Abstract
Background/Aims
Upregulated CD64 expression on neutrophils is the most useful marker for acute bacterial infections and systemic inflammation. However, it is unknown whether CD64 is involved in the pathogenesis of acute pancreatitis (AP). This study was designed to determine whether CD64 is implicated in severe acute pancreatitis (SAP), and thus, is a suitable marker for SAP.
Methods
SAP was induced in rats with an intraperitoneal injection of L-arginine. CD64 expression in the rat pancreas was determined by quantitative real-time polymerase chain reaction (qRT-PCR) and immunohistochemistry. Additionally, the CD64 mRNA expression in peripheral blood leukocytes from 21 patients with mild acute pancreatitis (MAP) and 10 patients with SAP was investigated at the time of admission and during remission by qRT-PCR.
Results
CD64 mRNA and protein expression in the pancreas was significantly higher in rats with SAP, compared to the controls. The CD64 expression was higher in the patients with SAP than in the patients with MAP. During remission, CD64 mRNA decreased in both the MAP and SAP patients. The area under the curve of CD64 expression for the detection of SAP was superior to both the Ranson and the Acute Physiology and Chronic Health Evaluation II scores.
Conclusions
The CD64 level was significantly increased in correlation with the disease severity in SAP and may act as a useful marker for predicting the development of SAP.
Keywords: Severe acute pancreatitis, CD64, Leukocytes
INTRODUCTION
Up to 20% of patients with acute pancreatitis (AP) may develop severe acute pancreatitis (SAP) with multiple organ failure and a high risk of mortality.1,2 Quantifying the severity of AP can indicate the appropriate monitoring of treatment and predict outcome. Identifying which patients will develop a complicated course might help physicians select patients who would benefit the most from close surveillance or aggressive intervention, which may not help all patients with pancreatitis. There is no optimal marker for the early prediction of which patients will develop SAP, although several classification models including a variety of clinical and laboratory factors are available. The Ranson scoring system,3 which is the most commonly used in clinical practice, is retrospective and focuses on alcoholic AP.4 Laboratory markers, such as white blood cell count, C-reactive protein (CRP), and procalcitonin, have a low specificity and show limited correlation with disease activity.
At present, it is widely accepted that AP is an inflammatory disorder. The premature activation of digestive enzymes within the pancreatic acinar cells is a critical initiating event that leads to autodigestion of the pancreas.5 Acinar cell injury leads to a local inflammatory reaction with inflammatory leukocyte infiltration.6 Intracapillary leukocyte accumulation represents an initial step of leukocyte activation during AP.7 Once recruited, activated leukocytes aggravate the local damage of the pancreatic tissue by releasing inflammatory cytokines.6,8 If this inflammatory reaction is very strong, it leads to a systemic inflammatory response syndrome (SIRS), which is ultimately responsible for the majority of the morbidity and mortality associated with AP.5,8,9 Systemic leukocyte activation is a direct consequence of SIRS, and if excessive, it can lead to distant organ damage and multiple organ dysfunction syndrome.5,8,9 Although leucocytes play an active role in the pathophysiology of SAP, it is difficult to predict SAP only by the peripheral leukocyte count.
CD64, the high-affinity receptor for immunoglobulin (Ig) G1 and IgG3, is mainly distributed on peripheral blood neutrophils and monocytes. Resting neutrophils express CD64 at very low levels, but the expression of this marker is strongly upregulated by the proinflammatory cytokines interferon-γ10 and granulocyte colony-stimulating factor.11,12 CD64 expression has been shown to closely correlate with macroscopic and histological evidence of inflammation. Upregulated CD64 expression on neutrophils is the most useful marker for acute bacterial infections,13–16 SIRS,17,18 and sepsis.19–21 However, whether CD64 is involved in the pathogenesis of AP is not known. This study was designed to determine whether CD64 is implicated in AP and is suitable as a marker of SAP.
MATERIALS AND METHODS
1. Animal care
Twenty adult male Sprague-Dawley rats with a median weight of 250 g were purchased from the Experimental Animal Center of the Third Military Medical University (Chongqing, China). There was no significant difference in animal weight between the study groups. Standard rat chow and tap water were provided ad libitum throughout the study period. The study was conducted following approval from the Scientific Investigation Board of the Third Military Medical University, Chongqing, China. The animals received humane care according to the criteria outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2. SAP model and study design
SAP was induced by an intraperitoneal injection of L-arginine in rats as previously described.22 The rats received 250 mg per 100 g body weight of 20% L-arginine hydrochloride in 0.9% sterile sodium chloride solution twice with a 1-hour interval between injections. The time of the last injection was designated as 0 hour. The animals were sacrificed 48 hours after the induction of experimental AP (or the commencement of the study in controls). Pancreas samples were collected immediately after animal sacrifice. The animals were randomly allocated into two groups as follows. Group 1 was the control group (n=10), and no intervention was undertaken in these animals until the time of sacrifice at 48 hours. Group 2 was the SAP group (n=10), and SAP was induced at the experiment onset (0 hour) as described above.
3. AP patients
The study was approved by the local research ethics committee. Informed consent was obtained from all subjects. Between January 2010 and August 2010, a total of 31 consecutive patients admitted to the Hospital of Chongqing with a clinical diagnosis of AP and aged more than 18 years old were included in the study. The inclusion and exclusion criteria are listed in Table 1. Venous blood samples were drawn from patients with AP at the time of admission and during remission.
Table 1.
The Inclusion and Exclusion Criteria
| Inclusion criteria |
| More than 18 years |
| Abdominal pain |
| Amylase >3 times upper normal limit |
| Onset of abdominal pain within 48 hours |
| Exclusion criteria |
| Acute pancreatitis due to surgery |
| Trauma |
| Cancer |
| Chronic pancreatitis |
The following clinical and laboratory data available at the time of admission and during remission were recorded: age, gender, etiology, amylase, leukocyte counts, glucose, CRP, computed tomography Balthazar score, Ranson score, and Acute Physiology and Chronic Health Evaluation (APACHE) II score. SAP was defined by meeting at least one of the criteria shown in Table 2.
Table 2.
The Definition of Severe Acute Pancreatitis
| Ranson signs ≥3 |
| APACHE-II score ≥3 |
| Organ failure: shock, PaO2 ≤60 mm Hg, creatinine >2.0 mg/L after rehydration, or gastrointestinal bleeding >500 mL/24 hr |
| Local complications: necrosis, abscess, or pseudocyst |
| Bedside index for severity in AP ≥3 |
| Computed tomography Balthazar score ≥4 (scores A to E were classified as 1–5 points, maximum score: 5) |
APACHE, Acute Physiology and Chronic Health Evaluation; AP, acute pancreatitis.
4. Immunohistochemistry
The pancreas was rapidly removed from the sacrificed rats and fixed in 4% paraformaldehyde overnight at 4°C. The fixed tissues were then embedded in paraffin and kept until use. Immunohistochemistry was performed to examine CD64 protein expression and localization in the tissues. Briefly, 4% paraformaldehyde-fixed and paraffin wax-embedded sections of the biopsy samples were dewaxed in xylol and rehydrated in a descending alcohol concentration series. The sections were washed in distilled water and microwave-based antigen retrieval was performed. Subsequently, the sections were washed in phosphate-buffered saline, preincubated with normal goat serum (10% in phosphate buffered saline [PBS]), and after three additional washes with PBS, incubated with the primary antibody (anti-CD64; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 hour. Endogenous peroxidase activity was blocked in 0.3% hydrogen peroxide in PBS, and the sections were incubated with biotinylated secondary antibody for 30 minutes (antirabbit; Boster, Wuhan, China). A qualitative examination of the histological samples was undertaken by a consulting histopathologist who was blind to specimen group allocation.
5. RNA quantification by real-time quantitative real-time polymerase chain reaction
Approximately 5.0 mL of ethylenediaminetetraacetic acid-anticoagulated whole blood from each patient or rat was centrifuged at 2,500 rpm for 5 minutes, and the plasma was isolated. The remaining blood cells were treated with 5 volumes of red blood cell lysis buffer (Sigma, St Louis, MO, USA) for 10 minutes and centrifuged. The peripheral blood lymphocytes (PBLs) obtained were suspended in TRIzol reagent (Invitrogen, Carls-bad, CA, USA) and frozen at −80°C.
Total RNA containing small RNAs was extracted from PBLs using TRIzol reagent according to the manufacturer’s protocol. A SYBR green quantitative real-time polymerase chain reaction (qRT-PCR) assay was used for RNA quantification in the plasma samples. In brief, 500 ng of RNA was polyadenylated by poly(A) polymerase and reverse transcribed to cDNA using the Prime-Script RT Reagent Kit (Takara, Liaoning, China) according to the manufacturer’s instructions. Real-time qRT-PCR was performed using SYBR® Premix Ex Taq (Takara, Dalian, China) with the manufacturer-provided primer and the RNA-specific forward primers using an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). The human RNA-specific primer sequences were as follows: CD64, 5′-ATG GCA CCT ACC ATT GCT CAG G-3′ and 5′-CCA AGC ACT TGA AGC TCC AAC TC-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-TTG GTA TCG TGG AAG GAC TCA TG-3′ and 5′-TCG CTG TTG AAG TCA GAG GAG AC-3′. The rat RNA-specific primer sequences were as follows: CD64, 5′-CTT CTC CTT CTA TGT GGG CAG T-3′ and 5′-GCT ACC TCG CAC CAG TAT GAT-3′, GAPDH, 5′-TTG GTA TCG TGG AAG GAC TCA TG-3′ and 5′-TCG CTG TTG AAG TCA GAG GAG AC-3′. The amplification profile was as follows: denaturation at 94°C for 5 minutes followed by 40 cycles of 94°C for 30 seconds, 60°C for 30 seconds and 72°C for 45 seconds. To correct for differences in the total cDNA concentrations of the different samples, gene-specific primers for the housekeeping gene GAPDH were used for normalization. The qRT-PCR analysis was performed on an Applied Biosystems 7500 system with SDS software using SYBR Green I chemistry. The samples were analyzed in triplicate, and cycle threshold values were used to calculate arbitrary mRNA concentrations using the relative standard curve method. The fold changes and mean standard errors for the target genes were obtained by averaging the ratios of the target genes to the internal control GAPDH.
6. Statistical analysis
Significance was determined by the Mann-Whitney U test, Pearson test, or Wilcoxon test where appropriate. Statistical significance was accepted at the p<0.05 level. All statistical calculations were performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. CD64 expression in SAP rats
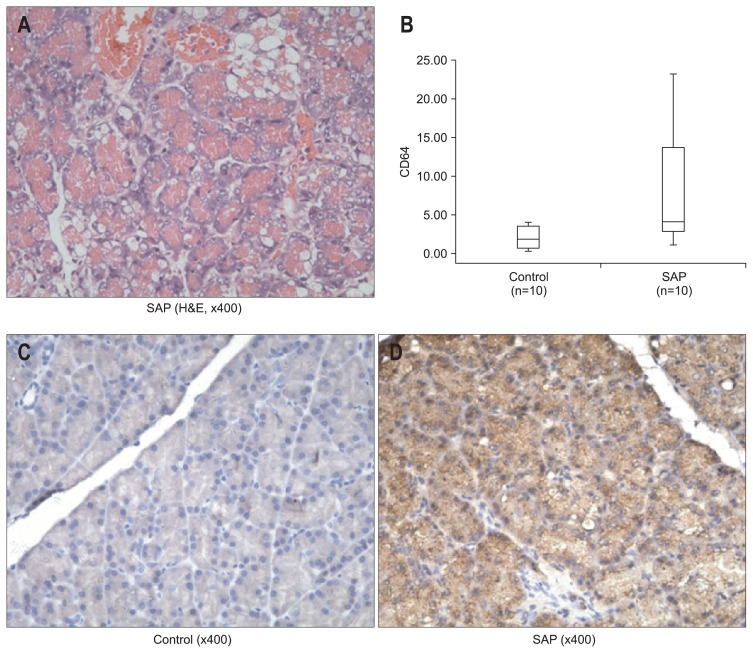
L-arginine-induced pancreatitis was used as the SAP model to investigate our hypothesis. As shown in Fig. 1A, light micrographs of the pancreas showed interstitial edema, inflammatory infiltrate, acinar cell necrosis, and adipose tissue in interstitial spaces. The CD64 mRNA level in the pancreas was significantly higher in the rat model of SAP compared to the control (p=0.028) (Fig. 1B). CD64 protein expression in the pancreas, identified by immunohistochemistry, was higher in SAP rats than in control rats (Fig. 1C and D).
Fig. 1.
(A) Pancreatic injury in L-arginine-induced experimental acute pancreatitis (H&E stain, ×400). Note the presence of edema, inflammatory cell infiltration, acinar cell degranulation, and dilatation. (B) CD64 expression by quantitative real-time polymerase chain reaction analysis in the control and severe acute pancreatitis (SAP) rats. CD64 mRNA expression was normalized to glyceraldehyde 3-phosphate dehydrogenase. The lines inside the boxes denote the medians. The boxes mark the interval between the 25th and 75th percentiles. The whiskers denote the interval from minimum to maximum. Statistically significant differences were determined using the Mann-Whitney U test. The CD64 expression was significantly higher in the SAP rats compared to the control rats (p=0.028). A comparison of positive pancreatic CD64 immunostaining (×400) in (C) the control and (D) SAP rats was performed. Pancreatic CD64 protein expression, as identified by immunohistochemistry, was higher in the SAP rats than in control rats.
2. Patient characteristics
The study included 31 patients, including six females (19%) and 25 males (81%). SAP was diagnosed in 10 patients (32.26%), and 21 patients (67.74%) were classified as having mild acute pancreatitis (MAP). The patient characteristics and laboratory variables at the time of inclusion are presented in Table 3.
Table 3.
The Patient Characters and Laboratory Variables at the Time of Study Inclusion
| Variable | Value |
|---|---|
| Patients | 31 (100) |
| Female sex | 6 (19) |
| Male sex | 25 (81) |
| Age, yr | 43 (18–82) |
| Etiology: | |
| Biliary | 5 (16) |
| Alcohol | 8 (26) |
| High fat diet | 9 (29) |
| Unknown | 9 (29) |
| Duration of pain before admission, hr | 24 (5–48) |
| Amylase, U/L | 382 (31–3,993) |
| WBC counts, 109/L | 13.10 (4.93–25.89) |
| CT Balthazar score | 3 (1–5) |
Data are presented as number (%) or median (range).
WBC, white blood cell; CT, computed tomography.
3. Expression of CD64 mRNA among patients with AP and its relationship with the Ranson and APACHE II scores
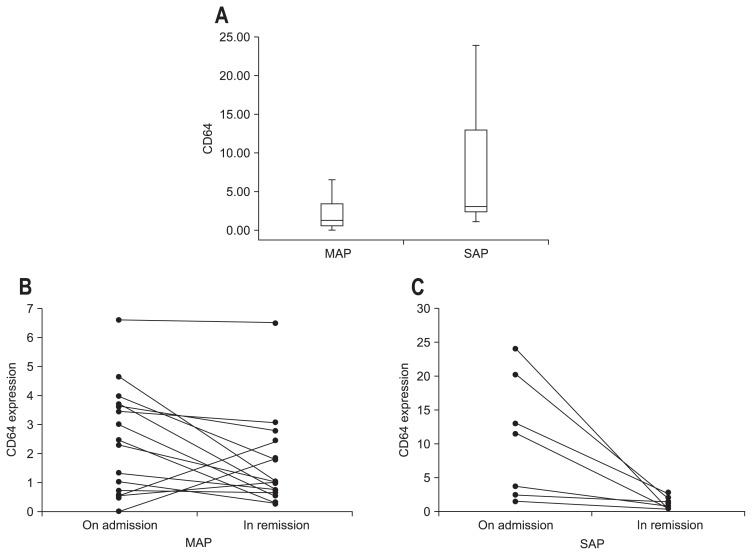
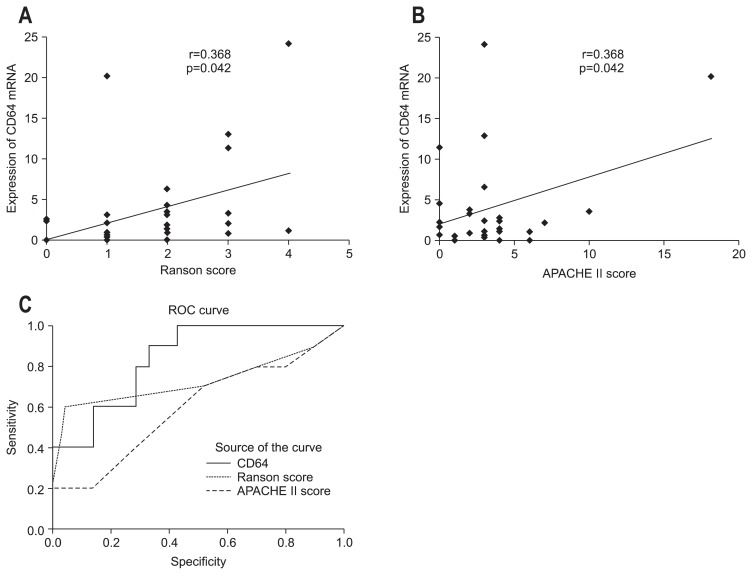
At the time of admission, CD64 expression was higher in the SAP patients compared to the MAP patients (p=0.015) (Fig. 2A). During remission, CD64 mRNA expression decreased in both the MAP (Fig. 2B) and SAP (Fig. 2C) groups (p=0.025 and p=0.008, respectively). CD64 mRNA expression in patients with AP at the time of admission correlated significantly with the Ranson score (r=0.368, p=0.042) (Fig. 3A) and APACHE score (r=0.368, p=0.042) (Fig. 3B). Fig. 3C shows the receiver operating characteristic curves of the CD64 mRNA level and the Ranson score at admission. The optimum cutoff level of CD64 was 2.362, with a sensitivity of 80% and specificity of 66.7%. The area under the curve (AUC) of CD64 (AUC, 0.838±0.071; 95% confidence interval [CI], 0.698 to 0.978) for the detection of SAP was superior to the Ranson score (AUC, 0.724±0.118; 95% CI, 0.492 to 0.956) and the APACHE II score (AUC, 0.593±0.115; 95% CI, 0.368 to 0.818) (Fig. 3C).
Fig. 2.
(A) CD64 expression by quantitative real-time polymerase chain reaction analysis and changes in the CD64 mRNA expression in patients with (B) mild acute pancreatitis (MAP) and (C) severe acute pancreatitis (SAP) at admission and during remission. Box plots of CD64 expression in MAP patients and SAP patients. CD64 mRNA expression was normalized to glyceraldehyde 3-phosphate dehydrogenase. The lines inside the boxes denote the medians. The boxes mark the interval between the 25th and 75th percentiles. The whiskers denote the interval from minimum to maximum. Statistically significant differences were determined using the Mann-Whitney U test (p=0.015). CD64 mRNA expression in patients with MAP and SAP was higher at admission than during remission (p=0.025 and p=0.008, respectively). Statistically significant differences were determined using Wilcoxon tests.
Fig. 3.
The correlations were determined between CD64 mRNA expression in leukocytes with (A) the Ranson score and (B) the Acute Physiology and Chronic Health Evaluation (APACHE) II score in patients with acute pancreatitis (AP) at admission. (C) Comparisons of receiver operating characteristic (ROC) curves of the APACHE II score, Ranson score, and CD64 mRNA expression in patients with AP at admission.
DISCUSSION
CD64 expression on the surface of neutrophils has been found to be an early marker of bacterial infection. In this study, we demonstrated that CD64 is a potential early diagnostic biomarker of SAP. CD64 was significantly upregulated in a rat model of SAP. We also showed that CD64 expression on the leukocytes of patients with AP increased significantly and correlated with disease severity. Furthermore, the CD64 level on leukocytes decreased during AP remission. These results suggest that an increase in CD64 expression may be relevant to pancreatic acinar cell injury in AP.
CD64 was initially reported in microbial inflammation and as a crucial mediator of septic shock. Furthermore, it has been shown that increased CD64 expression is associated with infectious and noninfectious inflammatory processes.23 We considered two possibilities concerning the increase of CD64 levels at admission (under sterile conditions). First, CD64 may be up-regulated because of nonmicrobial inflammation (systemic inflammatory response). A significant feature of AP is pancreatic inflammation with excessive leukocyte recruitment. Triggered by chemotactic signals, leukocytes arrest on the vascular endothelium and prepare for their migration into tissue.24 Ryschich et al.7 demonstrated that intracapillary leukocyte accumulation represents an initial step of leukocyte activation during AP, consisting of the rapid promotion of leukocyte adhesion to the capillary endothelium and the intraluminal intracapillary crawling of leukocytes, which leads to sustained capillary occlusion. Upregulated CD64 expression also plays important roles in phagocytosis, cytolysis, and the induction of inflammatory cytokines,25 which may play a critical role in leukocyte recruitment. Second, CD64 may be increased in response to undetected microbes or endotoxin in the very early phase of AP because bacterial DNA has been detected in patient serum in the early phase of AP without signs of sepsis.26 Therefore, it is conceivable that increased CD64 expression in the early phase of SAP is associated with noninfectious inflammation.
Several serum markers (CRP, procalcitonin, interleukin [IL]-6, and IL-8) and clinical scoring systems (the Ranson score and the APACHE II score) have been used to assess severity in AP.27 However, these approaches have limitations. Conventional severity scores consist of multiple factors (Ranson, 11 factors; and APACHE II, 14 factors) and are complicated. In our results, we evaluated the utility of CD64 as a marker for assessing SAP. Our data showed that CD64 was increased within 48 hours after onset and was highly correlated with the Ranson score and the APACHE II score, indicating that CD64 may be an early severity marker for SAP. Moreover, the AUC of CD64 for the detection of SAP was greater than the Ranson score and the APACHE II score. Therefore, it is evident that the usefulness of CD64 in detecting SAP is superior to that of the Ranson score and the APACHE II score.
CRP is an acute phase protein produced by the liver, and its levels are increased in a state of acute inflammation. Plasma CRP levels greater than 150 mg/L within the first 72 hours of AP correlate with the presence of necrosis. In this study, the median CRP level was 14.03 mg/L (range, 1.04 to 46 mg/L) on the first day of admission. CD64 did not correlate with CRP at admission (data not shown), which implies that CRP may not show a good prognostic accuracy for SAP on the first day of admission. Most recently, Cardoso et al.28 found that the optimal CRP for SAP was at 48 hours after hospital admission.
In conclusion, our results show that CD64 mRNA expression in peripheral blood leukocytes is closely related to the severity of AP. It may be a useful marker for predicting the development of SAP. Combining CD64 level with current score systems may predict SAP with higher accuracy.
ACKNOWLEDGEMENTS
This work was supported by grants from the Chongqing Science Foundation for Distinguished Young Scholars (CSTC, 2009BA5045).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Hasibeder WR, Torgersen C, Rieger M, Dünser M. Critical care of the patient with acute pancreatitis. Anaesth Intensive Care. 2009;37:190–206. doi: 10.1177/0310057X0903700206. [DOI] [PubMed] [Google Scholar]
- 2.Fenton-Lee D, Imrie CW. Pancreatic necrosis: assessment of outcome related to quality of life and cost of management. Br J Surg. 1993;80:1579–1582. doi: 10.1002/bjs.1800801228. [DOI] [PubMed] [Google Scholar]
- 3.Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69–81. [PubMed] [Google Scholar]
- 4.Gonzálvez-Gasch A, de Casasola GG, Martín RB, Herreros B, Guijarro C. A simple prognostic score for risk assessment in patients with acute pancreatitis. Eur J Intern Med. 2009;20:e43–e48. doi: 10.1016/j.ejim.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M, Wong FL, Cao Y, et al. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- 6.Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24( Suppl 1):45–51. doi: 10.1097/01.shk.0000191413.94461.b0. [DOI] [PubMed] [Google Scholar]
- 7.Ryschich E, Kerkadze V, Deduchovas O, et al. Intracapillary leucocyte accumulation as a novel antihaemorrhagic mechanism in acute pancreatitis in mice. Gut. 2009;58:1508–1516. doi: 10.1136/gut.2008.170001. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–125. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 10.Buckle AM, Hogg N. The effect of IFN-gamma and colony-stimulating factors on the expression of neutrophil cell membrane receptors. J Immunol. 1989;143:2295–2301. [PubMed] [Google Scholar]
- 11.Kerst JM, van de Winkel JG, Evans AH, et al. Granulocyte colony-stimulating factor induces hFc gamma RI (CD64 antigen)-positive neutrophils via an effect on myeloid precursor cells. Blood. 1993;81:1457–1464. [PubMed] [Google Scholar]
- 12.Kerst JM, de Haas M, van der Schoot CE, et al. Recombinant granulocyte colony-stimulating factor administration to healthy volunteers: induction of immunophenotypically and functionally altered neutrophils via an effect on myeloid progenitor cells. Blood. 1993;82:3265–3272. [PubMed] [Google Scholar]
- 13.Fjaertoft G, Håkansson LD, Pauksens K, Sisask G, Venge P. Neutrophil CD64 (FcgammaRI) expression is a specific marker of bacterial infection: a study on the kinetics and the impact of major surgery. Scand J Infect Dis. 2007;39:525–535. doi: 10.1080/00365540601113693. [DOI] [PubMed] [Google Scholar]
- 14.Simms HH, Frank MM, Quinn TC, Holland S, Gaither TA. Studies on phagocytosis in patients with acute bacterial infections. J Clin Invest. 1989;83:252–260. doi: 10.1172/JCI113867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyre PM, Campbell AS, Kniffin WD, Fanger MW. Monocytes and polymorphonuclear neutrophils of patients with streptococcal pharyngitis express increased numbers of type I IgG Fc receptors. J Clin Invest. 1990;86:1892–1896. doi: 10.1172/JCI114921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng PC, Li K, Wong RP, Chui KM, Wong E, Fok TF. Neutrophil CD64 expression: a sensitive diagnostic marker for late-onset nosocomial infection in very low birthweight infants. Pediatr Res. 2002;51:296–303. doi: 10.1203/00006450-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi SS, Lewis SM, Gant VA, Treacher D, Davis BH, Brown KA. Increased distribution and expression of CD64 on blood polymorphonuclear cells from patients with the systemic inflammatory response syndrome (SIRS) Clin Exp Immunol. 2001;125:258–265. doi: 10.1046/j.1365-2249.2001.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simms HH, D’Amico R. Polymorphonuclear leukocyte dysregulation during the systemic inflammatory response syndrome. Blood. 1994;83:1398–1407. [PubMed] [Google Scholar]
- 19.Layseca-Espinosa E, Pérez-González LF, Torres-Montes A, et al. Expression of CD64 as a potential marker of neonatal sepsis. Pediatr Allergy Immunol. 2002;13:319–327. doi: 10.1034/j.1399-3038.2002.01064.x. [DOI] [PubMed] [Google Scholar]
- 20.Streimish I, Bizzarro M, Northrup V, et al. Neutrophil CD64 as a diagnostic marker in neonatal sepsis. Pediatr Infect Dis J. 2012;31:777–781. doi: 10.1097/INF.0b013e318256fb07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soni S, Wadhwa N, Kumar R, et al. Evaluation of CD64 expression on neutrophils as an early indicator of neonatal sepsis. Pediatr Infect Dis J. 2013;32:e33–e37. doi: 10.1097/INF.0b013e31826faede. [DOI] [PubMed] [Google Scholar]
- 22.Tani S, Itoh H, Okabayashi Y, et al. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci. 1990;35:367–374. doi: 10.1007/BF01537416. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann JJ. Neutrophil CD64: a diagnostic marker for infection and sepsis. Clin Chem Lab Med. 2009;47:903–916. doi: 10.1515/CCLM.2009.224. [DOI] [PubMed] [Google Scholar]
- 24.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 25.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 26.de Madaria E, Martínez J, Lozano B, et al. Detection and identification of bacterial DNA in serum from patients with acute pancreatitis. Gut. 2005;54:1293–1297. doi: 10.1136/gut.2004.047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol. 2002;97:1309–1318. doi: 10.1111/j.1572-0241.2002.05766.x. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso FS, Ricardo LB, Oliveira AM, et al. C-reactive protein prognostic accuracy in acute pancreatitis: timing of measurement and cutoff points. Eur J Gastroenterol Hepatol. 2013;25:784–789. doi: 10.1097/MEG.0b013e32835fd3f0. [DOI] [PubMed] [Google Scholar]