Abstract
Endoscopic drainage for pancreatic and peripancreatic fluid collections (PFCs) has been increasingly used as a minimally invasive alternative to surgical or percutaneous drainage. Recently, endoscopic ultrasound-guided transluminal drainage (EUS-TD) has become the standard of care and a safe procedure for nonsurgical PFC treatment. EUS-TD ensures a safe puncture, avoiding intervening blood vessels. Single or multiple plastic stents (combined with a nasocystic catheter) were used for the treatment of PFCs for EUS-TD. More recently, the use of covered self-expandable metallic stents (CSEMSs) has provided a safer and more efficient approach route for internal drainage. We focused our review on the best approach and stent to use in endoscopic drainage for PFCs. We reviewed studies of EUS-TD for PFCs based on the original Atlanta Classification, including case reports, case series, and previous review articles. Data on clinical outcomes and adverse events were collected retrospectively. A total of 93 patients underwent EUS-TD of pancreatic pseudocysts using CSEMSs. The treatment success and adverse event rates were 94.6% and 21.1%, respectively. The majority of complications were of mild severity and resolved with conservative therapy. A total of 56 patients underwent EUS-TD using CSEMSs for pancreatic abscesses or infected walled-off necroses. The treatment success and adverse event rates were 87.8% and 9.5%, respectively. EUS-TD can be performed safely and efficiently for PFC treatment. Larger diameter CSEMSs without additional fistula tract dilation for the passage of a standard scope are needed to access and drain for PFCs with solid debris.
Keywords: Pancreatic pseudocyst, Walled-off necrosis, Endoscopic ultrasound-guided drainage, Metal stent, Endoscopic necrosectomy
INTRODUCTION
Peripancreatic fluid collections (PFCs) can develop secondary to either fluid leakage or liquefaction of pancreatic necrosis following acute pancreatitis, chronic pancreatitis, surgery, or abdominal trauma.1–4 Previously focusing on the original Atlanta Classification of acute pancreatitis,5 PFCs include acute fluid collections, acute and chronic pancreatic pseudocysts, pancreatic abscesses, and pancreatic necrosis. This original Atlanta Classification5 proposed the term “pancreatic abscess” to define a “localized collection of purulent material without significant necrotic material.” However, since this finding is extremely uncommon, the term “pancreatic abscess” was confusing even investigators of pancreatic diseases.
In 2013, the revised Atlanta Classification proposed to clarify several issues from the original Atlanta Classification.6 The revised Atlanta Classification classified local complications mostly followed by acute pancreatitis into four types according to pathological conditions and timing as follows: 1) acute peripancreatic fluid collection (APFC); 2) acute necrotic collection (ANC) (sterile or infected); 3) pancreatic pseudocyst (PP); and 4) walled-off necrosis (WON) (sterile or infected),6 In this classification, the term “pancreatic abscess” was removed and divided into infected PPs and WONs based on their component and radiologic images.6 Until 2013, an infected PP was lumped together with an infected WON in the same category as a pancreatic abscess. Thus, an infected ANC/PP or WON must be set apart from APFC, sterile PP or WON based on the revised Atlanta Classification6 because the strategy of treatment is markedly different (Table 1). The outcome of endoscopic drainage was significantly worse for WON compared with PP, with significantly fewer collection disappearances and more complications.7 Even if the PP should not always be treated according to the American Society for Gastrointestinal Endoscopy guideline,8 the indication for drainage of PP are symptoms (abdominal pain, early satiety), complications (infection, bleeding, rupture), obstruction of a surrounding hollow viscous (gastric, duodenal, or biliary obstruction), or enlarged PP. Drainage of PP was also recommended if the PPs were larger than 6 cm, continued to increase in size or did not resolve after 4 to 6 weeks7 as well as symptomatic lesions. Infected ANC was also recommended for drainage similarly to an infected PP. On the other hand, infected WONs, which consisted of a mature, encapsulated collection of pancreatic and/or peripancreatic necrosis that has developed a well-defined inflammatory wall, were recommended for not only drainage but also necrosectomy if needed.
Table 1.
Comparison of the Original and Revised Atlanta Classification
| Original Atlanta Classification (1993) | Revised Atlanta Classification (2012) | |
|---|---|---|
| Acute pancreatitis | Interstitial pancreatitis | Interstitial edematous pancreatitis |
| Sterile necrosis | Necrotizing pancreatitis (pancreatic necrosis and/or peripancreatic necrosis) | |
| Infected necrosis | Sterile necrosis | |
| Infected necrosis | ||
| Fluid collections during acute pancreatitis | Pancreatic pseudocyst | <4 Weeks after onset of acute pancreatitis |
| Pancreatic abscess | Acute peripancreatic fluid collection (APFC) | |
| Sterile necrosis | ||
| Infected necrosis | ||
| Acute necrotic collection (ANC) | ||
| Sterile necrosis | ||
| Infected necrosis | ||
| <4 Weeks after onset of acute pancreatitis | ||
| Pancreatic pseudocyst (PP) | ||
| Sterile necrosis | ||
| Infected necrosis | ||
| Walled-off pancreatic necrosis (WON) | ||
| Sterile necrosis | ||
| Infected necrosis |
At present, endoscopic drainages are popular as a minimally invasive alternative to surgical or percutaneous drainage for PFC management. Of the endoscopic drainages for PFCs, endoscopic ultrasound-guided transluminal drainage (EUS-TD) has become the standard and safe procedure in many centers for the nonsurgical treatment of PFCs because it can provide a safe puncture avoiding intervening blood vessels. Thus far, single or multiple plastic stents (combined with a nasocystic catheter) have commonly been used for the treatment of PFCs for EUS-TD. More recently, the use of covered self-expandable metallic stents (CSEMSs) has provided a safer and more efficient approach route for internal drainage.
In this review, we focus on the best approach and stent to use in endoscopic drainage for PFCs on the basis of the original Atlanta Classification5 because of lack of clinical results confirming the revised Atlanta Classification.6
OPTIMAL INTERVENTION FOR PFCs
A recent retrospective study regarding nonsurgical approaches–percutaneous versus endoscopic transmural drainage (conventional direct transluminal drainage by forward-viewing endoscopy [CTD] or EUS-TD)–to symptomatic PP revealed no significant difference between technical success rates in treating PP.9 However, percutaneous transmural drainage was associated with a higher reintervention rate, longer hospital stay, and increased number of follow-up abdominal imaging studies.9 Therefore, endoscopic transmural drainage should be the preferred modality for the drainage of symptomatic PP compared with percutaneous drainage. A recent prospective randomized controlled trial regarding surgical drainage versus EUS-TD for symptomatic PP revealed no difference in treatment success, complications, or reinterventions between the surgical and EUS-TD groups, the length of hospital stay was shorter, the physical and mental health scores were better, and the total mean costs were lower for the EUS-TD group.10 Because none of the patients randomized to EUS-TD developed PP recurrence at the follow-up evaluation, there was no evidence to suggest that surgical drainage is superior to EUS-TD for PP drainage. Thus, endoscopic drainage for PP drainage has become an effective alternative treatment to percutaneous and surgical drainage. Endoscopic drainage is now considered to be the first-line approach for treating symptomatic PP due to its less invasiveness, lower reinterventions, lower morbidity rate, and shorter hospital stay. In addition, endoscopic drainage of PP does not require general anesthesia. However, we should consider that surgical treatment still has an important role in terms of adjunctive or salvage therapy if endoscopic or percutaneous intervention fails.
OPTIMAL ENDOSCOPIC INTERVENTIONS FOR PFCs
Endoscopic drainage of PP consists of CTD, transpapillary drainage (TPD) and EUS-TD. In a web-based U.S. survey that identified the American Society for Gastrointestinal Endoscopy members who performed PP drainage in 2006, EUS-TD was used only by 56% of U.S. endoscopists and 43% by international endoscopists.11
TPD requires that the PP communicate with the main pancreatic duct and that it has few septations to permit complete drainage. Pancreatic duct strictures or disruption, if identified, may be dilated, after which a single plastic stent is placed into the main pancreatic duct. It is also crucial to evaluate for the presence of a pancreatic fistula, which if present, should be initially treated by pancreatic duct stenting. If the pancreatic fistula does not resolve after a prolonged period of pancreatic duct stenting, endoscopic sealing with N-butyl-2-cyanoacrylate can be considered.12 A recent prospective cohort study of patients with refractory pancreatic duct strictures revealed that the use of a wire-guided diathermic dilator is feasible and safe. Wire-guided diathermic dilator treatment may be considered a new standard alternative procedure when conventional dilation fails.13
EUS-TD of PP is an attractive endoscopic approach in patients who have a small window of entry based on computed tomography (CT) findings, particularly in the case of lack of an endoscopically defined area of luminal bulging, in unusual locations of PPs, with coagulopathy, with thrombocytopenia, with portal hypertension, with documented intervening vessels, in failed CTD or TPD and considering complication during CTD or TPD. A recent prospective randomized controlled trial regarding CTD versus EUS-TD revealed significant differences regarding technical success in treating PP.14 With regard to clinical outcomes (short-term and long-term results), however, there was no significant difference between CTD and EUS-TD.14 Therefore, for luminal bulging PPs, both CTD and EUS-TD can be selected and performed. However, for nonluminal bulging PPs or if CTD or TPD has failed, EUS-TD has the theoretical advantage of reducing the risk of bleeding, perforation, and infection compared with CTD. The first meta-analysis comparing the technical success and clinical outcomes of EUS-TD and CTD for PPs resulted in the same conclusion.15 Utilizing EUS-TD for PP has been shown to be the safest. A prerequisite for EUS-TD is the presence of a well-defined mature wall. The fluid collection must be accessible endoscopically, such as being located within 1 cm of the gastric or duodenal walls; paracolic collections cannot be accessed and would require adjunctive methods such percutaneous drainage.16 Thus, EUS-TD should be performed as a preferable approach to CTD or TPD (Table 2). To date, current reports in the literature regarding EUS-TD for PP have documented recent developments and improvement of outcomes.17,18
Table 2.
Advantages and Limitations of Conventional Transluminal, Transpapillary, and Endoscopic Ultrasound-Guided Transluminal Drainage
| Advantages | Limitations | |
|---|---|---|
| CTD | Widely used technique | Blind approach |
| For urgent treatment | Risk of bleeding | |
| Risk of perforation | ||
| Need for luminal bulging | ||
| Limited equipment and accessories | ||
| Oversight of MPD abnormality | ||
| TPD | Physiological flowing | Need to communicate with MPD |
| Possibility of resolution of MPD stricture | Noneffective for complex septations | |
| Diagnosable disconnected syndrome | Risk of exacerbation of pancreatitis | |
| A large variety of equipment | Long treatment period | |
| EUS-TD | Visualized approach | Required interventional expertise |
| Differential diagnosis during procedure | Limited equipment | |
| Ascertain the nature of a fluid collection | Oversight of MPD abnormality | |
| Available for nonluminal bulging lesion | ||
| Available in failed CTD or TPD | ||
| For urgent treatment |
CTD, conventional transluminal drainage; TPD, transpapillary drainage; EUS-TD, endoscopic ultrasound-guided transluminal drainage; MPD, main pancreatic duct.
OPTIMAL ENDOSCOPIC STENTS FOR EUS-TD
Currently, the type, size, and number of stents used for EUS-TD are the major concerns of interest. Traditionally, plastic pigtail stents provide highly secured drainage. The fistula tract between the gastrointestinal tract and the PP is maintained with the placement of double pigtail plastic stents for preventing dislocation and migration. Although double pigtail plastic stents have been used to provide drainage, occlusion rates are high and endoscopic access to the PP cavity via the fistula is limited because of its small caliber. Therefore, placement of multiple small-caliber (including simultaneous placement of a pigtail stent and a nasocystic drainage catheter) (Figs 1–3) or large-caliber pigtail plastic stents is required to maintain a large fistula for sufficient and effective drainage. However, small-caliber plastic stents are needed for multiple attempts and accesses to the cavity. These procedures may cause loss of the guidewire (failure of multiple stenting), proximal migration of the first stent into the cavity, additional time, and a more cumbersome procedure. On the other hand, large-caliber stents can be difficult to advance and deploy through the channel of the EUS scope.
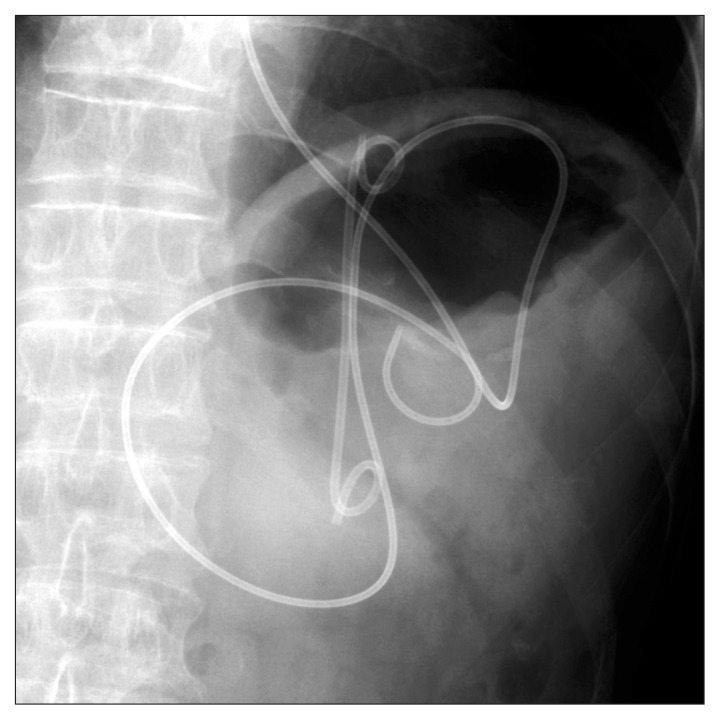
Fig. 1.
Radiograph showing a double pigtail plastic stent and a nasocystic catheter in the pancreatic pseudocyst.
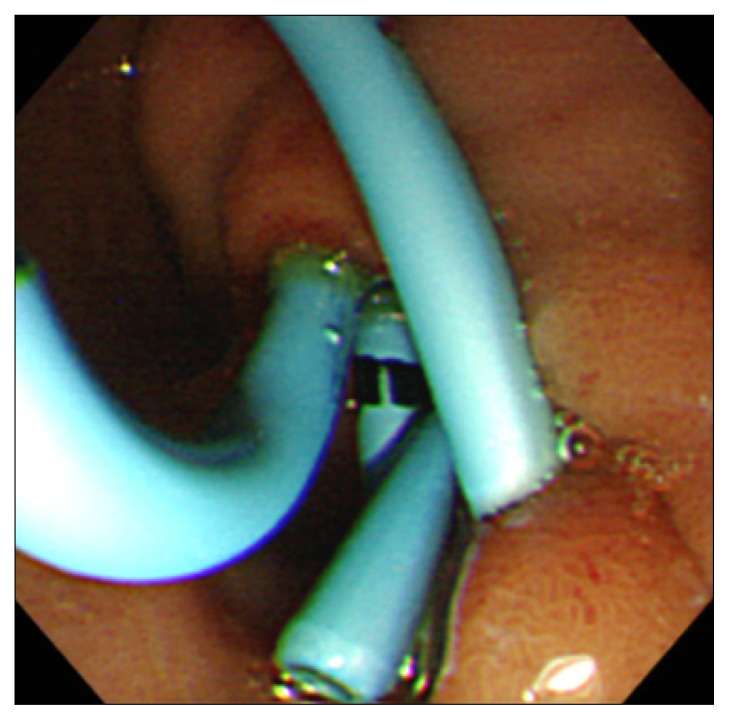
Fig. 3.
Endoscopic image showing a double pigtail stent in the pancreatic pseudocyst.
Recently, tubular CSEMSs (TCSEMS), which are used for the treatment of a biliary stricture, have been available for PP drainage instead of multiple plastic stents. The TCSEMS provide larger calibers than plastic stents, which might be advantageous for contaminated and excessive amounts of debris although it is much more expensive than plastic stents (Table 3). The TCSEMS can also reduce the risk of perforation, leakage and bleeding because of minimal dilation and sealing of the fistula tract including tamponade effects. Several reports of a case or case series of PP have indicated the utility of CSEMSs for drainage. A summary of these reports showed 93 patients with PP using CSEMS (Table 4).19–33 The technical success rate from published cases was 100% (93/93 PPs). PP resolution was achieved in 94.6% (88/93 PPs) with complete resolution in 90.6% (77/85 PPs). The complication rate was 21.1% (19/76 PPs). Among them, the most common complication was superinfection to PPs, with a mild degree of severity. On the other hand, the CSEMS migration rate was 3.9% (3/76 PPs) and the buried CSEMS rate was 2.6% (2/76 PPs). Partial or full CSEMS migration is a significant problem because CSEMSs are tubular conduits and do not have anchoring flanges. To prevent migration, the placement of a double pigtail stent or a nasocystic catheter through the CSEMS may be effective to serve as an anchoring effect. The currently available and used CSEMSs were designed for drainage related to a luminal stricture, but were not related to a transluminal route. Most previous reports involved a bile duct or an esophageal stent for drainage. When a bile duct or an esophageal stent is used for PP, the longer protrusion on both the gastrointestinal tract and the PP cavity sides entails a risk of contact ulceration, bleeding, or migration. They are not good options in cases when the PP is not firmly attached to the gastrointestinal wall, because they do not apply any anchorage force and the risk of leakage is high.30
Table 3.
Advantages and Limitations of Different Types of Stents
| Advantages | Limitations | |
|---|---|---|
| Plastic stent | Low cost | Small caliber |
| Easy extubation | Need for multiple stents | |
| Easy placement (small outer diameter) | Difficult placement (large caliber) | |
| Short patency | ||
| Poor visibility under fluoroscopy (during procedure) | ||
| Long treatment period | ||
| Possibility of fluid leak | ||
| Possibility of migration (during procedure) | ||
| Metallic stent | Large caliber | Difficult placement |
| Long patency | Expensive | |
| Easy shift to direct necrosectomy | Possibility of gatrointestinal tract injury | |
| Good visibility under fluoroscopy (during procedure) | Difficult extubation* | |
| Short treatment period | ||
| Prevents fluid leak | ||
| Hemostatic effect from puncture site |
Except for AXIOS stent.
Table 4.
Study Characteristics and Patient Outcome of Endoscopic Ultrasound-Guided Drainage of Pancreatic Pseudocyst Using a Self-Expandable Metallic Stent
| A. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Author (yr) | Journal | No. of patients | Size, cm | Timing of treatment | No. of sessions | Technical success (%) | Resolution success (%) | Complete resolution (%) | Time to resolution |
| Talreja (2008)19 | Gastrointest Endosc | 18 | 10±4 | Initial | 1 | 18 (100) | 17 (95) | 14 (78) | 77±80 Days (15–310) |
| Tarantino (2009)20 | Gastrointest Endosc | 1 | 18×15×3 | After multiple sessions | 1 | 1 (100) | 1 (100) | 1 (100) | 10 Days |
| Penn (2012)21 | Gastrointest Endosc | 20 | 13.4 (average) | Initial | 1 | 20 (100) | 17 (85) | 17 (85) | 101 Days |
| Itoi (2012)22 | Gastrointest Endosc | 15 | 9.8 (average) | Initial | 1 | 15 (100) | 15 (100) | 15 (100) | NA |
| Fabbri (2012)23 | Endoscopy | 12 | 11.8 (average) | Initial | 1 | 12 (100) | 11 (91.7) | 11 (91.7) | NA |
| Tarantino (2012)24 | World J Gastrointest Endosc | 1 | 20 | Initial | 1 | 1 (100) | 1 (100) | 1 (100) | 10 Days |
| Tarantino (2012)25 | Endoscopy | 1 | 20 | Initial | 1 | 1 (100) | 1 (100) | 1 (100) | 10 Days |
| Barresi (2012)26 | Ding Endosc | 1 | NA | Initial | 1–2 | 1 (100) | 1 (100) | 1 (100) | NA |
| Berzosa (2012)27 | Endoscopy | 4 | 13.4 (7.4–12.5) (average) | After multiple sessions | 1 | 4 (100) | 4 (100) | 4 (100) | NA |
| Weilert (2012)28 | Endoscopy | 8 | NA | Initial | 1 | 8 (100) | 8 (100) | NA | NA |
| Gornals (2012)29 | Endoscopy | 1 | 8×5 | Initial | 1 | 1 (100) | 1 (100) | 1 (100) | NA |
| Gornals (2013)30 | Surg Endosc | 4 | NA | Initial | 1 | 4 (100) | 4 (100) | 4 (100) | NA |
| Yamamoto (2013)31 | Gastrointest Endosc | 5 | 10.3 (average) | After multiple sessions | 1 | 5 (100) | 5 (100) | 7 (77.8) | NA |
| Téllez-Ávila (2013)32 | World J Gastrointest Endosc | 1 | 6×5 | After multiple sessions | 1 | 1 (100) | 1 (100) | 1 (100) | NA |
| Saxena (2014)33 | Gastrointest Endosc | 1 | 17×14 | Initial | 1 | 1 (100) | 1 (100) | 1 (100) | 4 Weeks |
| Total | 62 | 100% (64/64 pseudocysts) | 89% (57/64 pseudocysts) | 81.2% (52/64 pseudocysts) | |||||
| B. | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Author (yr) | GW size (inch) | Dilation devices | Type of SEMS | SEMS for use | Diameter/length, mm | Name of SEMS | Company name of SEMS | Plastic stent/placement position for SEMS |
| Talreja (2008)19 | 0.035 | Balloon/cystotome | FC | Bile duct | 10/60 | GORE® VIABIL® BILIARY ENDOPROSTHESIS | Conmed | Double-pig tail/alongside |
| Tarantino (2009)20 | 0.035 | Cystotome | PC | Bile duct | 10/40 | WALLSTENT™ Biliary RX Endoprosthesis | Boston Scientific | |
| Penn (2012)21 | 0.035 | Not used/balloon | FC | Bile duct | 10/40 | WALLFLEX™ Biliary RX stent | Boston Scientific | Double-pigtail/into |
| Itoi (2012)22 | 0.035 | Bougie/balloon/cystotome | FC | Exclusive use | 10/60 or 10/100 | AXIOS™ stent | Xlumena Inc. | - |
| Fabbri (2012)23 | 0.035 | Needle knife | FC | Bile duct | 10/40 or 10/60 | WALLFLEX™ Biliary RX stent or Niti-S | Boston Scientific or Taewoong Medical Co., Ltd. | - |
| Tarantino (2012)24 | 0.035 | Needle knife | FC | Bile duct | 8/40 | NA | Taewoong Medical Co., Ltd. | Straight/into |
| Tarantino (2012)25 | 0.035 | NA | FC | Bile duct | 10/20 | NA | Taewoong Medical Co., Ltd. | - |
| Barresi (2012)26 | NA | NA | PC | Bile duct | 10/40 | WALLFLEX™ Biliary RX stent | Boston Scientific | - |
| Berzosa (2012)27 | 0.035 | Needle knife | FC | Bile duct | 10/60 or 10/70 or 10/80 or 10/100 | GORE® VIABIL® BILIARY ENDOPROSTHESIS | Conmed | |
| Weilert (2012)28 | 0.035 | NAVIX | FC | Bile duct | 10/40 | WALLFLEX™ Biliary RX stent | Boston Scientific | - |
| Gornals (2012)29 | NA | NAVIX | FC | Exclusive use | 10/100 | AXIOS™ stent | Xlumena Inc. | - |
| Gornals (2013)30 | NA | NAVIX/balloon | FC | Exclusive use | 10/100 or 10/150 | AXIOS™ stent | Xlumena Inc. | - |
| Yamamoto (2013)31 | NA | Balloon | FC | Exclusive use | 16/20 | NAGI-stent | Taewoong Medical Co., Ltd. | NA/into |
| Téllez-Ávila (2013)32 | 0.035 | Needle knife/bougie/balloon | FC | Exclusive use | 10/30 | NAGI-stent | Taewoong Medical Co., Ltd. | - |
| Saxena (2014)33 | NA | Balloon | FC | Esophagus | 18/60 | Alimaxx, Bonastanet, WallFlex | Meritt, Endochoice, Boston Scientific | Double-pigtail/into |
| C. | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Author (yr) | Complication (%) | Details of complication | SEMS placement period | Recurrence | Convert to surgery | Morbidity | Observational period |
| Talreja (2008)19 | 8 (44) | Superinfection 5, Bleeding 2, Inward migration 1 | NA | - | 1 (5.6) | 1 (5.6) | 77±80 Days |
| Tarantino (2009)20 | - | - | 4 Weeks | - | - | - | 1 Month |
| Penn (2012)21 | 4 (20) | Superinfection 2, Post-EUS drainage fever 1, Post-ERCP pancreatitis 1 | 4–10 Weeks | 3 (15) | 3 (15) | - | NA |
| Itoi (2012)22 | 1 (6.7) | Migration 1 | 10–98 Days | - | - | - | NA |
| Fabbri (2012)23 | 2 (16.7) | Superinfection 1, FCSEMS removal impossible 1 | 28 Days | 1 (8.3) | 1 (5) | - | NA |
| Tarantino (2012)24 | - | - | 60 Days | - | - | - | 2 Months |
| Tarantino (2012)25 | - | - | NA | - | - | - | 3 Months |
| Barresi (2012)26 | 1 (100) | Buried 1 | NA | - | 1 (100) | - | - |
| Berzosa (2012)27 | - | - | NA | - | - | - | 19.8 Weeks (11–35) |
| Weilert (2012)28 | NA | NA | 7–10 Days | NA | NA | NA | NA |
| Gornals (2012)29 | 1 (100) | Tension pneumothorax 1 | 7 Days | - | - | - | 4 Months |
| Gornals (2013)30 | 1 (25) | Tension pneumothorax 1 | NA | NA | - | - | NA |
| Yamamoto (2013)31 | 1 (20) | Outward migration 1 | 31.8 Days (7–90) (average) | - | - | - | NA |
| Téllez-Ávila (2013)32 | - | - | NA | - | - | - | 6 Months |
| Saxena (2014)33 | - | - | NA | - | - | - | 12 Months |
| Total | 35.2% (19/54) | 8% (4/50) | 11.5% (6/52) | 1.85% (1/54) | |||
NA, data not available.
GW, guidewire; SEMS, self-expandable metallic stent; FC, fully covered; PC, partially covered; NA, data not available.
SEMS, self-expandable metallic stent; NA, data not available; EUS, endoscopic ultrasound; ERCP, endoscopic retrograde cholangiopancreatography; FCSEMS, fully covered self-expandable metallic stent.
More recently, new dedicated anchoring fully covered SEMSs (ACSEMSs) for PP have been developed, such as wide flared end (Fig. 4; (A, B) NAGI stent, Taewoong Medical Co., Ltd., Seoul, Korea,31 (C, D) BCF stent, M.I.Tech Co., Ltd., Seoul, Korea) or anchoring (Fig. 4; (E) AXIOS, Xlumena Inc., Mountain View, CA, USA)22 to prevent migration (Figs 5–7). These types of ACSEMS provide stent stability, minimize the risk of migration due to an anchoring effect, and maintain the larger SEMS lumen for passage, which may enable easy direct access into the PP cavity without a nonliquid component after expanding in full diameter.
Fig. 4.
(A, B) The new, fully-covered, self-expandable metallic stent (NAGI stent; Taewoong Medical Co., Ltd., Seoul, Korea). The NAGI stent consists of a fully-covered stent, 20-mm in length and 16-mm in diameter, with bilateral anchor flanges. The collapsible, braided stent is delivered through a 10.5-Fr catheter. The string is attached at the distal flange for stent removal. (C, D) The new, fully-covered, self-expandable metallic stent (BCF stent, M.I.Tech Co., Ltd., Seoul, Korea). The BCF stent consists of a fully-covered stent, 30- or 40-mm in length and 10-mm in diameter, with bilateral anchor flanges. The collapsible, braided stent is delivered through a 10.2-Fr catheter. The string is attached at the distal flange for stent removal. (E, F) The new, fully-covered, self-expandable metallic stent (AXIOS; Xlumena Inc., Mountain View, CA, USA). The AXIOS stent consists of a fully-covered, lumen-apposing stent, 6-, 8-, or 10-mm in length and 6-, 10-, or 15-mm in diameter, with dually-anchored flanges. The collapsible, braided stent is delivered through a 10.5-Fr catheter.
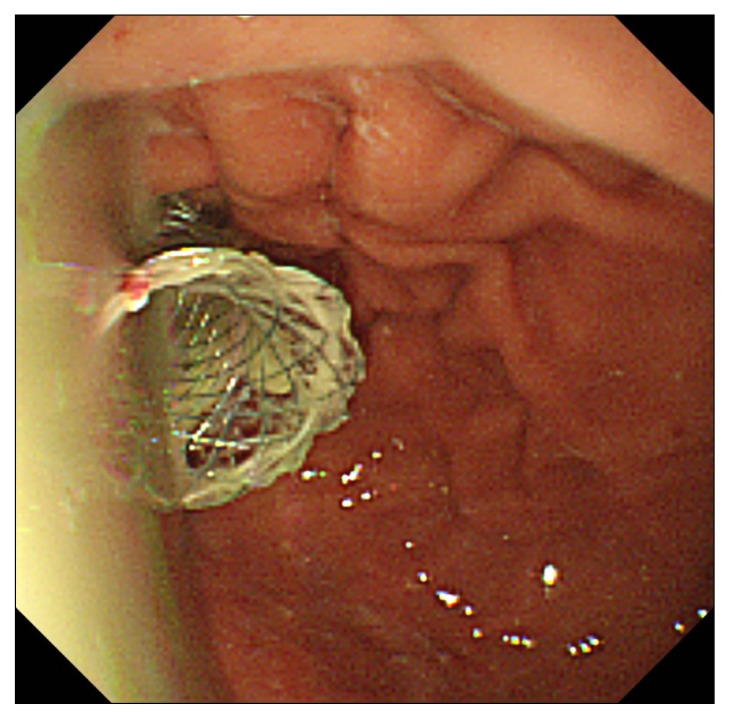
Fig. 5.
Endoscopic image showing a large amount of pus emerging from the NAGI stent (Taewoong Medical Co., Ltd., Seoul, Korea).
Fig. 7.
(A) EUS image showing AXIOS stent (Xlumena Inc., Mountain View, CA, USA) deployment. (B, C) Endoscopic image showing AXIOS stent during deployment. (D) Endoscopic image showing the endoscopic necrosectomy using the snare forceps through the AXIOS stent. stent placement
The question then is “what is the optimal stent for PP?” The answers to this question are straightforward. At the present, it is suggested that an ACSEMS like “yo-yo” shape22 is an ideal stent and is highly recommended for treating PP in terms of antimigration and the direct insertion of an endoscope through the ACSEMS. The stent anchors are designed to distribute pressure evenly on the luminal wall and securely anchor the stent, thus preventing migration. The proximal and distal anchor flanges are designed to hold the bile duct and duodenal wall in apposition, preventing leakage between the two nonadherent organs. Unfortunately, the ACSEMS is not available in Japan and Korea.
What remains controversial and yet to be determined are the appropriate period for stent placement and the optimal stent diameter. The recurrence of PFC requires further endoscopic, surgical, or percutaneous drainage. Stents for PFCs act as a conduit and facilitate drainage of pancreatic secretion from the disconnected gland. In a prospective randomized controlled trial involving the removal versus nonremoval of stents, the rate of PFC recurrence following stent removal was significantly higher, particularly in patients with main pancreatic duct rupture.34 It is likely that PFC resolution leads to the eventual adherence of the cavity wall, leading to the gradual migration of the stent toward the gastrointestinal lumen. Stent removal occurring before complete PFC collapse might lead to PFC recurrence, particularly if a communication exists between the PFC and the pancreatic duct.35 Prolonged transluminal stent placement has been adopted as a strategy to prevent PFC recurrence, that is, the stent remaining in its proper position reduces the recurrence rate of PFC.36 On the contrary, the appropriate duration of stent placement is recommended to be short (7 to 10 days) because of a significant risk of stent migration if the stents were left in place longer than 10 days.37 However, the short duration of stent placement may not be sufficient to create an adequately mature fistula tract that will consequently tolerate balloon dilation and direct endoscopic necrosectomy.28
CLINICAL IMPACT OF CSEMS FOR PFC TREATMENT
The clinical data on pancreatic abscess or infected WON are more limited and generally poor, owing to the need to remove abscess and necrotic debris, than in the case of PP drainage. EUS-TD for PP has recently become the preferred therapy. However, in collections with necrotic debris, the success rate falls with the drainage of cyst contents alone. Subsequent direct endoscopic necrosectomy has therefore been performed for an infected ANC, PP, or WON. We should consider direct endoscopic necrosectomy under the following conditions: 1) necrotizing pancreatitis is present; 2) US, EUS, CT, or magnetic resonance images show solid components in the fluid collection; and 3) acute inflammation suggesting an infected WON is present.38 Several sessions are necessary for sufficient necrosectomy to improve inflammation. For this technique, placement of multiple plastic stents and repeated large-diameter balloon dilatation are required in each session. Larger CSEMS allows further interventions using a conventional endoscope without multiple and repeated dilation.
Recently, a prospective randomized controlled trial of direct endoscopic drainage/necrosectomy of pancreatic abscess or infected WON versus surgical management has been performed.39 In this recent study involving patients with an infected WON, endoscopic necrosectomy reduced the proinflammatory response (serum interleukin-6) as well as the new-onset multiple organ failure, intra-abdominal bleeding requiring intervention, enterocutaneous fistula or perforation of a visceral organ requiring intervention and pancreatic fistula compared with surgical necrosectomy. In the study design, multiple plastic stenting for infected WON following repeated balloon dilation was performed. Therefore, large CSEMS was not used in that study.
A summary of studies reporting the use of CSEMS in 56 patients with pancreatic abscess or infected WONs is shown in Table 5.20,23,27,28,30,31,38,40–43 The technical success rate (100%, 57/57 pancreatic abscess or WONs) and the pancreatic abscess or infected WON complete resolution rate (87.8%, 43/49 pancreatic abscess or infected WONs) were high similarly to PPs. The complication rate was low (9.5%, 4/42 pancreatic abscess or infected WONs) compared with PPs. Larger diameter CSEMS without additional fistula tract dilation for the passage of a standard scope is needed to access and drain for pancreatic abscess or infected WONs with solid debris. During direct endoscopic necrosectomy through the CSEMS, such CSEMS interferes with the operation of the endoscope. On the other hand, a shorter SEMS is associated with a higher risk of migration. The SEMS length was selected on the basis of the size of the PP, pancreatic abscess or WON, with 1/3 to 1/2 of the SEMS protruding into the gastrointestinal tract at the level of the flared ends permitting apposition of the PP, pancreatic abscess or WON to the gastrointestinal tract.43 Commercially available biliary SEMSs neither offer a large diameter that allows a larger channel endoscope to be inserted in order to perform necrosectomy, nor permit complete apposition of the WONs to the wall of the gastrointestinal tract. Therefore, an anchoring FCSEMS particularly with a dumbbell shape is also strongly desired for treating infected ANC/PP or WONs.
Table 5.
Study Characteristics and Patient Outcome of Endoscopic Ultrasound-Guided Drainage of Pancreatic Abscess or Walled-Off Pancreatic Necrosis Using a Self-Expandable Metallic Stent
| A. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Author (yr) | Journal | No. of patients | Size, cm | Timing of treatment | No. of sessions | Technical success (%) | Resolution success (%) | Complete resolution | Time to resolution |
| Tarantino (2009)20 | Gastrointest Endosc | 1 | 18×15×3 | After multiple sessions | 1 | 1 (100) | 1 (100) | 1 (100) | 10 Days |
| Antillon (2009)40 | Gastrointest Endosc | 1 | NA | After multiple sessions | 1 | 1 (100) | 1 (100) | 1 (100) | NA |
| Tarantino (2010)41 | Pancreas | 1 | 17 | 2nd sessions | 2 (multiple gateways) | 1 (100) | 1 (100) | 1 (100) | 7 Days |
| Belle (2010)42 | Endoscopy | 4 | NA | Initial session | 1 | 4 (100) | 4 (100) | 4 (100) | NA |
| Fabbri (2012)23 | Endoscopy | 10 | 14.5 (average) | Initial session | 1 | 10 (100) | 7 (70) | 7 (70) | NA |
| Berzosa (2012)27 | Endoscopy | 2 | 5.9 (4.6–12) (average) | After multiple sessions | 2 (multiple gateways) | 2 (100) | 2 (100) | 2 (100) | 7 Weeks, 22 weeks |
| Weilert (2012)28 | Endoscopy | 8 | NA | Initial session | 1 | 8 (100) | NA | NA | NA |
| Itoi (2013)38 | J Hepatobiliary Pancreat Sci | 1 | NA | Initial session | 1 | 1 (100) | 1 (100) | 1 (100) | NA |
| Gornals (2013)30 | Surg Endosc | 5 | NA | After multiple sessions | 1 | 5 (100) | 5 (100) | 5 (100) | 3 Weeks |
| Yamamoto (2013)31 | Gastrointest Endosc | 4 | 20 (8–32) (average) | After multiple sessions | 1 | 4 (100) | 4 (100) | 4 (100) | NA |
| Sarkaria (2014)43 | J Clin Gastroenterol | 17 | 14.9±5.6 (8–29) (average) | Initial session | 1 | 17 (100) | 15 (88.2) | 15 (88.2) | NA |
| Total | 54 (56 WONs) | 100% (54/54 WONs) | 89.6% (43/48 WONs) | 89.6% (43/48 WONs) | |||||
| B. | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Author (yr) | GW size (inch) | Dilation devices | Type of SEMS | SEMS for use | Diameter/length (mm) | Name of SEMS | Company name of SEMS | Plastic stent/placement position for SEMS |
| Tarantino (2009)20 | 0.035 | Cystotome | PC | Bile duct | 10/40 | WALLSTENT™ Biliary RX Endoprosthesis | Boston Scientific | - |
| Antillon (2009)40 | NA | NA | NA | Esophagus | 22/70 | ALLIMAX-E Esophageal stent | Alveolus | Foley catheter and single PS (details unknown)/into |
| Tarantino (2010)41 | NA | Balloon | NA | NA | NA | NA | NA | - |
| Belle (2010)42 | NA | Needle knife/balloon | PC | Exclusive use | 18/60 or 20–25/50 | NA | NA | - |
| Fabbri (2012)23 | 0.035 | Needle knife | FC | Bile duct | 10/40 or 10/60 | WALLFLEX™ Biliary RX Endoprosthesis or Niti-S | Boston Scientific or Taewoong Medical Co., Ltd. | - |
| Berzosa (2012)27 | 0.035 | Needle knife /sphincterotome /balloon | FC | Bile duct | 10/60 or 10/70 or 10/80 or 10/100 | GORE® VIABIL® BILIARY ENDOPROSTHESIS | Conmed | - |
| Weilert (2012)28 | 0.035 | NAVIX | FC | Bile duct | 10/40 | WALLFLEX™ Biliary RX stent | Boston Scientific | - |
| Itoi (2013)38 | NA | Balloon | FC | Exclusive use | 10/40 | NAGI-stent | Taewoong Medical Co., Ltd. | - |
| Gornals (2013)30 | NA | NAVIX /balloon | FC | Exclusive use | 10/60 or 10/70 or 10/80 or 10/100 | GORE® VIABIL® BILIARY ENDOPROSTHESIS | Conmed | - |
| Yamamoto (2013)31 | NA | Balloon | FC | Exclusive use | 10/40 | WALLFLEX™ Biliary RX stent | Boston Scientific | PS (details NA)/into |
| Sarkaria (2014)43 | 0.035 | Balloon | FC | Esophagus | 10/100 | ALLIMAX-E Esophageal stent, Bonastanet®, WALLFLEX™ Biliary RX stent |
Alveolus, Standard Sci-Tech Inc., Boston Scientific | PS (details NA)/into |
| C. | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Author (yr) | Complication (%) | Details of complication | SEMS placement period | Recurrence | Convert to surgery (%) | Morbidity (%) | Observational period |
| Tarantino (2009)20 | - | - | 4 Weeks | - | - | - | 1 Month |
| Antillon (2009)40 | - | - | 2 Weeks | - | - | - | 2 Months |
| Tarantino (2010)41 | - | - | 15 Days | - | - | - | 1 Year |
| Belle (2010)42 | 1 (25) | Transitory obstruction 1 | NA | - | - | - | 4–147 Weeks |
| Fabbri (2012)23 | 1 (10) | Outward migration and sepsis 1 | 4 Weeks | NA | 1 (10) | - | NA |
| Berzosa (2012)27 | - | - | NA | - | - | - | 14.5 Weeks (average) |
| Weilert (2012)28 | NA | NA | 7–10 Days | - | NA | NA | NA |
| Itoi (2013)38 | - | - | 3 Weeks | - | - | - | NA |
| Gornals (2013)30 | - | - | NA | NA | - | - | NA |
| Yamamoto (2013)31 | 1 (25) | Bleeding 1 | 25–40 Days | - | 1 (25) | 1 (25) | NA |
| Sarkaria (2014)43 | 1 (5.9) | Perforation 1 | NA | NA | 2 (11.8) | NA | 237.6 Days (average) |
| Total | 9.5% (4/42) | 0% (0/22) | 9.5% (4/42) | 4% (1/25) | |||
NA, data not available; WON, walled-off pancreatic necrosis.
GW, guidewire; SEMS, self-expandable metallic stent; PC, partially covered; NA, data not available; PS, plastic stent; FC, fully covered.
SEMS, self-expandable metallic stent; NA, data not available.
TECHNICAL TIPS FOR DRAINAGE AND NECROSECTOMY OF TRICKY PFCs
The conventional single transluminal gateway drainage using transmural stenting (single or multiple plastic stents or large-bore SEMSs) has allowed the complete resolution of unilocural or uncomplicated PFCs. However, single gateway drainage for complicated or infected WONs is limited and often insufficient. Multilocular or huge infected WON requires multiple transluminal gateway drainage because of the presence of undrained subcavities.44–46 When subcavities or undrained areas of the main cavity are in a location far from the gastrointestinal tract, EUS-TD is not possible. Single transluminal gateway transcystic multiple drainage might be a better technique for these cases.45 If endoscopic intervention fails for complicated WON, the hybrid technique using endoscopic and percutaneous approaches is recommended and might be a better approach.
CONCLUSIONS
EUS-TD with SEMS placement for infected PP, pancreatic abscess or WONs is a technically feasible and apparently safe alternative to CTD and TD. EUS-TD with SEMS placement can be considered as the first-line therapy for PP. With increasing data showing better clinical outcome of EUS-TD with CSEMS, it is highly recommendable to conduct a prospective randomized controlled trial of plastic stent versus CSEMS for PP drainage to determine the long-term outcome and allow cost analysis. Finally, future clinical prospective studies should be conducted to validate local complications of acute pancreatitis on the basis of the revised Atlanta Classification.6
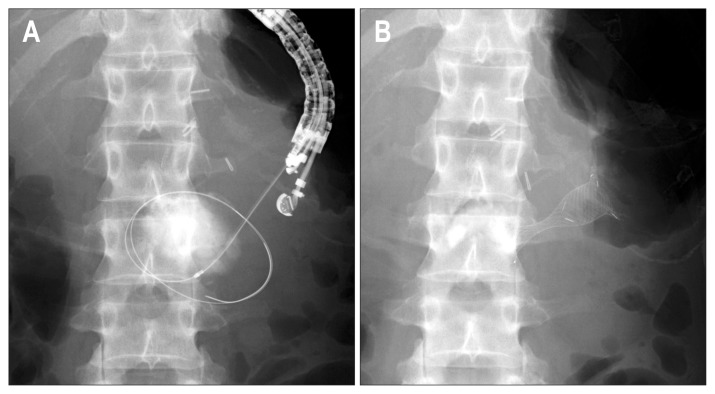
Fig. 2.
(A) Radiograph showing a double guidewire in the pancreatic pseudocyst. (B) After double guidewire placement, a double pigtail plastic stent was advanced into the pancreatic pseudocyst.
Fig. 6.
(A) Radiograph showing fistula dilation using a wire-guided 6-Fr diathermic dilator (Cysto-Gastro-Set; Endo-Flex, Voerde, Germany). (B) Radiograph showing the NAGI stent (Taewoong Medical Co., Ltd., Seoul, Korea) into the pancreatic pseudocyst.
AKCNOWLEDGEMENTS
The authors are indebted to Dr. Edward Barroga, Associate Professor and Senior Medical Editor of the Department of International Medical Communications of Tokyo Medical University for the editorial review of the manuscript.
Footnotes
CONFLICTS OF INTEREST
Drs. Kawakami and Itoi are consultants of Olympus Medical Systems Corporation, Tokyo, Japan, Taewoong Medical Co., Ltd., Seoul, Korea, and M.I.Tech Co., Ltd., Seoul, Korea. Dr. Kawakami is a consultant and gives lectures for Piolax Medical Devices, Kanagawa, Japan. Dr. Itoi gives lectures for Olympus and holds consultant and advisory board positions with Xlumena Inc., Mountain View, CA, USA. No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Baillie J. Pancreatic pseudocysts (Part I) Gastrointest Endosc. 2004;59:873–879. doi: 10.1016/S0016-5107(04)00354-2. [DOI] [PubMed] [Google Scholar]
- 2.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvanitakis M, Delhaye M, Chamlou R, et al. Endoscopic therapy for main pancreatic-duct rupture after Silastic-ring vertical gastro-plasty. Gastrointest Endosc. 2005;62:143–151. doi: 10.1016/S0016-5107(05)01627-5. [DOI] [PubMed] [Google Scholar]
- 4.Klöppel G. Pseudocysts and other non-neoplastic cysts of the pancreas. Semin Diagn Pathol. 2000;17:7–15. [PubMed] [Google Scholar]
- 5.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 6.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 7.Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–764. doi: 10.1053/gast.1996.v111.pm8780582. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson BC, Baron TH, Adler DG, et al. ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastro-intest Endosc. 2005;61:363–370. doi: 10.1016/S0016-5107(04)02779-8. [DOI] [PubMed] [Google Scholar]
- 9.Akshintala VS, Saxena P, Zaheer A, et al. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc. 2014;79:921–928. doi: 10.1016/j.gie.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogas-trostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583–590.e1. doi: 10.1053/j.gastro.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf TE, Baron TH. Endoscopic transmural drainage of pancreatic pseudocysts: results of a national and an international survey of ASGE members. Gastrointest Endosc. 2006;63:223–227. doi: 10.1016/j.gie.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Seewald S, Brand B, Groth S, et al. Endoscopic sealing of pancreatic fistula by using N-butyl-2-cyanoacrylate. Gastrointest Endosc. 2004;59:463–470. doi: 10.1016/S0016-5107(03)02708-1. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami H, Kuwatani M, Kawakubo K, et al. Transpapillary dilation of refractory severe biliary stricture or main pancreatic duct by using a wire-guided diathermic dilator (with video) Gastroin-test Endosc. 2014;79:338–343. doi: 10.1016/j.gie.2013.07.055. [DOI] [PubMed] [Google Scholar]
- 14.Park DH, Lee SS, Moon SH, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudo-cysts: a prospective randomized trial. Endoscopy. 2009;41:842–848. doi: 10.1055/s-0029-1215133. [DOI] [PubMed] [Google Scholar]
- 15.Panamonta N, Ngamruengphong S, Kijsirichareanchai K, Nugent K, Rakvit A. Endoscopic ultrasound-guided versus conventional transmural techniques have comparable treatment outcomes in draining pancreatic pseudocysts. Eur J Gastroenterol Hepatol. 2012;24:1355–1362. doi: 10.1097/MEG.0b013e32835871eb. [DOI] [PubMed] [Google Scholar]
- 16.Seewald S, Ang TL, Teng KC, Soehendra N. EUS-guided drainage of pancreatic pseudocysts, abscesses and infected necrosis. Dig Endosc. 2009;21( Suppl 1):S61–S65. doi: 10.1111/j.1443-1661.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- 17.Fabbri C, Luigiano C, Maimone A, Polifemo AM, Tarantino I, Cennamo V. Endoscopic ultrasound-guided drainage of pancreatic fluid collections. World J Gastrointest Endosc. 2012;4:479–488. doi: 10.4253/wjge.v4.i11.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singhal S, Rotman SR, Gaidhane M, Kahaleh M. Pancreatic fluid collection drainage by endoscopic ultrasound: an update. Clin Endosc. 2013;46:506–514. doi: 10.5946/ce.2013.46.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, Kahaleh M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video) Gastrointest Endosc. 2008;68:1199–1203. doi: 10.1016/j.gie.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Tarantino I, Barresi L, Fazio V, Di Pisa M, Traina M. EUS-guided self-expandable stent placement in 1 step: a new method to treat pancreatic abscess. Gastrointest Endosc. 2009;69:1401–1403. doi: 10.1016/j.gie.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Penn DE, Draganov PV, Wagh MS, Forsmark CE, Gupte AR, Chauhan SS. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 2012;76:679–684. doi: 10.1016/j.gie.2012.04.457. [DOI] [PubMed] [Google Scholar]
- 22.Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastroin-test Endosc. 2012;75:870–876. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Fabbri C, Luigiano C, Cennamo V, et al. Endoscopic ultrasound-guided transmural drainage of infected pancreatic fluid collections with placement of covered self-expanding metal stents: a case series. Endoscopy. 2012;44:429–433. doi: 10.1055/s-0031-1291624. [DOI] [PubMed] [Google Scholar]
- 24.Tarantino I, Di Pisa M, Barresi L, Curcio G, Granata A, Traina M. Covered self expandable metallic stent with flared plastic one inside for pancreatic pseudocyst avoiding stent dislodgement. World J Gastrointest Endosc. 2012;4:148–150. doi: 10.4253/wjge.v4.i4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarantino I, Traina M, Barresi L, Di Pisa M, Curcio G, Granata A. Treatment of infected pancreatic pseudocysts using a novel, dedicated covered self-expandable metal stent (CSEMS) with an effective antimigration system. Endoscopy. 2012;44(Suppl 2):UCTN:E147–E148. doi: 10.1055/s-0031-1291570. [DOI] [PubMed] [Google Scholar]
- 26.Barresi L, Tarantino I, Curcio G, Granata A, Traina M. Buried stent: new complication of pseudocyst drainage with self-expandable metallic stent. Dig Endosc. 2012;24:285. doi: 10.1111/j.1443-1661.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- 27.Berzosa M, Maheshwari S, Patel KK, Shaib YH. Single-step endoscopic ultrasonography-guided drainage of peripancreatic fluid collections with a single self-expandable metal stent and standard linear echoendoscope. Endoscopy. 2012;44:543–547. doi: 10.1055/s-0031-1291710. [DOI] [PubMed] [Google Scholar]
- 28.Weilert F, Binmoeller KF, Shah JN, Bhat YM, Kane S. Endoscopic ultrasound-guided drainage of pancreatic fluid collections with indeterminate adherence using temporary covered metal stents. Endoscopy. 2012;44:780–783. doi: 10.1055/s-0032-1309839. [DOI] [PubMed] [Google Scholar]
- 29.Gornals JB, Loras C, Mast R, Botargues JM, Busquets J, Castellote J. Endoscopic ultrasound-guided transesophageal drainage of a mediastinal pancreatic pseudocyst using a novel lumen-apposing metal stent. Endoscopy. 2012;44(Suppl 2):UCTN:E211–E212. doi: 10.1055/s-0032-1309384. [DOI] [PubMed] [Google Scholar]
- 30.Gornals JB, De la Serna-Higuera C, Sánchez-Yague A, Loras C, Sánchez-Cantos AM, Pérez-Miranda M. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013;27:1428–1434. doi: 10.1007/s00464-012-2591-y. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto N, Isayama H, Kawakami H, et al. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc. 2013;77:809–814. doi: 10.1016/j.gie.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Téllez-Ávila FI, Villalobos-Garita A, Ramírez-Luna MÁ. Use of a novel covered self-expandable metal stent with an anti-migration system for endoscopic ultrasound-guided drainage of a pseudocyst. World J Gastrointest Endosc. 2013;5:297–299. doi: 10.4253/wjge.v5.i6.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxena P, Kumbhari V, Khashab MA. EUS-guided drainage of a giant hemorrhagic pseudocyst by a through-the-scope esophageal metal stent. Gastrointest Endosc. 2014;79:202–203. doi: 10.1016/j.gie.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Arvanitakis M, Delhaye M, Bali MA, et al. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007;65:609–619. doi: 10.1016/j.gie.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 35.Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080–2088. doi: 10.1007/s11605-011-1621-8. [DOI] [PubMed] [Google Scholar]
- 36.Onodera M, Kawakami H, Kuwatani M, et al. Endoscopic ultrasound-guided transmural drainage for pancreatic fistula or pancreatic duct dilation after pancreatic surgery. Surg Endosc. 2012;26:1710–1717. doi: 10.1007/s00464-011-2097-z. [DOI] [PubMed] [Google Scholar]
- 37.Binmoeller KF. EUS-guided drainage of pancreatic fluid collections using fully covered self-expandable metal stents. Gastroenterol Hepatol (N Y) 2013;9:442–444. [PMC free article] [PubMed] [Google Scholar]
- 38.Itoi T, Nageshwar Reddy D, Yasuda I. New fully-covered self-expandable metal stent for endoscopic ultrasonography-guided intervention in infectious walled-off pancreatic necrosis (with video) J Hepatobiliary Pancreat Sci. 2013;20:403–406. doi: 10.1007/s00534-012-0551-5. [DOI] [PubMed] [Google Scholar]
- 39.Bakker OJ, van Santvoort HC, van Brunschot S, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053–1061. doi: 10.1001/jama.2012.276. [DOI] [PubMed] [Google Scholar]
- 40.Antillon MR, Bechtold ML, Bartalos CR, Marshall JB. Transgastric endoscopic necrosectomy with temporary metallic esophageal stent placement for the treatment of infected pancreatic necrosis (with video) Gastrointest Endosc. 2009;69:178–180. doi: 10.1016/j.gie.2008.03.1066. [DOI] [PubMed] [Google Scholar]
- 41.Tarantino I, Traina M, Barresi L, Volpes R, Gridelli B. Transgastric plus transduodenal necrosectomy with temporary metal stents placement for treatment of large pancreatic necrosis. Pancreas. 2010;39:269–270. doi: 10.1097/MPA.0b013e3181bb9636. [DOI] [PubMed] [Google Scholar]
- 42.Belle S, Collet P, Post S, Kaehler G. Temporary cystogastrostomy with self-expanding metallic stents for pancreatic necrosis. Endoscopy. 2010;42:493–495. doi: 10.1055/s-0029-1244021. [DOI] [PubMed] [Google Scholar]
- 43.Sarkaria S, Sethi A, Rondon C, et al. Pancreatic necrosectomy using covered esophageal stents: a novel approach. J Clin Gastroenterol. 2014;48:145–152. doi: 10.1097/MCG.0b013e3182972219. [DOI] [PubMed] [Google Scholar]
- 44.Varadarajulu S, Phadnis MA, Christein JD, Wilcox CM. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011;74:74–80. doi: 10.1016/j.gie.2011.03.1122. [DOI] [PubMed] [Google Scholar]
- 45.Mukai S, Itoi T, Sofuni A, et al. Novel single transluminal gateway transcystic multiple drainages after EUS-guided drainage for complicated multilocular walled-off necrosis (with videos) Gastrointest Endosc. 2014;79:531–535. doi: 10.1016/j.gie.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Mukai S, Itoi T, Sofuni A, et al. Expanding endoscopic interventions for pancreatic pseudocyst and walled-off necrosis. J Gastroenterol. Epub 2014 Apr 24. http://dx.doi.org/10.1007/s00535-014-0957-8. [DOI] [PubMed]