Diabetic nephropathy (DN), which is a major end-organ complication in diabetes, continues to be the most common cause of end-stage renal disease and accounts for >40% of patients on renal replacement therapy (1). Currently available factors that are routinely used in clinical practice to predict and monitor the progression of DN include degree of proteinuria and both glycemic and blood pressure control. In this issue of Diabetes, Riphagen et al. (2) performed a post hoc analysis of two large clinical trials—Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan (RENAAL) and Irbesartan Diabetic Nephropathy Trial (IDNT)—and demonstrated that serum bilirubin levels are inversely correlated with progression of DN. These findings are clinically important because they identify a potential biomarker and/or therapeutic target for DN, a disease that causes significant morbidity and mortality.
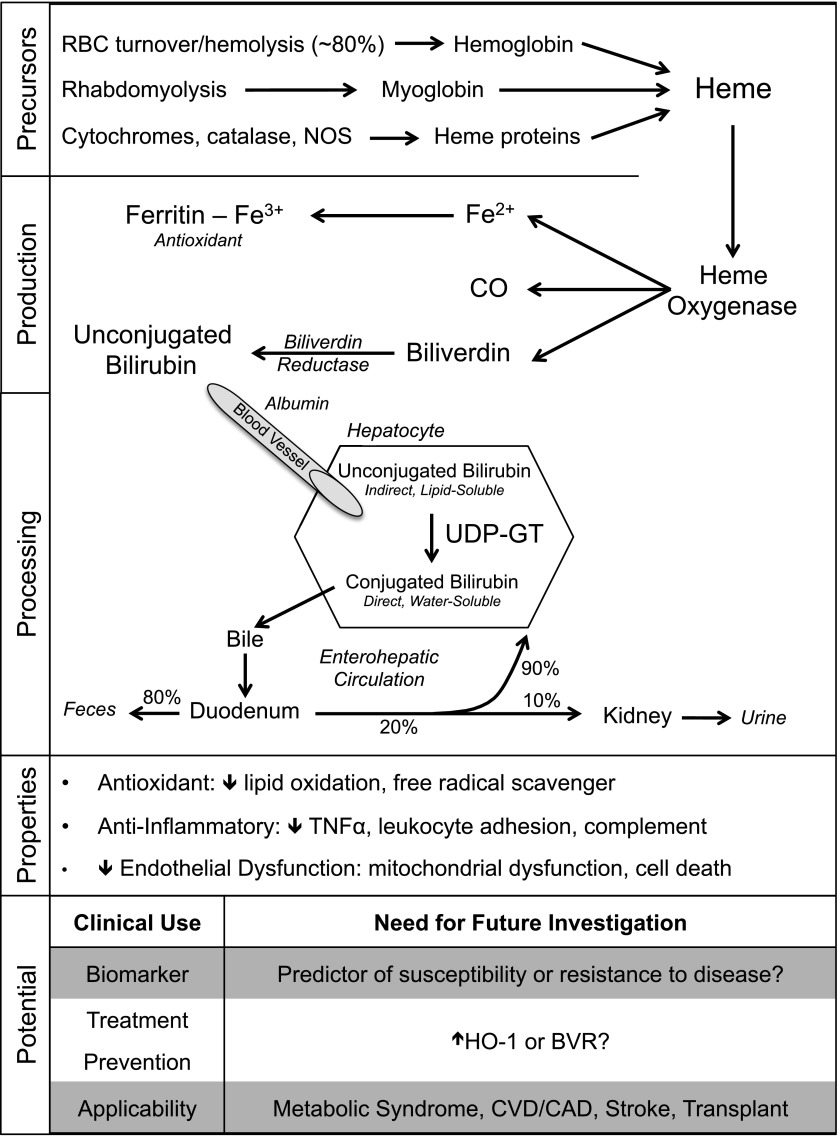
Bilirubin is generated when heme oxygenase (HO) catalyzes the degradation of heme (derived from heme proteins). This results in formation of biliverdin, which is rapidly converted into bilirubin by biliverdin reductase (Fig. 1) (3). Further processing of bilirubin occurs in hepatocytes, where unconjugated (lipid-soluble) bilirubin is conjugated by uridine diphosphate–glucuronosyl transferase (UDP-GT) to a water-soluble form for excretion. Total bilirubin is the sum of conjugated (direct) and unconjugated (indirect) bilirubin and generally ranges from 0.3 to 1.2 mg/dL in healthy individuals. In conditions such as erythroblastosis fetalis, hemolysis results in markedly elevated bilirubin, which causes kernicterus and neurological damage in neonates. Furthermore, genetic deficiency of UDP-GT in Crigler-Najjar syndrome type I results in severely elevated total bilirubin and is incompatible with life. Given the outcomes of these conditions, it is not surprising that bilirubin was long considered to be merely a toxic byproduct of heme degradation. However, unconjugated bilirubin levels are positively correlated with plasma antioxidant capacity (4), and moderate elevations in serum total bilirubin have been associated with reduced susceptibility to several common diseases (Table 1). Although a protective role for bilirubin in kidney disease has been established in animal studies (5,6), human data are lacking in this area.
Figure 1.
Schematic of bilirubin generation and conjugation in the liver. The physiological properties of bilirubin may explain its ability to protect against the progression of DN. These properties make bilirubin a potential clinical biomarker or therapeutic target for a variety of disease states. BVR, biliverdin reductase; CAD, coronary artery disease; CO, carbon monoxide; CVD, cardiovascular disease; Fe2+, ferrous iron; Fe3+, ferric iron; NOS, nitric oxide synthase; RBC, erythrocyte; TNFα, tumor necrosis factor-α.
Table 1.
Clinical conditions associated with serum bilirubin levels
| Condition | Strength of evidence |
|---|---|
| Cardiovascular disease | |
| Atherosclerosis | +++ |
| In-stent restenosis | + |
| Cerebral vascular disease (stroke) | ++ |
| Metabolic syndrome | ++ |
| Diabetes complications | |
| DN | ++ |
| Retinopathy | + |
| Chronic kidney disease | + |
| Transplant rejection | + |
| Autoimmunity | |
| Systemic lupus erythematosus | + |
| Rheumatoid arthritis | + |
Riphagen et al. (2) investigated the association between serum bilirubin levels and progression of DN in a post hoc analysis of 1,498 patients in the RENAAL trial (7). Data from this study were then independently verified using the IDNT (8). In both trials, the renal end point was examined over a ≥2.5-year follow-up period and defined as a doubling of serum creatinine or end-stage renal disease requiring dialysis or transplantation. The authors’ findings demonstrate, for the first time, a significant and graded inverse association between baseline total bilirubin levels and progression of DN in patients between 30 and 70 years of age. This association remained significant after adjustment for potentially confounding patient characteristics and risk factors for renal disease, and it was independent of treatment with placebo or angiotensin receptor blocker. While serum total bilirubin levels decreased significantly from baseline during the first year of the study, this change was not associated with progression of DN and may be explained by the observed concomitant decrease in serum hemoglobin levels, an observation that was exacerbated by angiotensin receptor blocker therapy (losartan, RENAAL; irbesartan, IDNT) relative to control (2). These findings suggest that measurement of bilirubin levels may identify subjects at risk for progression of DN.
Confirmation of initial findings using the IDNT and the relatively large sample size strengthens the findings reported by Riphagen et al. However, several limitations inherent to the design of this study deserve mention. Given that the authors were constrained to the data gathered in the RENAAL trial and the IDNT, only total bilirubin levels in a narrow range (<1.5 times the upper limit of normal) were studied. To translate these findings to clinical practice, future studies are needed to define an optimal range of total bilirubin in which progression of DN is prevented. In addition, only total bilirubin levels were measured, without distinguishing between conjugated versus unconjugated bilirubin. This is particularly important since conjugation of bilirubin appears to significantly affect its functional characteristics. Moderate unconjugated hyperbilirubinemia (ranging between 1.2 and 6.0 mg/dL) that is seen in patients with Gilbert syndrome confers protection from cardiovascular disease (9). Such a protective effect has not been reported in patients with conjugated hyperbilirubinemia caused by obstructive jaundice or genetic deficiencies such as Dubin-Johnson syndrome (4,10). An additional limitation of this study is that its design limits the ability to establish a causal link between serum total bilirubin and protection from progression of DN.
Nonetheless, this report raises questions about how bilirubin is generated and why its concentration in serum varies between individuals. The HO enzyme system, comprised of two isoforms, HO-1 and HO-2, degrades the noxious pro-oxidant heme moiety, resulting in the production of biliverdin, carbon monoxide, and iron (11). Biliverdin is converted to bilirubin by biliverdin reductase, an enzyme that has key roles in cell signaling via the protein kinase C pathway (12). It has been suggested that individuals with higher HO-1 expression also have increased total bilirubin (13). Therefore, whether elevated serum bilirubin directly provides protection from disease or is simply a marker of increased HO-1 expression remains to be verified experimentally. While HO-2 is expressed constitutively, HO-1 expression is induced by numerous stimuli that impose cellular oxidative stress. Genetic polymorphisms in the human HO-1 proximal promoter—specifically, length variations in a GT repeat region—affect basal and inducible HO-1 expression and correlate with several diseases, including chronic kidney disease (14). Animal models have demonstrated that increased HO-1 expression confers protection in many diseases. Several ongoing clinical trials targeting the HO-1 pathway are focused on diseases where an association between increased serum bilirubin levels and protection from disease was demonstrated (15,16) (Table 1). It is important to underscore that similar to the underlying vasculopathy in diabetes complications, many diseases that are associated with bilirubin levels are also mediated by vascular injury (Table 1). This commonality among diverse pathologies supports a protective role for bilirubin at the level of the vasculature—the endothelium and/or vascular smooth muscle.
In diabetes, the protective properties of bilirubin are likely due to its potent antioxidant properties (Fig. 1). Bilirubin inhibits lipid peroxidation and attenuates LDL oxidation (17). Chronic hyperglycemia results in the generation of reactive oxygen species (e.g., superoxide), predominantly by the mitochondria. This pathway is particularly prominent in endothelial cells and leads to vascular dysfunction, a major underlying feature in both diabetes and its complications (18). Targeting mitochondrial oxidative stress prevents DN in mice (19). In addition, bilirubin derived from HO enzyme activity or administered exogenously prevents endothelial cell death in diabetic rats (20). Exogenous bilirubin also preserves mitochondrial integrity (5). Aortic rings isolated from hyperbilirubinemic Gunn rats, which have a genetic deficiency in UDP-GT, exhibit reduced levels of superoxide production and a blunted tonic response to angiotensin II infusion (21). These findings were recently corroborated in humans with Gilbert syndrome, where hyperbilirubinemia was associated with decreased indices of oxidative stress and enhanced endothelium-dependent vasodilation (22). Further, the protective properties of bilirubin in vascular disease have been confirmed in other models of systemic hypertension (23) as well as a model of balloon injury (24).
Given the emerging understanding of the protective properties of bilirubin and its broad association with disease in clinical trials, the results published in this issue of Diabetes have the potential to positively impact the management of patients with diabetes and its complications. Importantly, bilirubin measurement is inexpensive, performed routinely, and accessible to most medical centers. Therefore, the findings reported by Riphagen et al. (2) are widely applicable, especially given the staggering burden of DN. This study also demonstrates that serum bilirubin levels can be used by clinicians to prognosticate the progression of DN. However, additional work must be done to understand the full clinical potential of bilirubin as a marker in monitoring DN (Fig. 1). In the future, studies should be designed to determine the reliability of bilirubin in estimating the rate of DN progression for the purpose of treatment planning. Animal models of hyperbilirubinemia such as the Gunn rat in the context of diabetic kidney disease may be an important area for investigation. Future research should focus on clearly defining how (prognostic versus therapeutic), when (specific disease states to which bilirubin is causally linked), and why (mechanism) bilirubin may be a valuable clinical tool in assessing and managing the progression of DN.
Article Information.
Funding. This work was supported by National Institutes of Health grants R01-DK-59600, R01-DK-083390, and P30-DK-079337 (to A.A.); and American Heart Association grant 13PRE17000013 (to T.D.H.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 2845.
References
- 1.American Diabetes Association Executive summary: Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riphagen IJ, Deetman PE, Bakker SJL, et al. Bilirubin and progression of nephropathy in type 2 diabetes: a post-hoc analysis of RENAAL with independent replication in IDNT. Diabetes 2014;63:2845–2853 [DOI] [PubMed] [Google Scholar]
- 3.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem 1969;244:6388–6394 [PubMed] [Google Scholar]
- 4.Vítek L, Jirsa M, Brodanová M, et al. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis 2002;160:449–456 [DOI] [PubMed] [Google Scholar]
- 5.Adin CA, Croker BP, Agarwal A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 2005;288:F778–F784 [DOI] [PubMed] [Google Scholar]
- 6.Fujii M, Inoguchi T, Sasaki S, et al. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int 2010;78:905–919 [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators The losartan renal protection study—rationale, study design and baseline characteristics of RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan). J Renin Angiotensin Aldosterone Syst 2000;1:328–335 [DOI] [PubMed] [Google Scholar]
- 8.Rodby RA, Rohde RD, Clarke WR, et al. For the Collaborative Study Group The Irbesartan type II diabetic nephropathy trial: study design and baseline patient characteristics. Nephrol Dial Transplant 2000;15:487–497 [DOI] [PubMed] [Google Scholar]
- 9.Bosma PJ, Chowdhury JR, Bakker C, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 1995;333:1171–1175 [DOI] [PubMed] [Google Scholar]
- 10.Schwertner HA, Vítek L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: possible protective effects and therapeutic applications of bilirubin. Atherosclerosis 2008;198:1–11 [DOI] [PubMed] [Google Scholar]
- 11.Balla J, Vercellotti GM, Jeney V, et al. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol Nutr Food Res 2005;49:1030–1043 [DOI] [PubMed] [Google Scholar]
- 12.Gibbs PE, Tudor C, Maines MD. Biliverdin reductase: more than a namesake - the reductase, its Peptide fragments, and biliverdin regulate activity of the three classes of protein kinase C. Front Pharmacol 2012;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YH, Chau LY, Chen JW, Lin SJ. Serum bilirubin and ferritin levels link heme oxygenase-1 gene promoter polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes Care 2008;31:1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YH, Kuo KL, Hung SC, Hsu CC, Chen YH, Tarng DC. Length Polymorphism in Heme Oxygenase-1 and Risk of CKD among Patients with Coronary Artery Disease. J Am Soc Nephrol. 24 April 2014 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang SJ, Lee C, Kruzliak P. Effects of serum bilirubin on atherosclerotic processes. Ann Med 2014;46:138–147 [DOI] [PubMed] [Google Scholar]
- 16.Hull TD, Agarwal A, George JF. The mononuclear phagocyte system in homeostasis and disease: a role for heme oxygenase-1. Antioxid Redox Signal 2014;20:1770–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–1046 [DOI] [PubMed] [Google Scholar]
- 18.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chacko BK, Reily C, Srivastava A, et al. Prevention of diabetic nephropathy in Ins2(+/)⁻(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J 2010;432:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodella L, Lamon BD, Rezzani R, et al. Carbon monoxide and biliverdin prevent endothelial cell sloughing in rats with type I diabetes. Free Radic Biol Med 2006;40:2198–2205 [DOI] [PubMed] [Google Scholar]
- 21.Pflueger A, Croatt AJ, Peterson TE, et al. The hyperbilirubinemic Gunn rat is resistant to the pressor effects of angiotensin II. Am J Physiol Renal Physiol 2005;288:F552–F558 [DOI] [PubMed] [Google Scholar]
- 22.Maruhashi T, Soga J, Fujimura N, et al. Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in Gilbert syndrome. Circulation 2012;126:598–603 [DOI] [PubMed] [Google Scholar]
- 23.Nath KA, d’Uscio LV, Juncos JP, et al. An analysis of the DOCA-salt model of hypertension in HO-1-/- mice and the Gunn rat. Am J Physiol Heart Circ Physiol 2007;293:H333–H342 [DOI] [PubMed] [Google Scholar]
- 24.Peyton KJ, Shebib AR, Azam MA, Liu XM, Tulis DA, Durante W. Bilirubin inhibits neointima formation and vascular smooth muscle cell proliferation and migration. Front Pharmacol 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimm H, Yun JE, Jo J, Jee SH. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke 2009;40:3422–3427 [DOI] [PubMed] [Google Scholar]
- 26.Lee JP, Kim do H, Yang SH, et al. Serum bilirubin affects graft outcomes through UDP-glucuronosyltransferase sequence variation in kidney transplantation. PLoS ONE 2014;9:e93633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheriyath P, Gorrepati VS, Peters I, et al. High Total Bilirubin as a Protective Factor for Diabetes Mellitus: An Analysis of NHANES Data From 1999 - 2006. J Clin Med Res 2010;2:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]