Abstract
Background/Aims
High intensity focused ultrasound (HIFU) utilizes a targeted extracorporeal focused ultrasound beam to ablate neoplastic pancreatic tissue. We used an in vitro model to examine the effects of bone, metallic stents, plastic stents, metal plates, and cyst-like lesions on HIFU treatment.
Methods
HIFU was delivered to the phantom models implanted with foreign bodies, and the location, shape, and size of the ablated zones were evaluated.
Results
Bone and metallic plates reflected the ultrasound beam, shifting the ablation zone from the focal zone to the prefocal area. In the phantoms containing metal stent, plastic stent, and cyst, most of the ablative energy was reflected to the prefocal area by the surface, with the remainder penetrating through the phantom. The area of the ablated margins was significantly larger in size and volume than the intended focal ablation zone.
Conclusions
During HIFU therapy, artificial or anatomical barriers could affect the direction of the ultrasound beams, shifting the ablation zone from the focal area to a prefocal site with a larger than expected ablation zone. These factors should be considered prior to HIFU treatment for pancreatic tumors because they could limit ablation success, in addition to causing complications.
Keywords: High-intensity focused ultrasound ablation, Cysts, Metal stent, Phantom
INTRODUCTION
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related deaths in the United States.1 Patients with pancreatic cancer show very poor prognosis with an overall 1-, 3-, and 5-year survival rates of 15%, 5%, and 4%, respectively in the EUROCARE study.1 Although surgical resection provides the best opportunity for cure, only 10% to 20% of these patients present initially with potentially resectable tumors whilst the majority with inoperable locally advanced or metastatic disease.2
Treatment of locally advanced pancreatic cancer is quite challenging to most clinicians. Many studies have evaluated local ablation therapies, including cryosurgery,3,4 radiofrequency ablation therapy,5 and high intensity focused ultrasound (HIFU) therapy.6,7 HIFU is a noninvasive extracorporeal therapy that uses ultrasound beams with short wavelengths and MHz frequencies directed to small volume target areas creating focal ablation. The absorption of high intensity acoustic energy by the target tissues generates thermal heat giving rise to coagulation necrosis. It is an image-guided treatment modality that allows for accurate targeting focused into a small area provided there is an acoustic window to allow propagation of the ultrasound beams.
Recent HIFU applications have focused on tumors located in the solid organs of the abdomen.8–12 There have been several case series reporting on the use of HIFU to treat patients with pancreatic adenocarcinoma. In general, these reports suggest that HIFU is an effective therapy for palliation of pain and in some cases there were significant reductions of tumor load with an objective response rates ranging from 14.6% to 74%.13–16
However, as the pancreas is a retroperitoneal organ located adjacent to important major organs and blood vessels, potential complications could arise as a result of reflection, refraction, and absorption of high intensity acoustic energy as it propagates through the acoustic window. In addition, the presence of bones or gas pockets located in the ultrasound beam path around the target area could lead to collateral thermal tissue damage due to the increase in energy delivered to the interface of soft tissue with air or bone.17,18 Similar consequences may be seen in the presence of metallic or plastic stents inserted for complicated locally advanced pancreatic cancers.
Hence, these external factors could potentially interfere with the propagation path of the HIFU beam hindering treatment success. Therefore, we used an in vitro model to evaluate the influence of these external factors during HIFU tumor ablation.
MATERIALS AND METHODS
1. Phantom fabrication
Phantoms were constructed from polyacrylamide gel mixed with bovine serum albumin (BSA), a protein used as a temperature-sensitive indicator.19 Polyacrylamide gels were made by linking copolymerization of acrylamide and N, N-methylene bisacrylamide in an aqueous medium.19 The mixture comprised of 7% acrylamide and 9% BSA. The phantoms were transparent at room temperature. Following HIFU treatment, it would show a localized well-defined opaque lesion as a result of BSA denaturation. The formation of the opaque lesions could also be observed in real-time as hyperechoic areas in B mode ultrasound images.
To simulate in vivo condition, either bone, metal plate, metal stent, plastic stent, or a rubber balloon filled with distilled water to mimic a cyst was inserted into the phantoms. Integrated B mode ultrasound was used to evaluate optically denatured areas and to measure the three-dimensional axes of the opaque lesions formed after HIFU ablation.
2. HIFU treatment system
The FEP-BY02 HIFU system (YDME, Beijing, China) was used for this experiment and has two HIFU transducers; a lower and an upper transducer. We used only the upper HIFU transducer, which is constructed of 251 individual lead zirconate titanate crystal elements and driven in phase at 1 MHz, together with an integrated B mode ultrasound imaging probe (Logiq 5; General Electric Healthcare, Seongnam, Korea). The HIFU transducer had an aperture of 37 cm and a focal distance of 25.5 cm. The focus has a −6 dB beam width of 1.6 mm and axial length of 10 mm. Treatment was delivered using a computer program that allows the operator to identify the target region and specify the electrical power delivered by the HIFU transducer as well as to input the pulse length, duty factor, interval spacing between treatment sites and number of pulses per treatment site.
Therefore we standardized and controlled the main treatment parameters by delivering HIFU treatment at a power of 400 W, a 60 pulse per treatment spot, a unit transmission time of 150 ms, and an intermission time of 150 ms. The size of the target area was set at 13.4×13.4×15.2 mm. This study was approved by the Institutional Review Board at Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
3. Statistical analysis
Mean±SD of the X, Y, and Z axes were used for descriptive statistics. Size increments following HIFU ablation were assessed using the Wilcoxon signed rank test statistical analysis for paired data. All statistical analyses were performed using the SPSS version 18.0 statistical package (IBM Co., Armonk, NY, USA).
RESULTS
1. Ablation shapes and focal zone displacement
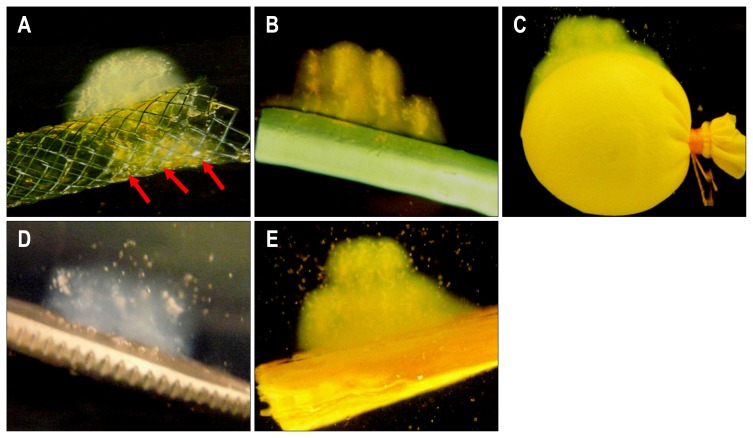
This study revealed that ultrasound beam penetration was affected by the presence of bone, metal plate, metal stent, plastic stent, and cyst-like lesion, hence altering and causing displacement of the focal ablation zone. In phantoms containing metal stents (Fig. 1A), the ultrasound beam was reflected by the metallic mesh and accumulated largely in the prefocal zone. A small fraction of the ultrasound beam penetrated into the metallic membrane, forming a small ablated zone in the focal area within the lumen (arrows). In phantoms containing plastic stents, most of the ultrasound beam was integrated in the prefocal zone (Fig. 1B). In phantoms containing cysts-like lesion, HIFU ablation was reflected mainly onto the surface surrounding the cyst resulting in a wide elliptical shaped ablation zone (Fig. 1C). Meanwhile, in phantoms containing metal plates (Fig. 1D) and bones (Fig. 1E), most of the ultrasound beam was reflected and caused shifting of the ablation zone from the focal area to the prefocal area. In all models, there were no ablated lesions in the postfocal zone.
Fig. 1.
(A) Ablation shapes and focal zone movement. The ultrasound beam partially penetrated into the metal stent (arrows). High-intensity focused ultrasound energy was mainly reflected onto the surface of the (B) plastic stent and (C) cyst model. The ultrasound beam reflected from the surface of the (D) bone and (E) metal was integrated entirely in the prefocal zone, whereas the ablation occurred in the opposite direction.
2. Areas of ablation
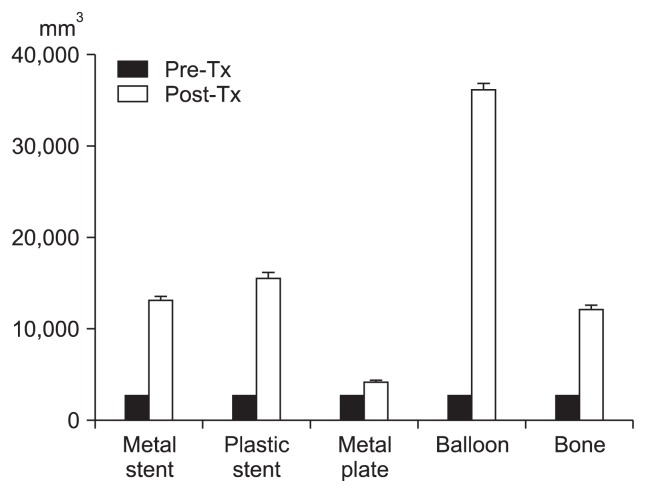
In all phantom models, HIFU treatment resulted in a larger volume of ablation than the intended target volume (Fig. 2). The presence of a metal stent, plastic stent, metal plate, cyst-like lesion, and bone also significantly increased the sizes and three-dimensional axes values beyond the targeted ablation focus (Table 1).
Fig. 2.
Volumetric changes between the pretreatment (pre-Tx) target zone and posttreatment (post-Tx) ablation zone.
Table 1.
Size of the Target and Ablation Zones in Various Phantom Models (n=10 per model)
| Target zone, mm | Ablation zone, mm | Size difference (post-Tx–pre-Tx), mm | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| X | Y | Z | X | Y | Z | X, p-value* | Y, p-value* | Z, p-value* | |
| Metal stent | 13.61±0.49 | 13.61±0.49 | 15.27±0.28 | 23.83±9.94 | 24.18±12.35 | 19.23±2.97 | 12.02, 0.017 | 14.54, 0.093 | 4.62, 0.009 |
| Plastic stent | 13.43±0.04 | 13.43±0.04 | 15.25±0.31 | 27.27±8.52 | 27.02±9.32 | 19.34±3.30 | 15.57, 0.007 | 13.59, 0.005 | 4.09, 0.005 |
| Metal plate | 13.65±0.47 | 13.65±0.47 | 15.36±0.93 | 16.13±7.09 | 16.08±7.46 | 21.92±10.04 | 3.38, 0.008 | 3.24, 0.005 | 6.18, 0.333 |
| Cyst | 13.40±0 | 13.40±0 | 15.20±0 | 36.32±2.360 | 35.47±1.799 | 28.10±3.097 | 22.92, 0.005 | 22.07, 0.005 | 12.9, 0.005 |
| Bone | 12.26±3.78 | 12.25±3.78 | 13.79±4.21 | 21.70±10.95 | 21.71±10.09 | 21.16±8.16 | 10.61, 0.012 | 10.58, 0.009 | 7.37, 0.005 |
Data are presented as mean±SD.
Tx, treatment.
Wilcoxon signed rank test (p<0.05).
DISCUSSION
HIFU offers tremendous potential as an ablative treatment modality for solid organ tumors using focused extracorporeal ultrasound beams.12 HIFU is noninvasive and thought to be a safe therapy. However, there are certain limitations and risks associated with its use. The high intensity ultrasound beams could produce burns in the tissues that lie between the transducer and the target area. Complications arose as a result of reflection, refraction, or absorption of the acoustic energy along the beam pathway or adjacent to the target ablation zone.8 Thermal injury could occur at either the shallower areas of the target lesion or unwanted deep penetration over the target area.
In this study, the presence of biliary, pancreatic and even duodenal stents acts as external factors that could contribute to a significant role in planning for HIFU treatment. Metal stents have a metallic mesh composed of nitinol, whereas plastic stents are made of polytetrafluoroethylene. This study revealed that the nitinol metallic mesh reflected ultrasound energy to the pre-focal zone, with a small fraction of this energy passing through. Likewise, the phantom models with the plastic stents showed similar prefocal zone ablation whilst the cyst-like lesions which are fluid-filled cavities reflected the acoustic energy creating an elliptical ablation zone around the cyst surface. As expected, both the metal and bone fragments inserted in the phantoms impeded and reflected the HIFU energy beam resulting in a pre-focal area of ablation.
In all cases, the area of ablation was significantly greater in volume and three-dimensional axes dimension than the intended target volume area. Similarly, this is a result of reflection and refraction of acoustic energy beyond and away from the focal area resulting in a dispersed and widened area of ablation to the prefocal zones.
Therefore, present study demonstrates the significance of external factors that can impede the propagation of acoustic energy and shift the ablation area to the prefocal region giving rise to untargeted heating and potentially undesirable adjacent tissue thermal injury. In clinical practice, the anatomical considerations that would have similar interaction with the ultrasound beams include the skin, soft tissue with variable attenuation and densities, i.e., peritoneum, pleura, or abdominal wall, the interface reflection between soft tissue with air in the bowel or stomach, vertebra especially with tumors located near the vertebral column and the rib cage. Hwang et al.20 describes that the treatment area must be well visualized on pretreatment ultrasound images for treatment to proceed, otherwise, collateral damage is typically observed around the intended treatment region. The observation that pre-treatment image planning can be used to predict changes in the HIFU lesion production and to prevent collateral damge is important.20 Thereby, it is imperative that careful patient selection and preoperative evaluation should be carried out with great care prior to HIFU.
Another factor that play a significant role is the aperture of the transducer used to deliver HIFU ablation. A transducer with a wide aperture would reduce the damage to surrounding soft tissue and allow higher acoustic energy to be concentrated to the ablation focus. However, the large acoustic window required for a wider aperture is more likely to encounter interference or anatomical barriers. While a narrower and smaller aperture reduces the possible obstacle to the energy pathway, the concentrated ultrasound beam has a very high thermal ablation energy delivered to a small focal point that may give a rise to higher complication rates and possibly induce intraprocedural pain.
Although phantom models do not reflect the true in vivo clinical picture, this study suggests that the presence of artificial and anatomical structures could affect the propagation of the ultrasound beams altering the target ablation focus. Careful evaluation and attention to these factors during the planning stage of HIFU for patients with locally advanced pancreatic tumors may avert major harmful complications.
ACKNOWLEDGEMENTS
This study was supported by grant (2010–201) from Asan Institute for Life Sciences and grant (2E21250-09-091) from Korea Institute of Science & Technology.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Faivre J, Forman D, Estève J, Obradovic M, Sant M. Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe: EUROCARE Working Group. Eur J Cancer. 1998;34:2184–2190. doi: 10.1016/S0959-8049(98)00330-X. [DOI] [PubMed] [Google Scholar]
- 2.Cardenes HR, Chiorean EG, Dewitt J, Schmidt M, Loehrer P. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist. 2006;11:612–623. doi: 10.1634/theoncologist.11-6-612. [DOI] [PubMed] [Google Scholar]
- 3.Kovach SJ, Hendrickson RJ, Cappadona CR, et al. Cryoablation of unresectable pancreatic cancer. Surgery. 2002;131:463–464. doi: 10.1067/msy.2002.121231. [DOI] [PubMed] [Google Scholar]
- 4.Korpan NN. Cryosurgery: ultrastructural changes in pancreas tissue after low temperature exposure. Technol Cancer Res Treat. 2007;6:59–67. doi: 10.1177/153303460700600202. [DOI] [PubMed] [Google Scholar]
- 5.Varshney S, Sewkani A, Sharma S, et al. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP. 2006;7:74–78. [PubMed] [Google Scholar]
- 6.Wang X, Sun J. High-intensity focused ultrasound in patients with late-stage pancreatic carcinoma. Chin Med J. 2002;115:1332–1335. [PubMed] [Google Scholar]
- 7.Wu F, Wang ZB, Zhu H, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236:1034–1040. doi: 10.1148/radiol.2362041105. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol. 2003;76:590–599. doi: 10.1259/bjr/17150274. [DOI] [PubMed] [Google Scholar]
- 9.McDannold N, Tempany CM, Fennessy FM, et al. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology. 2006;240:263–272. doi: 10.1148/radiol.2401050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebillard X, Gelet A, Davin JL, et al. Transrectal high-intensity focused ultrasound in the treatment of localized prostate cancer. J Endourol. 2005;19:693–701. doi: 10.1089/end.2005.19.693. [DOI] [PubMed] [Google Scholar]
- 11.Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226:897–905. doi: 10.1148/radiol.2271020395. [DOI] [PubMed] [Google Scholar]
- 12.Wu F. Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim Invasive Ther Allied Technol. 2006;15:26–35. doi: 10.1080/13645700500470124. [DOI] [PubMed] [Google Scholar]
- 13.Xie DR, Chen D, Teng H. A multicenter non-randomized clinical study of high intensity focused ultrasound in treating patients with local advanced pancreatic carcinoma. Chin J Clin Oncol. 2003;30:630–634. [Google Scholar]
- 14.Xiong LL, He CJ, Yao SS, et al. The preliminary clinical results of the treatment for advanced pancreatic carcinoma by high intensity focused ultrasound. Chin J Gen Surg. 2005;16:345–347. [Google Scholar]
- 15.Xu YQ, Wang GM, Gu YZ, Zhang HF. The acesodyne effect of high intensity focused ultrasound on the treatment of advanced pancreatic carcinoma. Clin Med J China. 2003;10:322–323. [Google Scholar]
- 16.Yuan C, Yang L, Yao C. Observation of high intensity focused ultrasound treating 40 cases of pancreatic cancer. Chin J Clin Hep. 2003;19:145. [Google Scholar]
- 17.Myers MR. Transient temperature rise due to ultrasound absorption at a bone/soft-tissue interface. J Acoust Soc Am. 2004;115:2887–2891. doi: 10.1121/1.1707091. [DOI] [PubMed] [Google Scholar]
- 18.Hynynen K. Hot spots created at skin-air interfaces during ultrasound hyperthermia. Int J Hyperthermia. 1990;6:1005–1012. doi: 10.3109/02656739009140983. [DOI] [PubMed] [Google Scholar]
- 19.Lafon C, Zderic V, Noble ML, et al. Gel phantom for use in high-intensity focused ultrasound dosimetry. Ultrasound Med Biol. 2005;31:1383–1389. doi: 10.1016/j.ultrasmedbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Hwang JH, Wang YN, Warren C, et al. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol. 2009;35:967–975. doi: 10.1016/j.ultrasmedbio.2008.12.006. [DOI] [PubMed] [Google Scholar]