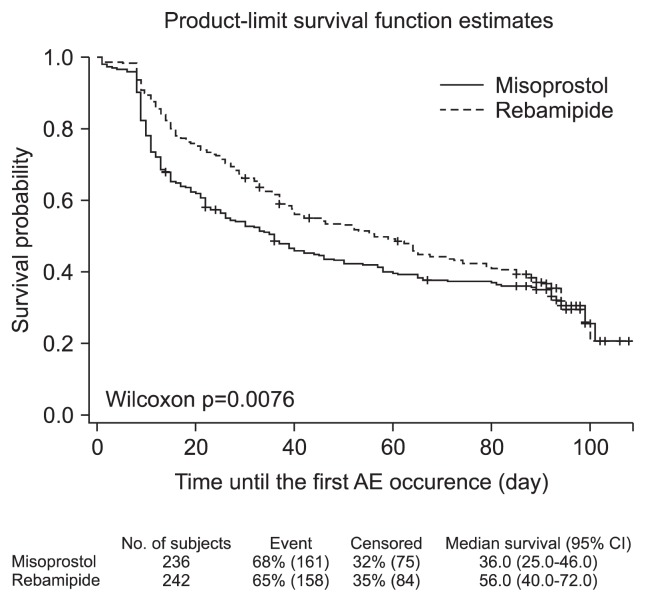
The misoprostol group may have more patients who withdrew early due to gastrointestinal pain because of gastrointestinal symptoms from incipient gastric ulcers. Furthermore, the time to the first AE in the patients who dropped out was 36 days for the misoprostol group and 56 days for the rebamipide group, and the more rapid onset of AEs in the misoprostol group may have affected the withdrawal rate (Fig. 3). In addition to misoprostol, PPI cotherapy is used to prevent NSAID-induced gastrointestinal injury. According to recent data from Cochrane, the prevalence of endoscopically confirmed gastric ulcers after 12 weeks of NSAIDs and PPI cotherapy is 12.8% to 19.1%.20 In case of misoprostol, the gastric ulcer prevalence due to NSAIDs has been found to be 8.1% to 10.5%.17 When a group of patients at high risk for gastrointestinal complications received NSAIDs for 12 weeks in the current study, the occurrence rate of gastric ulcer in the rebamipide group was 11.1% in the PP analysis set, which did not differ from previously reported occurrence rate of gastric ulcer for PPIs. This result suggests that rebamipide is able to prevent gastric ulcer complications with an efficacy that is similar to that of PPIs when it is administered for 12 weeks to patents at high risk for NSAIDs complications. Furthermore, the use of rebamipide could be a cost-effective method of preventing NSAID-induced gastrointestinal complications in Korea.
Fig. 3.
Time to first adverse event (AE) (safe population).
Even when we regarded the withdrawal due to gastrointestinal injuries (the major AE for misoprostol) as a treatment failure, the therapeutic failure rates in the misoprostol and the rebamipide groups (13.1% and 13.6%, respectively) were not significantly different. However, the severity of gastrointestinal symptoms in the rebamipide group was significantly lower during the 12 weeks of NSAIDs administration. Therefore, rebamipide can effectively improve the gastrointestinal symptoms that occur with NSAIDs use. Because misoprostol decreases medication compliance due to gastrointestinal symptoms from the drug itself, rebamipide is thought to be a more appropriate medication for preventing NSAIDs complications. The rebamipide group also had significantly lower antacid consumption. Thus, the clinical usefulness of rebamipide is thought to be superior to that of misoprostol for patients who take NSAIDs for extended periods.
According to the safety analysis, nausea was more common in the misoprostol group compared with the rebamipide group. Although ADRs during the NSAIDs cotherapy occurred less frequently in the rebamipide group compared with the misoprostol group, the difference between the two groups was not significant. This result was confirmed in the Cochrane Database of Systematic Reviews and in the existing literature.20,21 The Cochrane Database of Systematic Reviews has reported that misoprostol increases the dropout risk due to AEs (especially nausea, diarrhea, and abdominal pain) by 1.6-fold.20 Moreover, in a 6-month study of the prevention of serious NSAID-induced gastrointestinal complications, the early dropout rate due to AEs was found to be higher in the misoprostol group (27.4%, 1,210/4,404 subjects) than the placebo group (20.1%, 896/4,439 subjects) (p<0.001).21 Consequently, rebamipide can be safely used in clinical practice, even when administered with NSAIDs for 12 weeks.
For patients who require continuous administration of NSAIDs, the ability of rebamipide to reduce gastric ulcers and gastrointestinal symptoms has been recognized, and the results are similar to the previously reported effects of PPIs. In particular, misoprostol (a comparator drug) was associated with a high dropout rate due to gastrointestinal side effects, whereas the rebamipide group was proven to not be inferior to the misoprostol group in the ITT analysis, in which withdrew cases were regarded as treatment failures. The misoprostol group had a more rapid onset of AEs and more high-severity AEs. To a certain extent, these results are believed to be responsible for the high dropout rate and the low compliance in the misoprostol group, whereas rebamipide was determined to have high clinical safety and efficacy for patients who use NSAIDs for extended periods.
In conclusion, cotherapy with the mucosal protective agent rebamipide can safely and effectively prevent gastric ulcers in high-risk patients who require long-term NSAID administration.
ACKNOWLEDGEMENTS
The authors would like to thank the following researchers their participation and assistance with this study:
Myung-Gyu Choi, Department of Gastroenterology, Seoul St. Mary’s Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
Yeong-Wook Song, Department of Rheumatology, Seoul National University Hospital, Seoul, Korea.
Jin Hong Kim, Department of Gastroenterology, Ajou University Hospital, Suwon, Korea.
Seung-Cheol Shim, Department of Rheumatology, Eulji University Hospital, Daejeon, Korea.
Sang Young Seol, Department of Gastroenterology, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
Jung-Soo Song, Department of Rheumatology, Chung-Ang University College of Medicine, Seoul, Korea.
Jun-Ki Min, Department of Rheumatology, Bucheon St. Mary’s Hospital, The Catholic University of Korea College of Medicine, Bucheon, Korea.
Han Joo Baek, Department of Rheumatology, Gachon University of Medicine and Science, Incheon, Korea.
Seung Jae Hong, Department of Rheumatology, Kyung Hee University School of Medicine, Seoul, Korea.
Sang-Heon Lee, Department of Rheumatology, Konkuk University School of Medicine, Seoul, Korea.
Hyun Ok Kim, Department of Rheumatology, Gyeongsang National University School of Medicine, Jinju, Korea.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Raskin JB, White RH, Jackson JE, et al. Misoprostol dosage in the prevention of nonsteroidal anti-inflammatory drug-induced gastric and duodenal ulcers: a comparison of three regimens. Ann Intern Med. 1995;123:344–350. doi: 10.7326/0003-4819-123-5-199509010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Targownik LE, Thomson PA. Gastroprotective strategies among NSAID users: guidelines for appropriate use in chronic illness. Can Fam Physician. 2006;52:1100–1105. [PMC free article] [PubMed] [Google Scholar]
- 3.Seager JM, Hawkey CJ. ABC of the upper gastrointestinal tract: indigestion and non-steroidal anti-inflammatory drugs. BMJ. 2001;323:1236–1239. doi: 10.1136/bmj.323.7323.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballinger AB, Kumar PJ, Scott DL. Misoprostol in the prevention of gastroduodenal damage in rheumatology. Ann Rheum Dis. 1992;51:1089–1093. doi: 10.1136/ard.51.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldkind L, Simon LS. Patients, their doctors, nonsteroidal anti-inflammatory drugs and the perception of risk. Arthritis Res Ther. 2006;8:105. doi: 10.1186/ar1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia Rodríguez LA, Hernández-Díaz S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res. 2001;3:98–101. doi: 10.1186/ar146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkey CJ. Non-steroidal anti-inflammatory drugs and the gastric mucosa: mechanisms of damage and protection. Aliment Pharmacol Ther. 1988;2( Suppl 1):57–64. doi: 10.1111/j.1365-2036.1988.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 8.Bäck M, Yin L, Ingelsson E. Cyclooxygenase-2 inhibitors and cardiovascular risk in a nation-wide cohort study after the withdrawal of rofecoxib. Eur Heart J. 2012;33:1928–1933. doi: 10.1093/eurheartj/ehr421. [DOI] [PubMed] [Google Scholar]
- 9.Roulet L, Vernaz N, Giostra E, Gasche Y, Desmeules J. Adverse effects of proton pump inhibitors: should we worry about long-term exposure? Rev Med Interne. 2012;33:439–445. doi: 10.1016/j.revmed.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43(9 Suppl):5S–13S. [PubMed] [Google Scholar]
- 11.Iijima K, Ichikawa T, Okada S, et al. Rebamipide, a cytoprotective drug, increases gastric mucus secretion in human: evaluations with endoscopic gastrin test. Dig Dis Sci. 2009;54:1500–1507. doi: 10.1007/s10620-008-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudis K, Sakamoto C. The role of cyclooxygenase in gastric mucosal protection. Dig Dis Sci. 2005;50( Suppl 1):S16–S23. doi: 10.1007/s10620-005-2802-7. [DOI] [PubMed] [Google Scholar]
- 13.Kim HK, Kim JI, Kim JK, et al. Preventive effects of rebamipide on NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig Dis Sci. 2007;52:1776–1782. doi: 10.1007/s10620-006-9367-y. [DOI] [PubMed] [Google Scholar]
- 14.Naito Y, Yoshikawa T, Iinuma S, et al. Rebamipide protects against indomethacin-induced gastric mucosal injury in healthy volunteers in a double-blind, placebo-controlled study. Dig Dis Sci. 1998;43(9 Suppl):83S–89S. [PubMed] [Google Scholar]
- 15.Park SH, Cho CS, Lee OY, et al. Comparison of prevention of NSAID-induced gastrointestinal complications by rebamipide and misoprostol: a randomized, multicenter, controlled trial-STORM STUDY. J Clin Biochem Nutr. 2007;40:148–155. doi: 10.3164/jcbn.40.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanza FL, Royer GL, Jr, Nelson RS, Chen TT, Seckman CE, Rack MF. A comparative endoscopic evaluation of the damaging effects of nonsteroidal anti-inflammatory agents on the gastric and duodenal mucosa. Am J Gastroenterol. 1981;75:17–21. [PubMed] [Google Scholar]
- 17.Graham DY, Agrawal NM, Roth SH. Prevention of NSAID-induced gastric ulcer with misoprostol: multicentre, double-blind, placebo-controlled trial. Lancet. 1988;2:1277–1280. doi: 10.1016/S0140-6736(88)92892-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Lee YC, Jeon SW, et al. Guidelines of prevention and treatment for NSAID-related peptic ulcers. Korean J Gastroenterol. 2009;54:309–317. doi: 10.4166/kjg.2009.54.5.309. [DOI] [PubMed] [Google Scholar]
- 19.Graham DY, Agrawal NM, Campbell DR, et al. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med. 2002;162:169–175. doi: 10.1001/archinte.162.2.169. [DOI] [PubMed] [Google Scholar]
- 20.Rostom A, Dube C, Wells G, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev. 2002;4:CD002296. doi: 10.1002/14651858.CD002296. [DOI] [PubMed] [Google Scholar]
- 21.Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:241–249. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]