Abstract
Hepatocellular adenoma (HCA) is an uncommon benign hepatic tumor, and the use of oral contraceptives is known to contribute to the development of HCA. Recently, a genotype and phenotype classification system for HCA was suggested, and malignant transformation to hepatocellular carcinoma (HCC) was shown to be strongly associated with activating mutations in β-catenin. Here, we report three cases of HCA in Korean patients: 7-cm, inflammatory and β-catenin-activated HCA with HCC transformation in a 46-year-old man; 13-cm, β-catenin-activated HCA with cytological atypia in a 23-year-old woman; and 10-cm, pigmented, inflammatory and β-catenin-activated HCA in a 36-year-old man. All cases exhibited the nuclear expression of β-catenin and diffuse cytoplasmic expression of glutamine synthetase upon immunohistochemical staining. All tumors were completely resected, and the patients were followed for 3 to 6 years with no evidence of local recurrence or metastasis.
Keywords: Adenoma, liver cell, Beta catenin, Carcinoma, hepatocellular, Glutamine synthetase
INTRODUCTION
Hepatocellular adenoma (HCA) is an uncommon benign liver tumor, constituting 2% of all liver neoplasms.1 It is known to occur mainly in women with a history of oral contraceptive use.2 Although a rare occurrence, hepatocellular carcinoma (HCC) can arise from HCA.3 Recent studies have revealed that β-catenin mutation is the leading cause for the malignant transformation of HCA to HCC.4–6 Here we report three cases of β-catenin activated HCA.
CASE REPORTS
1. Case 1
A 46-year-old man showed a hepatic mass found during a routine check-up. His past history was unremarkable and he had no history of alcohol or drug abuse. Laboratory findings were all within normal ranges, including aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase, lactate dehydrogenase, total bilirubin, and total protein. Hepatitis B surface antigen and antihepatitis C virus antibody were negative, but anti-HBs antibody was positive. Serum levels of α-fetoprotein and protein-induced by vitamin K absence or antagonist II were normal.
Liver dynamic computed tomography (CT) showed an arterial enhancing 6-cm sized lesion without washout in portal and delayed phase. Gadobenate dimeglumine (Gd-BOPTA) enhanced magnetic resonance imaging (MRI) showed slight hyposignal intensity in T1-weighted image, and arterial enhancement without washout on portal phase. In 3-hour delayed hepatobiliary phase, the lesion was hypointense. In T2-weighted image, the lesion was diffusely hyperintense with poorly defined hyperintense foci. The findings were characteristic of HCA. However, there was a focal washout area within the enhancing mass, which may suggest HCC (Fig. 1A–E).
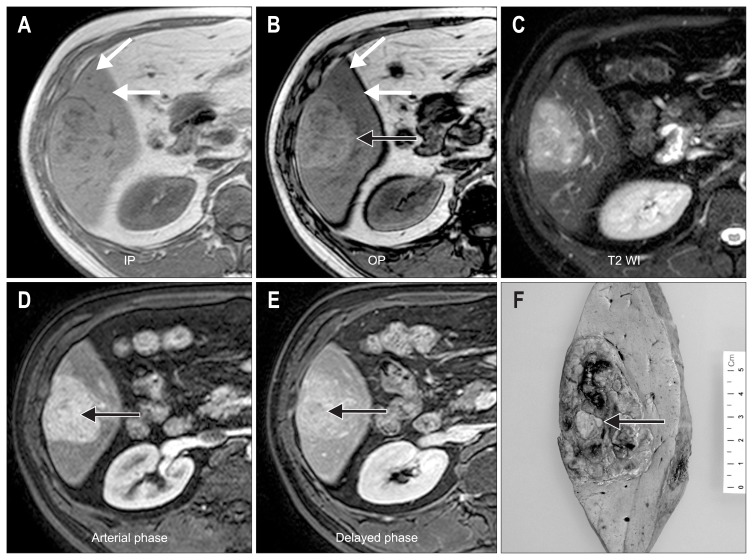
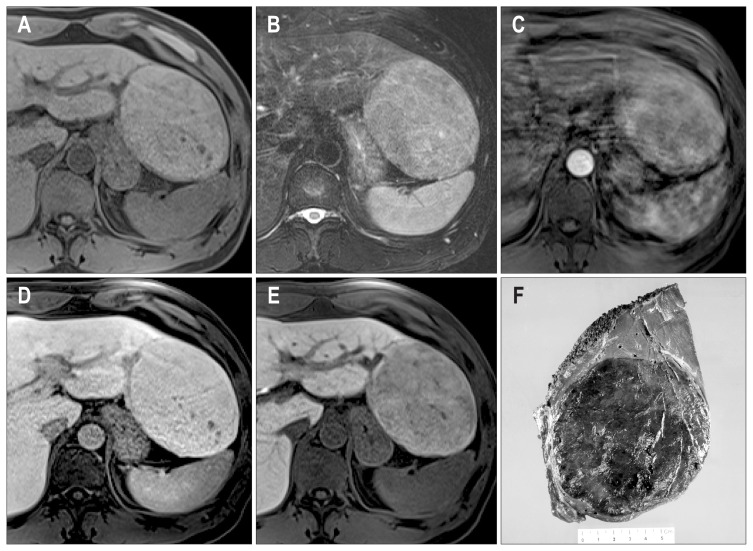
Fig. 1.
(A–E) The radiologic features of case 1. In-phase (IP) (A) and opposed-phase (OP) (B) T1-weighted, gradient echo images show an iso-intense lesion on IP images, whereas a hyperintense mass (black arrow) appears on the OP image due to decreased signal in the surrounding liver (white arrows). A T2-weighted image (T2 WI) (C) shows a slightly hyperintense mass. An axial, gadobenate dimeglumine-enhanced, T1-weighted, gradient echo image in the arterial phase (D) shows a homogeneous enhancement of the mass (arrow). An axial, gradient echo image in the delayed phase (E) shows no washout of the contrast. These features are consistent with hepatocellular adenoma; however, a washout area within the enhanced mass is suspicious for hepatocellular carcinoma (arrow). (F) Gross features of case 1. The resected specimen demonstrates a well-defined and multilobular mass measuring 7×5 cm, and a yellowish nodule is noted at the center (arrow).
Right lobectomy was performed and the specimen revealed a multilobular mass measuring 7×5 cm with a nodule-in-nodule pattern (Fig. 1F). Microscopically, the mass showed a well-defined border with an expanding growth pattern (Fig. 2A). The outer nodules, the major part of the tumor, were composed of hepatocyte-like cells with mild nuclear atypia (Fig. 2B). The tumor cells were arranged in trabecular cords of one- or two-cell thickness. There were several abnormal vessels with mono-nuclear infiltrates (Fig. 2C). In contrast, the inner nodule showed an acinar pattern and prominent cellular atypia with stromal invasion (Fig. 2D). The tumor cells of the outer and inner nodules showed positivity for serum amyloid protein A (SAA) (Fig. 2E), diffuse strong cytoplasmic expression of glutamine synthetase (GS) (Fig. 2F) and nuclear expression of β-catenin (Fig. 2G). In contrast, the expression of heat shock protein 70 (HSP-70) was found in only the inner nodule (Fig. 2H). The nonneoplastic liver showed mild macrovesicular steatosis. Taken together, the inner nodule was discerned to be a malignant transformation of HCA. The patient has been followed up regularly for 6 years with no evidence of metastasis or recurrence after operation.
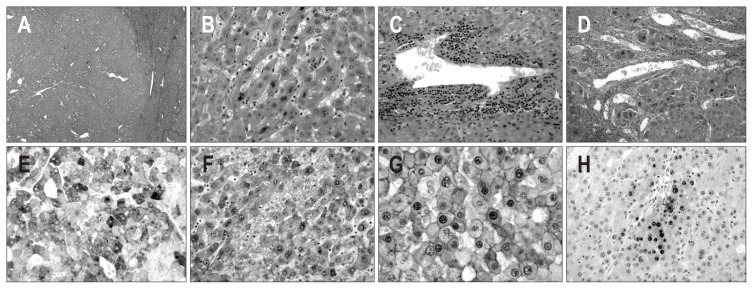
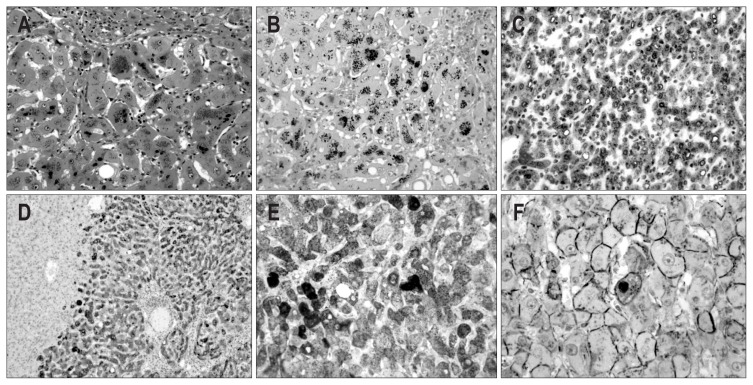
Fig. 2.
Microscopic and immunohistochemical features of case 1. (A) The mass shows a well-defined border with an expanding growth pattern (H&E stain, ×40). (B) The tumor cells of the outer nodule show a trabecular pattern of one- or two-cell thickness with mild nuclear atypia (H&E stain, ×200). (C) Several abnormally shaped blood vessels with infiltration of mononuclear inflammatory cells are noted within the tumor (H&E stain, ×200). (D) The tumor cells of the inner nodule show stromal invasion (H&E stain, ×200). (E, F) The tumor cells of the outer and inner nodules show diffuse and strong cytoplasmic expression of serum amyloid protein A (E) and glutamine synthetase (F) (×200). (G) The tumor cells of the outer and inner nodules show diffuse nuclear expression of β-catenin (×400). (H) The tumor cells of the inner nodule show the focal expression of heat shock protein 70 (×200).
2. Case 2
A 23-year-old woman presented with a huge hepatic mass. Her past medical history was unremarkable and she had no history of oral contraceptive use. The laboratory findings were normal. Liver dynamic CT showed an approximately 13-cm mass replacing almost entire right hepatic lobe. The mass showed arterial enhancement and washout on portal and delay phase. On MRI, the mass showed slight low signal intensity in T1-weighted image and gross fat component in dual-echo T1-weighted image. On T2-weighted image, the lesion showed slight high signal intensity, and T2 hyperintense, delayed enhancing central scar. With gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid (Gd-EOB-DTPA) injection, the mass showed arterial enhancement, subsequent slight washout, and hypointensity on hepatobiliary phase (Fig. 3).
Fig. 3.
Magnetic resonance images of case 2 showing an in-phase image (A) and an out-of-phase image (B) of a dual-echo T1-weighted image, a T2-weighted image (C), arterial phase (D), delayed phase (E), and hepatobiliary images (F). There is an arterial enhancement, subsequent washout, and slight hypointensity on the hepatobiliary phase image.
Right lobectomy was performed. Macroscopic examination revealed a well-delimited tumor measuring 13 cm in size. Microscopically, the tumor was composed of hepatocyte-like cells with abundant and eosinophilic granular cytoplasm. Most tumor cells showed a bland-looking appearance, but focal areas exhibited an acinar pattern and nuclear pleomorphism (Fig. 4A). Immunohistochemistry revealed focal nuclear expression of β-catenin (Fig. 4B), diffuse expression of GS (Fig. 4C) and focal expression of HSP-70 (Fig. 4D). The nonneoplastic liver showed mild nonspecific reactive hepatitis. Taken together, the findings were consistent with β-catenin activated HCA with cytological atypia. The patient has been followed up for 3 years with no evidence of recurrence or metastasis of the tumor.
Fig. 4.
Microscopic and immunohistochemical features of case 2. (A) Focal areas exhibit an acinar pattern and nuclear pleomorphism (H&E stain, ×200). (B) The tumor cells reveal the focal nuclear expression of β-catenin (×400), diffuse and strong cytoplasmic expression of glutamine synthe-tase (C, ×200), and nuclear expression of heat shock protein 70 (D, ×200).
3. Case 3
A 36-year-old man presented with an incidental hepatic mass. His past history was unremarkable and laboratory findings were normal. Gd-EOB-DTPA enhanced MRI showed a mass at left lateral segment. The mass was slightly hyperintense in T2-weighted image with several poorly defined bright foci, and isointense in T1-weighted image, and arterial enhancement and slight washout on late dynamic phase. The mass was hypointense at hepatobiliary phase image. On MRI, no gross fat or hemorrhage was noted in the tumor (Fig. 5A–E).
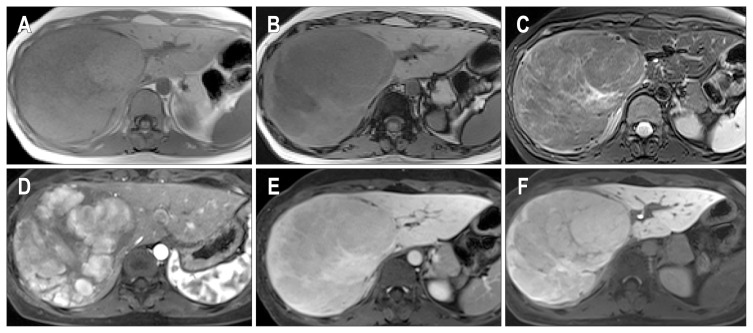
Fig. 5.
(A–E) Gadoxetic acid enhanced magnetic resonance imaging of case 3. There is a 10-cm mass lesion in the left lateral segment with an isointense signal in the T1-weighted image (A), diffuse high signal intensity and several bright foci in the T2-weighted image (B), arterial enhancement with slight washout (D), and poor contrast uptake on the hepatobiliary phase image (E). (F) The gross features of case 3. The cut surface of the mass is well-delimited and dark green in color with focal areas of congestion and hemorrhage.
Left lobectomy was performed. Macroscopically, the specimen comprised a huge mass, measuring 10 cm in size, with a cut surface showing a dark-green color and focal areas of congestion (Fig. 5F). Microscopically, the nuclei of tumor cells were slightly larger than those of the nontumor hepatocytes. Moderate mononuclear inflammatory cell infiltrates were found around abnormally shaped blood vessels. In the cytoplasm of the tumor cells, dark-brown colored granular pigment was observed (Fig. 6A), which was positive with Fontana-Masson stain (Fig. 6B), mimicking Dubin-Johnson-like pigment. Additionally, diffuse immunoexpression of SAA (Fig. 6C) and C-reactive protein (CRP) (Fig. 6D) was noted. GS was diffusely positive (Fig. 6E) and there was rare nuclear expression of β-catenin (Fig. 6F). The nonneoplastic liver showed mild nonspecific reactive hepatitis. These features suggested the diagnosis of inflammatory and β-catenin-activated HCA. The patient has been followed up for a period of 3 years with no evidence of local recurrence or metastasis after operation.
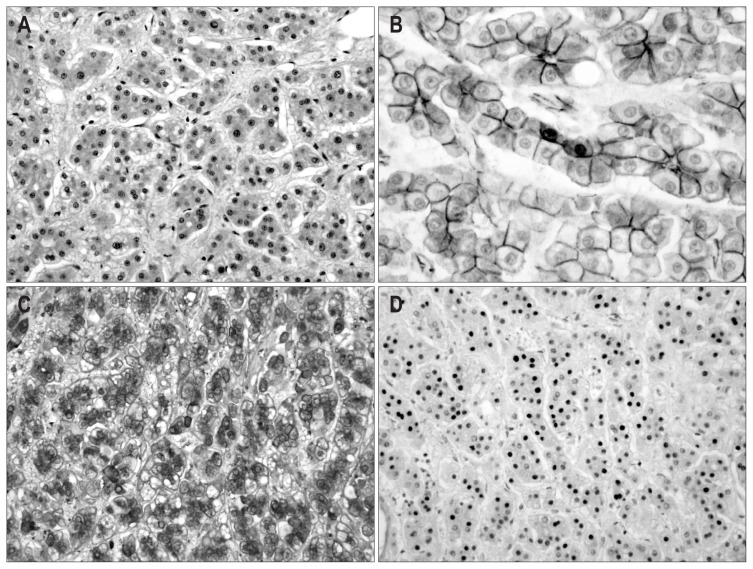
Fig. 6.
Microscopic and immunohistochemical features of case 3. (A) The tumor cells are composed of hepatocyte-like cells with mild atypia. Dark brown granular pigment is present in the cytoplasm of the tumor cells (H&E stain, ×200). (B) The pigment is positive by Fontana-Masson staining (×200). The cytoplasm of the tumor cells is diffusely positive for serum amyloid protein A (C, ×200), C-reactive protein (D, ×100), and glutamine synthetase (E, ×200). The tumor cells show the focal nuclear expression of β-catenin (F, ×400).
DISCUSSION
HCA is a rare benign tumor that usually develops in noncirrhotic liver, and is more frequently found in women than in men.7 The use of oral contraceptives is widely accepted as contributing to the development of HCA, and the natural history of HCAs is closely correlated with the dose and duration of oral contraceptive used.2 An increased risk of HCA is also associated with anabolic androgens, metabolic disorders, and vascular diseases.8,9 Recent reports from Eastern countries, however, suggest that HCAs exhibit significant epidemiological differences between Eastern and Western patients. In Eastern countries, HCAs have been reported mainly in males, and the degree of association with the use of oral contraceptives is much lower than that in Western populations. A review of 206 Asian patients reported a male preponderance of 59.7% (123/206) and only 13.2% (11/83) of female patients had a history of using oral contraceptives.10
Based on genotype-phenotype features, HCAs can be sub-categorized as follows: 1) HCA with mutations in hepatocyte nuclear factor 1α (HNF1α); 2) HCA with mutations in the β-catenin gene; 3) HCA with inflammatory features; and 4) HCA, not otherwise specified.4,6 Mutations in HNF1α, observed in approximately 35% of cases of HCA, are considered the least likely to involve malignant transformation. The presence of activating mutation in β-catenin, however, has been identified in about 10% of all HCA cases, and is highly correlated with malignant transformation.4,5 Inflammatory HCAs are characterized as displaying mononuclear infiltrates and immunohistochemically positivity for SAA and CRP. About one tenth of these HCAs have been reported to comprise mutations in the β-catenin gene.6 All cases of the present report were considered to be indicative of β-catenin activated HCA; the first and the third cases were demonstrative of HCA with inflammatory features. The first case showed features of malignant transformation with a nodule-in-nodule pattern and stromal invasion. In the others, worrisome features suggesting HCC were present, but there was no definite evidence of malignant transformation. All patients have shown no disease recurrence or metastasis for at least 3 years after treatment.
Distinguishing HCA from well-differentiated HCC is very important for the treatment of the patients, but it is difficult and sometimes impossible to attain, even in resected specimens. Histological features suggestive of HCC include liver cell plates of more than three cells thick, increased cellularity with high nuclear/cytoplasm ratio, and stromal invasion of tumor cells.11 Immunohistochemically, application of identification of glypi-can-3 expression is recommended to detect well-differentiated HCC. However, its usefulness is limited because it has been reported to be negative in one third of HCCs.12 Therefore, it is more helpful to apply a combination of HCC markers including glypican-3, HSP-70, and GS to distinguish well differentiated HCC from HCA.13 Identification of β-catenin gene mutation is also important as β-catenin activation in HCA is associated with risk of malignant potential, and it shows an aberrant nuclear and cytoplasmic expression of β-catenin and a diffuse, strong expression of GS.5 Therefore, combined application of identification of β-catenin and GS expression may be more effective in distinguishing β-catenin activated HCA from other subtypes of HCA.
Interestingly, Dubin-Johnson-like pigment was observed in the third case of the present study. The pigment showed larger and darker granules than lipofuscin, and was positive for Fontana-Masson stain. Recent study has indicated that Dubin-Johnson pigment is actually derived from lipofuscin, atypical bile pigments, or porphyrins, and may arise as the result of a defect in biliary excretion.14 In the literature, among seven series, eight cases of pigmented HCA have been reported, and one of those was diagnosed as HCC.15–17
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Ishak KG. Hepatic lesions caused by anabolic and contraceptive steroids. Semin Liver Dis. 1981;1:116–128. doi: 10.1055/s-2008-1040724. [DOI] [PubMed] [Google Scholar]
- 2.Baum JK, Bookstein JJ, Holtz F, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2:926–929. doi: 10.1016/S0140-6736(73)92594-4. [DOI] [PubMed] [Google Scholar]
- 3.Ito M, Sasaki M, Wen CY, et al. Liver cell adenoma with malignant transformation: a case report. World J Gastroenterol. 2003;9:2379–2381. doi: 10.3748/wjg.v9.i10.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 5.Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 6.Bioulac-Sage P, Laumonier H, Laurent C, Zucman-Rossi J, Balabaud C. Hepatocellular adenoma: what is new in 2008. Hepatol Int. 2008;2:316–321. doi: 10.1007/s12072-008-9075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronald M, Woodfield J, McCall J, Koea J. Hepatic adenomas in male patients. HPB (Oxford) 2004;6:25–27. doi: 10.1080/13651820310020846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen C, Lawson D, DeRose PB. Sex and androgenic steroid receptor expression in hepatic adenomas. Hum Pathol. 1998;29:1428–1432. doi: 10.1016/S0046-8177(98)90011-9. [DOI] [PubMed] [Google Scholar]
- 9.Kwon JE, Park YN. Hepatic adenomatosis in glycogen storage disease. Korean J Hepatol. 2008;14:108–112. doi: 10.3350/kjhep.2008.14.1.108. [DOI] [PubMed] [Google Scholar]
- 10.Lin H, van den Esschert J, Liu C, van Gulik TM. Systematic review of hepatocellular adenoma in China and other regions. J Gastroenterol Hepatol. 2011;26:28–35. doi: 10.1111/j.1440-1746.2010.06502.x. [DOI] [PubMed] [Google Scholar]
- 11.Park YN, Kojiro M, Di Tommaso L, et al. Ductular reaction is helpful in defining early stromal invasion, small hepatocellular carcinomas, and dysplastic nodules. Cancer. 2007;109:915–923. doi: 10.1002/cncr.22460. [DOI] [PubMed] [Google Scholar]
- 12.Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 2008;21:1011–1018. doi: 10.1038/modpathol.2008.85. [DOI] [PubMed] [Google Scholar]
- 13.Di Tommaso L, Franchi G, Park YN, et al. Diagnostic value of HSP70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology. 2007;45:725–734. doi: 10.1002/hep.21531. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura T, Alroy J, Gatmaitan Z, et al. Defective biliary excretion of epinephrine metabolites in mutant (TR-) rats: relation to the pathogenesis of black liver in the Dubin-Johnson syndrome and Corriedale sheep with an analogous excretory defect. Hepatology. 1992;15:1154–1159. doi: 10.1002/hep.1840150629. [DOI] [PubMed] [Google Scholar]
- 15.Hechtman JF, Raoufi M, Fiel MI, et al. Hepatocellular carcinoma arising in a pigmented telangiectatic adenoma with nuclear β-catenin and glutamine synthetase positivity: case report and review of the literature. Am J Surg Pathol. 2011;35:927–932. doi: 10.1097/PAS.0b013e318218ca3f. [DOI] [PubMed] [Google Scholar]
- 16.Masuda T, Beppu T, Ikeda K, et al. Pigmented hepatocellular adenoma: report of a case. Surg Today. 2011;41:881–883. doi: 10.1007/s00595-010-4344-7. [DOI] [PubMed] [Google Scholar]
- 17.Vij M, Patra S, Rela M. Pigmented hepatocellular adenoma with complete CD34 immunostaining pattern: a diagnostic dilemma. Indian J Pathol Microbiol. 2012;55:528–530. doi: 10.4103/0377-4929.107801. [DOI] [PubMed] [Google Scholar]