Abstract
Hypoglycemic detection at the portal-mesenteric vein (PMV) appears mediated by spinal afferents and is critical for the counter-regulatory response (CRR) to slow-onset, but not rapid-onset, hypoglycemia. Since rapid-onset hypoglycemia induces Fos protein expression in discrete brain regions, we hypothesized that denervation of the PMV or lesioning spinal afferents would suppress Fos expression in the dorsal medulla during slow-onset hypoglycemia, revealing a central nervous system reliance on PMV glucosensors. Rats undergoing PMV deafferentation via capsaicin, celiac-superior mesenteric ganglionectomy (CSMG), or total subdiaphragmatic vagotomy (TSV) were exposed to hyperinsulinemic–hypoglycemic clamps where glycemia was lowered slowly over 60–75 min. In response to hypoglycemia, control animals demonstrated a robust CRR along with marked Fos expression in the area postrema, nucleus of the solitary tract, and dorsal motor nucleus of the vagus. Fos expression was suppressed by 65–92% in capsaicin-treated animals, as was epinephrine (74%), norepinephrine (33%), and glucagon (47%). CSMG also suppressed Fos expression and CRR during slow-onset hypoglycemia, whereas TSV failed to impact either. In contrast, CSMG failed to impact upon Fos expression or the CRR during rapid-onset hypoglycemia. Peripheral glucosensory input from the PMV is therefore required for activation of hindbrain neurons and the full CRR during slow-onset hypoglycemia.
Introduction
Iatrogenic hypoglycemia has emerged as the primary obstacle for achieving glycemic control in insulin-dependent diabetic patients (1). Since establishing euglycemia holds the potential to ameliorate many of the serious microvascular complications, there has been a renewed interest in understanding the mechanisms underlying hypoglycemic detection. Originally viewed as the domain of the ventromedial hypothalamus, it is now known that glucose-sensing neurons are widely distributed within the central nervous system (CNS) as well as in the periphery (2). In addition to the ventromedial hypothalamus, neurons responding specifically to hypoglycemia have been identified within the paraventricular nucleus, lateral hypothalamic area, arcuate nucleus, area postrema (AP), nucleus of the solitary tract (NTS), and dorsal motor nucleus of the vagus (DMX). Peripheral glucose sensors responding to hypoglycemia have been identified in the carotid bodies (3) and portal-mesenteric vein (PMV) (4,5). The relative importance of the various glucose-sensing loci is an area of considerable debate and may in part depend upon the rate at which hypoglycemia develops. We have shown that portal-mesenteric glucose sensors are particularly important for the detection of slow-onset hypoglycemia (5). Lesioning portal-mensenteric glucose sensors essentially eliminated sympathoadrenal responses to hypoglycemia and severely constrained glucose counter regulation. In contrast, rapid-onset hypoglycemia elicited a counter-regulatory response (CRR) that appears largely independent of peripheral glucose sensory input.
While peripheral and central glucose sensors may subserve different roles, it has been proposed that these various glucose-sensing loci comprise an integrated neural network for defending the glycemic status of the body (6–8). However, these models for the portohepatic–brain–sympathoadrenal neural axis were based primarily on observations of glucose elevations in the portohepatic circulation. Increasing portal vein glucose levels inhibits the firing of hepatic vagal afferents (9), inhibits glucose-excited neurons in the lateral hypothalamic area and NTS (10,11), reduces the adrenal nerve firing rate (6), and suppresses appetite (12–14). Since sectioning the hepatic vagus eliminates most of these responses, it is generally presumed that glucose sensory input from the portal vein is conveyed to the CNS via glucose-sensitive vagal afferents. However, CRRs to hypoglycemia have repeatedly been shown to be unaffected by vagotomy (15–17), despite the fact that PMV glucose sensors play a critical role in the sympathoadrenal response to hypoglycemia (5,18,19). Alternatively, evidence suggests hypoglycemic detection at the PMV may be mediated by spinal afferents (17), though any connection with brain glucose sensory loci remains speculative.
The early gene product Fos is widely used to map functional neural networks underlying many stress responses, including hypoglycemia (20). Utilizing this approach, investigators have identified two primary levels of the CNS activated by hypoglycemia. In the hindbrain, Fos is observed primarily in the AP, NTS, and DMX, while activation is also observed in a number of forebrain regions, including the hypothalamus (13,21,22). However, these studies have relied on injections of insulin or 2-deoxyglucose, both of which induce hypoglycemia or glucopenia rapidly and may obviate peripheral glucose sensory input (5). Here we test the hypothesis that activation of the CNS during slow-onset hypoglycemia is dependent upon PMV glucose sensory input via spinal afferents, but not during rapid-onset hypoglycemia.
Research Design and Methods
Animals
Three experimental studies used conscious unrestrained male Wistar rats (weight = 254.4 ± 2.87 g; total n = 43). Animals were housed in individual cages, fed ad libitum, and on 12-h light–dark cycles. All surgical and experimental procedures were preapproved by the University of Southern California Institutional Animal Care and Use Committee.
Surgical Procedures
One week before experiments, all animals were chronically cannulated under single-dose anesthesia (3:3:1 ketamine HCl, xylazine, acepromazine malate; 0.1 mL/0.1 kg body weight) administered intramuscularly. Cannulas were inserted into the left jugular vein (dual cannula, Silastic ID = 0.025 cm) for peripheral administration of glucose and insulin and the right carotid artery (Clay Adams PE-50) for arterial blood sampling. At that time, a laparotomy was performed, and the abdominal contents were reflected and covered with sterile gauze soaked in warm saline (37°C). Animals then underwent one of three separate denervation procedures, with control animals (n = 16) undergoing an analogous sham operation.
Experiment 1
Portal-mesenteric sensory denervation (capsaicin; n = 5) was effected via topical application of a 1% capsaicin solution (vehicle solution 10% ethanol and 10% Tween80 in 0.9% saline) as previously described (5,19). Briefly, the portal and superior mesenteric veins were isolated, and small strips of filter paper were measured to fit the length and width of the vessels then soaked in a 1% capsaicin solution and placed on the veins for 15 min with care taken to shield surrounding tissues from exposure to the capsaicin. Strips were removed thereafter, and the veins were rinsed thoroughly with saline. Control animals (n = 5) underwent a sham operation where only vehicle solution was applied.
Experiment 2
To eliminate spinal afferent innervation of the portal and superior mesenteric veins, a celiac-superior mesenteric ganglionectomy (CSMG; n = 5) was performed. Following the laparotomy, the celiac and superior mesenteric ganglia were located on the ventral aspect of the descending aorta, caudal to the celiac artery, and rostral to the superior mesenteric artery. The ganglia were then gently removed by blunt dissection, severing all visible connections (17). To eliminate vagal afferent innervation of the PMV, a total subdiaphragmatic vagotomy (TSV; n = 5) was performed involving bilateral sectioning of the anterior and posterior trunks of the vagus nerve located along the esophagus just caudal to the diaphragm (17). Control animals (n = 5) underwent a sham operation in which the subdiaphragmatic branches of the vagus and CSMG were exposed but not severed.
Experiment 3
A CSMG was performed as above (n = 6) with control animals (n = 6) undergoing a sham operation.
Following denervation procedures, the stomach and intestines were returned to the abdominal cavity and the abdominal musculature sutured to close the cavity. Ventral skin incisions were closed separately with individual sutures, reinforced with suture glue (Nexaband) and swabbed with an antibacterial agent (Betadine). Cannulas were tunneled subcutaneously, exteriorized at the back of the neck, and incased in an infusion harness (Instech Laboratories). Animals were allowed 6 days to recover from surgery and to regain their original body weight. No significant differences in body weight were observed between experimental groups on the day of experiments. Sixteen hours prior to experiments, all access to food (but not water) was removed.
In Vivo Clamps
Slow-Onset Hyperinsulinemic–Hypoglycemic Clamps (Experiments 1 and 2)
On the day of the experiment, all animals were exposed to the same protocol to induce hypoglycemia. Animals were placed in an infusion chamber and their jugular catheters attached to extensions from a dual-channel infusion swivel connected to infusion pumps for insulin and glucose infusions. Animals were allowed to rest for 60 min (−90 to −30 min) before sampling was initiated. Basal arterial samples were drawn at −30 and 0 min for analysis of glucose, insulin, glucagon, and catecholamines. At minute 0, whole-body hypoglycemia was induced slowly via insulin infusion (25 mU·kg−1·min−1) and variable exogenous glucose infusion (20% dextrose). Glucose infusion was slowly decreased to achieve deep hypoglycemia, 2.6 mmol/L, within 60–75 min. During this time, additional glucose samples were drawn every 10 min to control the rate of glycemic decline. Glucose infusions were adjusted thereafter to sustain hypoglycemia at 2.6 mmol/L until minute 105. Arterial plasma samples for glucose and catecholamines were taken at minutes 60, 75, 90, and 105 of the clamp. The volume of blood sampled was replaced with an equal volume of whole blood from donor animals. An additional sample was taken at minute 105 for the determination of glucagon and insulin concentrations.
Hyperinsulinemic–Euglycemic Clamps (Experiment 1 Only)
To ascertain the potential impact of hyperinsulinemia versus hypoglycemia on brain Fos expression, hyperinsulinemic–euglycemic clamps were performed on a separate group of sham control animals (n = 6). One week later, they underwent identical clamp and sampling protocols to those described above except that euglycemia (∼6.5 mmol/L) was maintained throughout the entire 105-min experiment utilizing variable glucose (20%) infusions.
Rapid-Onset Hyperinsulinemic–Hypoglycemic Clamps (Experiment 3)
Animals were placed in an infusion chamber, and catheters were connected as described above. Animals were allowed 55 min to rest (−100 to −45 min) before insulin (25 mU·kg−1·min−1) and glucose infusions (variable) were initiated and a hyperinsulinemic–euglycemic clamp established from minute −45 to 0. At minute 0, the glucose infusion was reduced to achieve the hypoglycemic nadir, 2.5 mmol/L, by minute 20, which was sustained for the remainder of the clamp (20–110 min). Sampling for glucose analysis was performed every 5–15 min between minutes −45 and 110. Larger arterial samples (250 μL) were drawn at minutes −45, 0, 20, 40, 60, 90, and 110 for catecholamine analysis, with insulin and glucagon samples taken at minutes 0 (basal) and 110 (deep hypoglycemia).
Tissue Processing and Immunocytochemistry
At the terminus of the clamp, rats were killed (sodium pentobarbital, overdose) and rapidly decapitated; brains were collected and fixed in paraformaldehyde, sectioned, and then processed as previously described (23). In brief, 30 μm coronal hindbrain sections were cut between levels 68–71 of the Swanson rat brain atlas (24). Sections were reacted for 72 h at 4°C with a rabbit polyclonal Fos antibody (Ab-5; 1:50 K, Chemicon) followed by a biotinylated goat anti-rabbit IgG. The immunocomplexes were then conjugated to streptavidin-peroxidase (Vector) and developed with a 0.5 mg/mL solution of 3,3-diaminobenzidine tetrahydrochloride containing 0.1 µL/mL hydrogen peroxide followed by 0.5% cobalt acetate (Sigma-Aldrich) for 10 min. Sections were then mounted on gelatin-coated slides, air-dried overnight, dehydrated in alcohols, cleared in xylenes, and cover slipped. To limit the impact of possible processing variations on the results, all sections from a single experiment were processed together. Under these circumstances, immunocytochemistry is a semiquantitative method, so comparing the numbers of Fos between separate experiments is not possible.
Sections were photographed using bright-field illumination with a Nikon Microphot SA microscope and a SPOT RT Digital Camera (Diagnostics Instruments Inc.) using SPOT Image (version 3.5.5, Mac OS). Brightness and contrast of the photomicrographs were matched across sections using the Photoshop CS3 (www.adobe.com) Curves and Brightness/Contrast tools. For each animal, the section corresponding to level 70 of Swanson (24) was identified, and the AP, NTS, and DMX were demarcated using local cytoarchitectural features in adjacent thionin-stained sections. Fos-ir nuclei in these three regions on both sides of the brain were counted manually.
Analytical Procedures
Glucose was assayed by the glucose oxidase method (YSI). Catecholamines were analyzed utilizing a single-isotope radioenzymatic method (25). Insulin and glucagon samples were assayed using commercially available radioimmunoassay kits (Linco Research and MP Biomedicals).
Data Analysis
Results were expressed as mean ± SEM. Comparisons among treatments over time were made utilizing repeated-measures ANOVA with post hoc analysis via Bonferroni test for multiple comparisons (Prism, GraphPad). For comparisons between groups under basal and hypoglycemic conditions or hypoglycemia alone, two-way ANOVA or one-way ANOVAs were used, respectively, with Bonferroni post hoc comparisons where appropriate.
Results
Effect of PMV Deafferentation on Hindbrain Fos Expression and CRR Hormone Responses to Slow-Onset Hypoglycemia
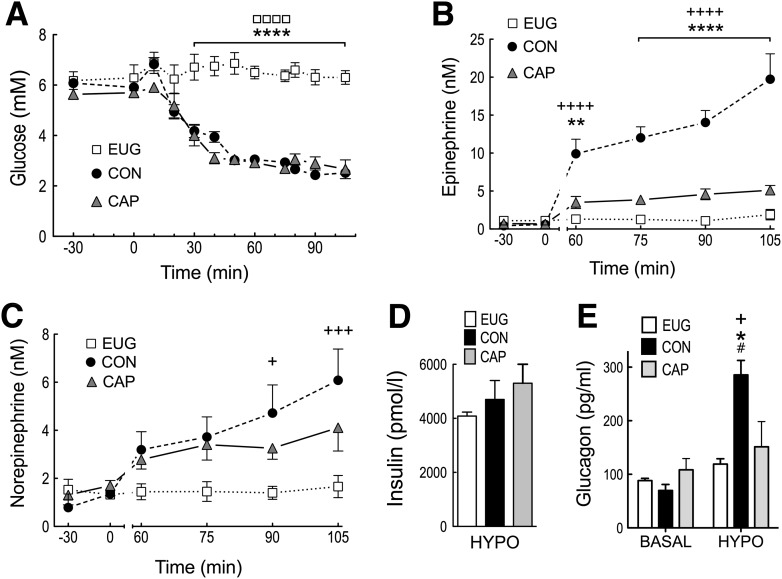
Insulin infusion led to an increase in the arterial plasma insulin concentration, 4,930 ± 699 pmol/L that was not significantly different between groups (P > 0.10) (Fig. 1). Basal glucose concentrations were 6.23 ± 0.43, 6.08 ± 0.13, and 5.63 ± 0.18 mmol/L for euglycemia, control, and capsaicin, respectively (not significant; P > 0.10). By design, euglycemic animals were clamped at glucose concentrations not significantly different from basal, while glucose was allowed to fall in a controlled manner over the next 60 min for both control and capsaicin, reaching hypoglycemic nadirs of 2.52 ± 0.15 and 2.66 ± 0.37 mmol/L, respectively (not significant for control versus capsaicin; P > 0.10).
Figure 1.
Arterial glucose (A), epinephrine (B), and norepinephrine (C) at basal (−30 to 0 min) and during a slow-onset (−0.05 mmol/L/min) hyperinsulinemic–hypoglycemic clamp (0–105 min). Insulin (D) during the hyperinsulinemic–hypoglycemic period, and glucagon (E) at the end of the basal (0 min) and hypoglycemic (105 min) periods. Open squares and bars represent euglycemia, closed black circles and bars represent control, and closed gray triangles and bars represent capsaicin treatment. All values are expressed as mean ± SEM. +P < 0.05; +++P < 0.001; ++++P < 0.0001 for control vs. euglycemia. *P < 0.05; **P < 0.01; ****P < 0.0001 for control vs. capsaicin. ☐☐☐☐P < 0.0001 for euglycemia vs. capsaicin. #P < 0.001 for basal vs. hypoglycemia. CAP, capsaicin; CON, control; EUG, euglycemia; HYPO, hypoglycemia.
As expected, euglycemic animals demonstrated epinephrine, norepinephrine, and glucagon values that were not significantly different from basal over the course of the experiment. In response to insulin-induced hypoglycemia, control animals demonstrated a robust sympathoadrenal response, where plasma epinephrine concentrations increased from 0.44 ± 0.10 at basal to 19.73 ± 3.35 nmol/L by minute 105. In contrast, ablation of PMV capsaicin-sensitive afferents led to a 74% suppression of the epinephrine response (5.11 ± 0.62 vs. 19.73 ± 3.35 nmol/L; P < 0.0001) by minute 105. Norepinephrine responses to hypoglycemia in control were significantly elevated above euglycemia (P < 0.001) but failed to rise significantly in capsaicin animals (P > 0.05). Basal glucagon values averaged 88.8 ± 12.4 pg/mL and were not significantly different between euglycemia, control, and capsaicin (P > 0.10). In response to hypoglycemia, control animals demonstrated a fourfold increase in glucagon that was significantly greater than capsaicin or euglycemia (P < 0.05). Deafferentation of the PMV via capsaicin prevented the hypoglycemia-induced rise in glucagon, i.e., values for capsaicin at hypoglycemia were not significantly different from euglycemia (P > 0.10).
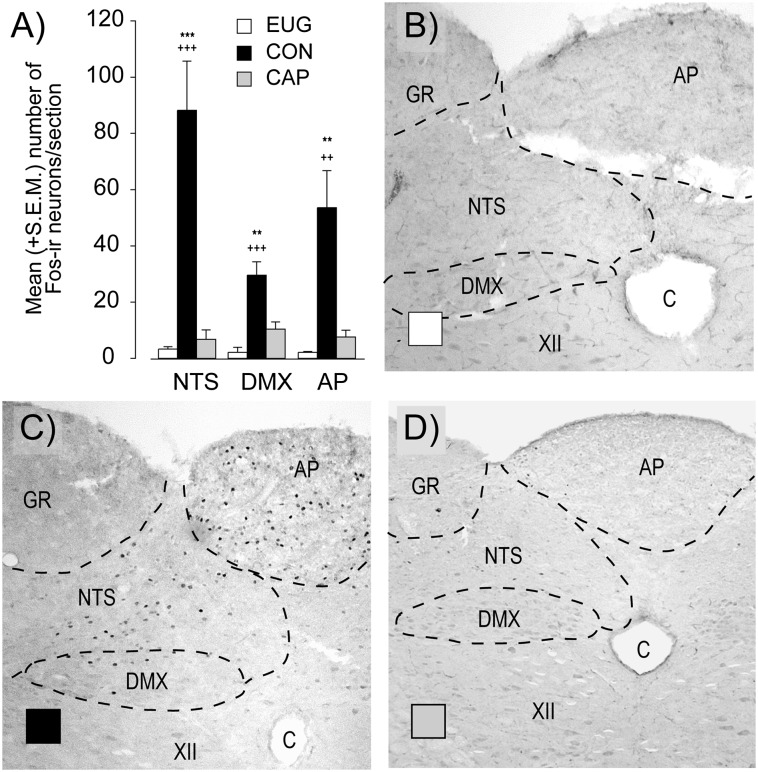
Euglycemia failed to induce significant Fos expression in the hindbrain, while hypoglycemia in nerve-intact animals (control) led to a 11-, 22-, and 23-fold increase in Fos expression in the NTS (P < 0.001), DMX (P < 0.001), and AP (P < 0.01), respectively. As with the hormonal responses above, deafferentation of the PMV led to an 86, 65, and 92% suppression in Fos-labeled nuclei within the AP (P < 0.01), DMX (P < 0.01), and NTS (P < 0.001) (Fig. 2), respectively.
Figure 2.
Fos-immunoreactive neurons within the NTS, DMX, and AP sampled at the end of a slow-onset (−0.05 mmol/L/min) hypoglycemic clamp (105 min) (A). Open squares represent euglycemia, closed black bars represent control, and closed gray bars represent capsaicin. Photomicrographs of representative hindbrain sections for euglycemia (B), control (C), and capsaicin (D) with the AP, NTS, DMX, hypoglossal nucleus, gracile nucleus, and central canal indicated. All values are expressed as mean ± SEM. **P < 0.01; ***P < 0.001 for control vs. capsaicin. ++P < 0.01; +++P < 0.001 for control vs. euglycemia. C, central canal; CAP, capsaicin; CON, control; EUG, euglycemia; GR, gracile nucleus; XII, hypoglossal nucleus.
Effect of Subdiaphragmatic Vagotomy Versus CSMG on Hindbrain Fos Expression in Response to Slow-Onset Hypoglycemia
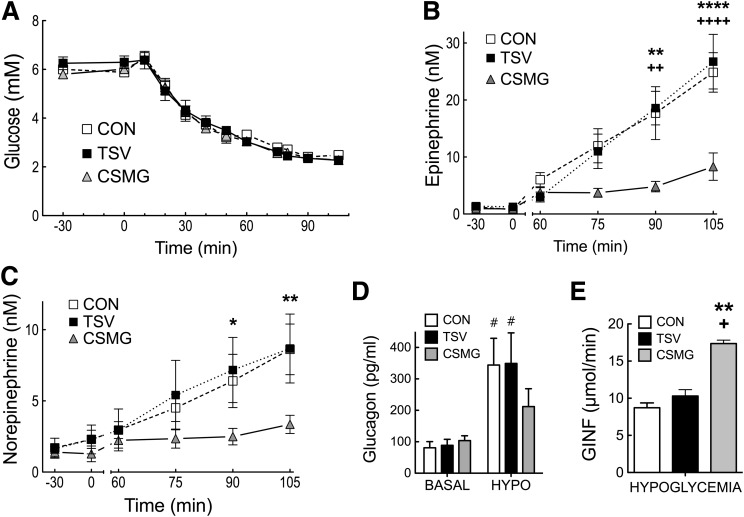
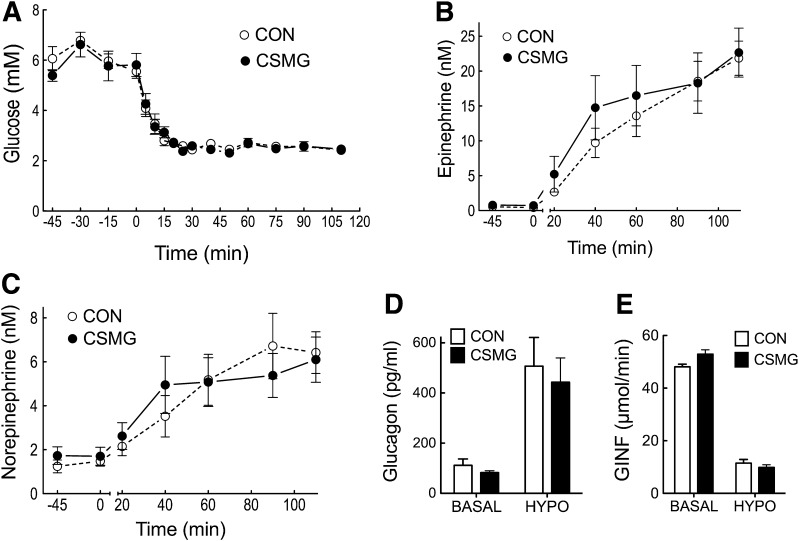
Insulin infusion increased plasma insulin values for control, CSMG, and TSV animals to 4,673 ± 437, 5,367 ± 54, and 4,615.17 ± 337 pmol/L, respectively (not significant; P > 0.10). Plasma glucose was matched in all groups during the clamp and slowly reduced from a mean value of 6.01 ± 0.10 mmol/L to a hypoglycemic nadir of 2.49 ± 0.05 mmol/L by minute 75 and maintained until minute 105 (not significant between groups; P > 0.10) (Fig. 3).
Figure 3.
Arterial glucose (A), epinephrine (B), and norepinephrine (C) at basal (−30 to 0 min) and during a slow-onset (−0.05 mmol/L/min) hyperinsulinemic–hypoglycemic clamp (0–105 min). Glucagon (D) at the end of the basal (0 min) and hypoglycemic (105 min) periods, and glucose infusion rate (E) during hypoglycemia (75–105 min). Open squares and bars represent control, closed black circles and bars represent TSV, and closed gray triangles and bars represent CSMG. All values are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ****P < 0.0001 for control vs. CSMG. +P < 0.05; ++P < 0.01; ++++P < 0.0001 for TSV vs. CSMG. #P < 0.05 for basal vs. hypoglycemia. CON, control; HYPO, hypoglycemia; GINF, glucose infusion rate.
Whole-body hypoglycemia increased arterial epinephrine concentrations 25-fold in control animals from a basal value of 1.00 ± 0.33 nmol/L to 24.84 ± 3.46 nmol/L by minute 105. TSV animals demonstrated a similar epinephrine response, with a 20-fold increase from basal, reaching a peak value of 26.73 ± 4.79 nmol/L, not significantly different from control (P > 0.05). In contrast, animals with CSMG, thereby lesioning spinal innervation to the PMV, experienced a 68% suppression in the peak epinephrine response to hypoglycemia compared with control or TSV animals (P < 0.0001). Groups demonstrated similar norepinephrine responses to hypoglycemia, with control and TSV demonstrating a 4.5-fold increase above basal at minute 105, while peak norepinephrine responses for CSMG were suppressed by 61% compared with control values (P < 0.01). In response to hypoglycemia, glucagon values increased to 344.02 ± 85.03 and 349 ± 97.10 pg/mL for control and TSV, respectively, while CSMG reached a value of 211.99 ± 54.64 pg/mL (not significant; P > 0.05). Consistent with the counter-regulatory hormone responses, the glucose infusion rate necessary to sustain deep hypoglycemia was significantly elevated in CSMG animals when compared with control (P < 0.01) and TSV (P < 0.05).
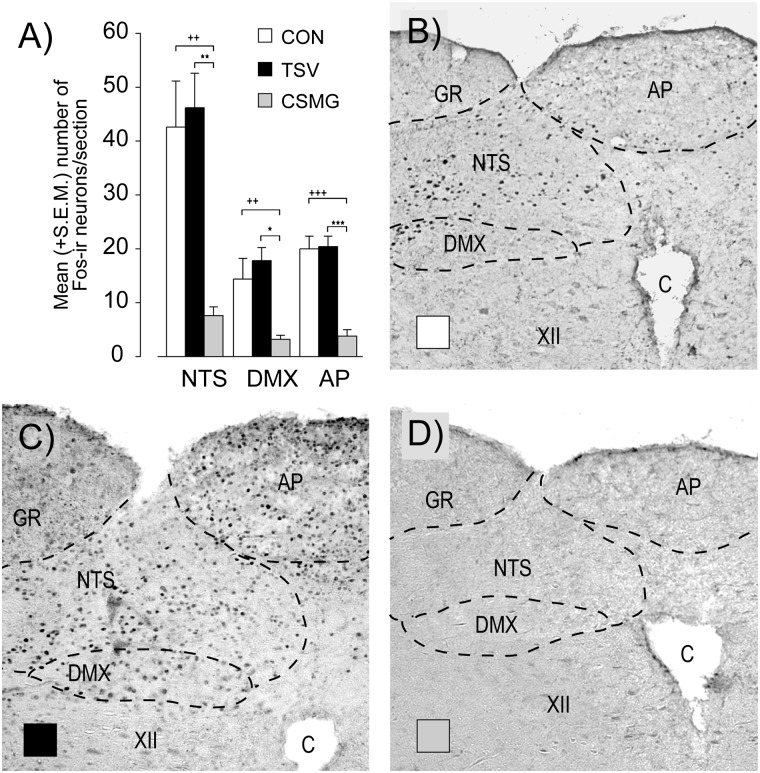
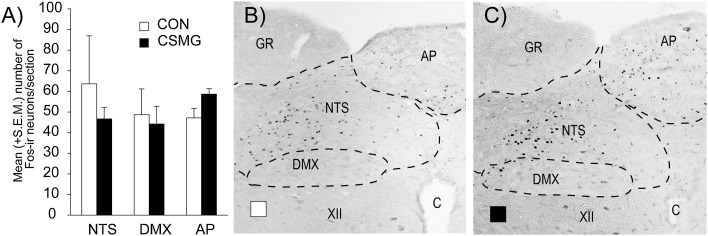
As with the previous experiment, control animals demonstrated significant Fos expression in several discrete hindbrain regions, i.e., NTS, DMX, and AP (Fig. 4). In accordance with their CRRs, TSV animals demonstrated Fos labeling in the hindbrain that was not significantly different from control (P > 0.10), while CSMG animals demonstrated an 81, 78, and 82% reduction in Fos-labeled nuclei for the AP (P < 0.001), DMX (P < 0.05), and NTS (P < 0.01), respectively.
Figure 4.
Fos-immunoreactive neurons within the NTS, DMX, and AP sampled at the end of a slow-onset (−0.05 mmol/L/min) hypoglycemic clamp (105 min) (A). Open bars represent control, closed black bars represent TSV, and closed gray bars represent CSMG. Photomicrographs of representative hindbrain sections for control (B), TSV (C), and CSMG (D) with the AP, NTS, DMX, hypoglossal nucleus, gracile nucleus, and central canal indicated. All values are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 for control vs. CSMG. ++P < 0.01; +++P < 0.001 for TSV vs. CSMG. C, central canal; CON, control; GR, gracile nucleus; XII, hypoglossal nucleus.
Effect of Ablating Spinal Sensory Output From the PMV via CSMG on Hindbrain Fos Expression in Response to Rapid-Onset Hypoglycemia
Insulin infusion increased plasma insulin concentrations from a mean basal value of 45.5 ± 5.0 pmol/L to a hyperinsulinemic plateau of 5,194 ± 510 pmol/L. No significant differences were observed in basal (P > 0.10) or terminal (P > 0.10) insulin concentrations between control and CSMG groups. In both groups, arterial glucose was rapidly lowered from a mean value of 5.66 ± 0.2 mmol/L to 2.44 ± 0.08 mmol/L within 20 min (Fig. 5). By design, these arterial glucose values were matched; thus there were no significant differences between control and CSMG animals at any sampling point.
Figure 5.
Arterial glucose (A), epinephrine (B), and norepinephrine (C) during a hyperinsulinemic–euglycemic clamp (−45 to 0 min) and during a rapid-onset (−0.16 mmol/L/min) hyperinsulinemic–hypoglycemic clamp (0–110 min). Glucagon (D) at the end of the basal (0 min) and hypoglycemic (110 min) periods, and glucose infusion rate (E) during euglycemia (−45 to 0 min) and deep hypoglycemia (60–110 min). Open circles and bars represent control, and closed circles and bars represent CSMG. All values are expressed as mean ± SEM. CON, control; GINF, glucose infusion rate; HYPO, hypoglycemia.
In response to rapid-onset hypoglycemia, epinephrine values in control animals increased ∼40-fold from 0.55 ± 0.14 to 21.85 ± 2.45 nmol/L by minute 110. In contrast to the results for slow-onset hypoglycemia (Fig. 3), CSMG animals demonstrated epinephrine responses to rapid-onset hypoglycemia that were not significantly different from control (P > 0.05). As with epinephrine, norepinephrine and glucagon both demonstrated robust responses to hypoglycemia in control animals that were not significantly impacted by CSMG. Consistent with their CRRs to rapid-onset hypoglycemia, the glucose infusion rates were not significantly different between groups at any point during the clamp. Substantial Fos expression was observed in the hindbrain following rapid-onset hypoglycemia but was not significantly different between control and CSMG (Fig. 6).
Figure 6.
Fos-immunoreactive neurons within the NTS, DMX, and AP sampled at the end of a rapid-onset (−0.16 mmol/L/min) hypoglycemic clamp (110 min) (A). Open bars represent control, and closed bars represent CSMG. Photomicrographs of representative hindbrain sections for control (B) and CSMG (C) with the AP, NTS, DMX, hypoglossal nucleus, gracile nucleus, and central canal indicated. All values are expressed as mean ± SEM. C, central canal; CON, control; GR, gracile nucleus; XII, hypoglossal nucleus.
Discussion
The existence of a hepatoportal–brain–sympathoadrenal neural network was postulated over 20 years ago (6,7,26), but its relevance to hypoglycemic detection remained speculative, with little compelling supportive evidence (8,27). Toward this end, we now provide the first evidence that activation of hindbrain neurons by slow-onset hypoglycemia (−0.05 mmol/L/min) requires glucosensory input from peripheral glucose sensors located in the PMV. When hypoglycemia developed slowly over 60 min, portal-mesenteric deafferentation via capsaicin dramatically suppressed hindbrain Fos-ir neuronal activation in regions that contain glucose-sensing neurons, i.e., the NTS (↓92%), DMX (↓65%), and AP (↓86%). Concomitant with reduced hindbrain Fos expression, there was substantial blunting of the sympathoadrenal response, i.e., epinephrine (↓74%) and norepinephrine (↓32%), together with a 47% reduction in the glucagon response to slow-onset hypoglycemia. Furthermore, severing all vagal input below the diaphragm had no significant effect on hindbrain Fos expression, while CSMG produced a reduction comparable with PMV deafferentation via capsaicin. Thus, as has been shown for the sympathoadrenal response, the ascending trajectory used for portal-mesenteric hypoglycemic sensory information to the hindbrain appears to be spinal, not vagal. Finally, we demonstrated that both hindbrain neuronal activation and the sympathoadrenal response to rapid-onset hypoglycemia (−0.16 mmol/L/min) appear independent of peripheral glucosensory input. These data provide compelling evidence that a functional PMV–hindbrain–sympathoadrenal network is required for CRRs to slow-onset hypoglycemia.
With the possible exception of spinal afferents, the critical components of a PMV–hindbrain–sympathoadrenal neural network are well described. The NTS is the major recipient of the sensory input from both vagal and spinal afferents innervating a variety of systemic organs (28). It also receives afferent input from adjacent glucosensory structures, e.g., the AP, a circumventricular organ ostensibly capable of detecting changes in circulating plasma glucose levels (29). Adachi et al. (10,30) have identified glucose-sensing neurons in various regions of the hindbrain, including the NTS, AP, and DMX. The DMX provides parasympathetic preganglionic control to the gut and other organs. Direct microinjection of 5-thioglucose into the brain reveals numerous hindbrain glucopenic sensing loci, primarily in the ventrolateral and dorsomedial medulla proximal to the AP or within the NTS (31). Most of these same cell groups demonstrate Fos activation in response to 2-deoxyglucose injection (32). Adrenomedullary chromaffin cells are innervated by sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord (33). In turn, these are innervated by a small number of hindbrain structures: the caudal raphe nuclei, the ventromedial and rostral ventrolateral medulla, and the A5 cell group (34). Antidopamine-β-hydroxylase saporin injected into the spinal cord selectively eliminates those hindbrain catecholaminergic neurons critical to the sympathoadrenal response to 2-deoxyglucose (35). Further, experiments with decerebrate animals show that the hindbrain alone is sufficient to retain the sympathoadrenal response to a 2-deoxyglucose challenge (36). Thus critical elements already exist for a simple neural network that may be sufficient to monitor and maintain euglycemia in the face of moderate or slowly developing hypoglycemic challenges.
Prior models describing a portahepatic–CNS–sympathoadrenal neural axis assumed that all visceral glucose sensory input to the brain was effected via vagal afferents (6–8). While there is little doubt that glucose-sensing vagal afferents exist, their role in hypoglycemic detection remains questionable (15–17). Outside of two extreme hypoglycemic/glucopenic conditions, i.e., 0 mmol/L glucose and large portal 2-deoxyglucose injections (9,37), evidence for vagal afferent glucose sensing has been restricted to euglycemia and hyperglycemia (9,38,39). Vagotomies, both acute and chronic, have repeatedly failed to affect CRRs to hypoglycemia (15–17). That the vagal glucose-sensing afferents of the PMV may subserve a different function, e.g., hyperglycemic sensing, is supported by observations indicating a linear response for these neurons across a wide range of glucose concentrations, 0–27 mmol/L (9,38,39). Alternatively, CSMG suppresses CRRs to hypoglycemia (17). While vagal afferents of the celiac branch are likely severed in this procedure, this occurs caudal to the total subdiaphragmatic lesion (which has no impact on CRR), suggesting that the impact of CSMG is achieved through the sectioning of spinal glucose sensory afferents. Consistent with a spinal origin, peak epinephrine, norepinephrine, and glucagon values for CSMG and capsaicin animals were not significantly different in the current study (the apparent discrepancy in Figs. 1C and 3C derive from the slightly lower control norepinephrine values, though again control peak values were not significantly different). Additional support for a spinal origin of those glucose sensors critical for detecting hypoglycemia derives from the effectiveness of topical capsaicin in ablating PMV glucose sensing. Capsaicin acts through the TRPV1 receptor, which is largely restricted to spinal afferents at the level of the abdomen (40). Calcitonin gene-related peptide, a marker for spinal afferents at this level (41), is largely eliminated from the PMV by topical capsaicin or CSMG, but not TSV, along with the sympathoadrenal response to hypoglycemia (5,19,42). Spinal afferents are known to innervate a number of visceral organs and associated vasculature, and while most often associated with mechanoreception or nociception, there is evidence for their association with a variety of chemoreceptors, e.g., osmoreceptors (43) and glucose sensors (44).
Why the CNS requires PMV glucose sensory input for slow-onset hypoglycemic detection when it possesses a host of different glucosensing neurons remains unclear. Perhaps CNS glucose sensors are insensitive to slow or small glycemic changes, thereby serving as a “fail safe” mechanism for catastrophic declines in glucose. Decreasing the responsiveness of these neurons to any single molecule might, in turn, allow them to respond or integrate a variety of incoming signals to help achieve the appropriate motor response. Indeed, many of these neurons have been characterized as metabosensors responding to a variety of substrates and signaling molecules (45,46). As such, their role in glycemic detection may be secondary to other homeostatic parameters, e.g., energy expenditure. Alternatively, central glucose sensors may serve a synergistic and equally important role with peripheral glucose sensors in responding to hypoglycemia. For example, central glycemia may serve as a reference for integrative elements of the brain against which peripheral glycemic input is measured to establish appropriate CRRs. In this capacity, central glucose sensors might also act primarily to suppress CRRs once euglycemia is reestablished following a hypoglycemic event. This would further explain why clamping either portal-mesenteric or brain glycemia during general systemic hypoglycemia is equally effective in blunting the CRR (47,48).
What is clear from experiment 3 is that the brain can defend itself against large, rapid declines in blood glucose without portal-mesenteric glucose sensory input. Hindbrain Fos activation, as well as the hormonal CRRs, were unaffected by CSMG following rapid-onset hypoglycemia (Figs. 5 and 6). Since visceral spinal afferents synapse with secondary spinal neurons projecting to the hindbrain, these lesions most likely leave hindbrain neurons intact. Ritter et al. (31) have shown that neurons in the hindbrain respond to direct application of the 5-thioglucose (a glucopenic agent). Given that Fos expression in rapid- and slow-onset hypoglycemia occurs in the same loci, i.e., AP, NTS, and DMX, it may be that when there is a catastrophic decline in blood glucose, the same neurons that receive input from the PMV are fully capable of initiating a CRR without that input. However, it is not certain that these are the same neurons or that they are necessarily responding directly to local hypoglycemia; this hypothesis remains to be tested. Elucidating the neurons involved will require phenotyping, neuroanatomical tracing, and markers of neuronal activation. While Fos provides one measure of neuronal activation, it does not always correlate with neuronal firing rate, rather c-fos transcription results from elevated intracellular calcium subsequent to receptor activation (49).
In summary, peripheral glucose sensory input from the PMV is essential to activate hindbrain neurons in response to slow-onset hypoglycemia. Furthermore, PMV sensory neurons that provide hypoglycemic input to the hindbrain are not vagal in origin, but rather they appear to be spinal afferents. These findings are consistent with our earlier observations showing that portal-mesenteric denervated and CSMG, but not vagotomized, animals display diminished CRRs to slow-onset hypoglycemia (5,17,19). However, when the rate of fall in glycemia is rapid, i.e., −0.2 mmol/L/min, activation of both hindbrain neurons and CRRs are independent of portal-mesenteric glucose sensory input. Given the frequency of slow-onset hypoglycemia among insulin-dependent patients with diabetes (50), the PMV–hindbrain–sympathoadrenal glucosensory neural network constitutes a critical element in the body’s defense against insulin-induced hypoglycemia.
Article Information
Funding. This work was supported by grants from the National Institutes of Health (DK062471 [C.M.D.] and NS029728 [A.G.W.]) and the JDRF (1-2007-605 [C.M.D.] and 1-2008-710 [A.G.W.]).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.B. performed all surgeries, conducted experiments, analyzed samples, interpreted data, and assisted with preparation of the manuscript. A.V.M. and M.S. assisted with hormone assays and data analysis and edited the manuscript. A.M.K. provided assistance with immunocytochemical procedures and edited the manuscript. A.G.W. supervised the Fos immunocytochemistry and its analysis, assisted with microscopy, and edited the manuscript. C.M.D. designed and supervised all studies, assisted with data analysis and interpretation, and prepared the manuscript. C.M.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
References
- 1.Cryer PE. Hypoglycemia in diabetes: pathophysiological mechanisms and diurnal variation. Prog Brain Res 2006;153:361–365 [DOI] [PubMed] [Google Scholar]
- 2.Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol 2010;31:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardal R, López-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci 2002;5:197–198 [DOI] [PubMed] [Google Scholar]
- 4.Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes 1997;46:1521–1525 [DOI] [PubMed] [Google Scholar]
- 5.Saberi M, Bohland M, Donovan CM. The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of portal-superior mesenteric vein glucose sensing. Diabetes 2008;57:1380–1386 [DOI] [PubMed] [Google Scholar]
- 6.Niijima A. Nervous regulation of metabolism. Prog Neurobiol 1989;33:135–147 [DOI] [PubMed] [Google Scholar]
- 7.Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev 1987;3:185–206 [DOI] [PubMed] [Google Scholar]
- 8.Thorens B, Larsen PJ. Gut-derived signaling molecules and vagal afferents in the control of glucose and energy homeostasis. Curr Opin Clin Nutr Metab Care 2004;7:471–478 [DOI] [PubMed] [Google Scholar]
- 9.Niijima A. Afferent impulse discharges from glucoreceptors in the liver of the guinea pig. Ann N Y Acad Sci 1969;157:690–700 [DOI] [PubMed] [Google Scholar]
- 10.Adachi A, Shimizu N, Oomura Y, Kobáshi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett 1984;46:215–218 [DOI] [PubMed] [Google Scholar]
- 11.Shimizu N, Oomura Y, Novin D, Grijalva CV, Cooper PH. Functional correlations between lateral hypothalamic glucose-sensitive neurons and hepatic portal glucose-sensitive units in rat. Brain Res 1983;265:49–54 [DOI] [PubMed] [Google Scholar]
- 12.Friedman MI, Granneman J. Food intake and peripheral factors after recovery from insulin-induced hypoglycemia. Am J Physiol 1983;244:R374–R382 [DOI] [PubMed] [Google Scholar]
- 13.Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci 1993;4:93–106 [DOI] [PubMed] [Google Scholar]
- 14.Novin D, Sanderson J, Gonzalez M. Feeding after nutrient infusions: effects of hypothalamic lesions and vagotomy. Physiol Behav 1979;22:107–113 [DOI] [PubMed] [Google Scholar]
- 15.Jackson PA, Pagliassotti MJ, Shiota M, Neal DW, Cardin S, Cherrington AD. Effects of vagal blockade on the counterregulatory response to insulin-induced hypoglycemia in the dog. Am J Physiol 1997;273:E1178–E1188 [DOI] [PubMed] [Google Scholar]
- 16.Jackson PA, Cardin S, Coffey CS, et al. Effect of hepatic denervation on the counterregulatory response to insulin-induced hypoglycemia in the dog. Am J Physiol Endocrinol Metab 2000;279:E1249–E1257 [DOI] [PubMed] [Google Scholar]
- 17.Fujita S, Donovan CM. Celiac-superior mesenteric ganglionectomy, but not vagotomy, suppresses the sympathoadrenal response to insulin-induced hypoglycemia. Diabetes 2005;54:3258–3264 [DOI] [PubMed] [Google Scholar]
- 18.Hevener AL, Bergman RN, Donovan CM. Portal vein afferents are critical for the sympathoadrenal response to hypoglycemia. Diabetes 2000;49:8–12 [DOI] [PubMed] [Google Scholar]
- 19.Fujita S, Bohland M, Sanchez-Watts G, Watts AG, Donovan CM. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol Endocrinol Metab 2007;293:E96–E101 [DOI] [PubMed] [Google Scholar]
- 20.Pacák K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 2001;22:502–548 [DOI] [PubMed] [Google Scholar]
- 21.Yuan P-Q, Yang H. Neuronal activation of brain vagal-regulatory pathways and upper gut enteric plexuses by insulin hypoglycemia. Am J Physiol Endocrinol Metab 2002;283:E436–E448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurst P, Garfield AS, Marrow C, Heisler LK, Evans ML. Recurrent hypoglycemia is associated with loss of activation in rat brain cingulate cortex. Endocrinology 2012;153:1908–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan AM, Watts AG. Intravenous 2-deoxy-D-glucose injection rapidly elevates levels of the phosphorylated forms of p44/42 mitogen-activated protein kinases (extracellularly regulated kinases 1/2) in rat hypothalamic parvicellular paraventricular neurons. Endocrinology 2004;145:351–359 [DOI] [PubMed] [Google Scholar]
- 24.Swanson LW. Brain maps III. 3rd ed. Oxford, Elsevier Academic Press, 2004 [Google Scholar]
- 25.Peuler JD, Johnson GA. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci 1977;21:625–636 [DOI] [PubMed] [Google Scholar]
- 26.Oomura Y, Yoshimatsu H. Neural network of glucose monitoring system. J Auton Nerv Syst 1984;10:359–372 [DOI] [PubMed] [Google Scholar]
- 27.McCrimmon R. The mechanisms that underlie glucose sensing during hypoglycaemia in diabetes. Diabet Med 2008;25:513–522 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz GJ. Integrative capacity of the caudal brainstem in the control of food intake. Philos Trans R Soc Lond B Biol Sci 2006;361:1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young AA. Brainstem sensing of meal-related signals in energy homeostasis. Neuropharmacology 2012;63:31–45 [DOI] [PubMed] [Google Scholar]
- 30.Adachi A, Kobashi M, Funahashi M. Glucose-responsive neurons in the brainstem. Obes Res 1995;3(Suppl. 5):735S–740S [DOI] [PubMed] [Google Scholar]
- 31.Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res 2000;856:37–47 [DOI] [PubMed] [Google Scholar]
- 32.Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res 1998;805:41–54 [DOI] [PubMed] [Google Scholar]
- 33.Llewellyn-Smith IJ. Anatomy of synaptic circuits controlling the activity of sympathetic preganglionic neurons. J Chem Neuroanat 2009;38:231–239 [DOI] [PubMed] [Google Scholar]
- 34.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res 1989;491:274–296 [DOI] [PubMed] [Google Scholar]
- 35.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol 2001;432:197–216 [DOI] [PubMed] [Google Scholar]
- 36.DiRocco RJ, Grill HJ. The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation. Science 1979;204:1112–1114 [DOI] [PubMed] [Google Scholar]
- 37.Niijima A. The effect of D-glucose on the firing rate of glucose-sensitive vagal afferents in the liver in comparison with the effect of 2-deoxy-D-glucose. J Auton Nerv Syst 1984;10:255–260 [DOI] [PubMed] [Google Scholar]
- 38.Niijima A. The effect of endogenous sugar acids on the afferent discharge rate of the hepatic branch of the vagus nerve in the rat. Physiol Behav 1988;44:661–664 [DOI] [PubMed] [Google Scholar]
- 39.Niijima A. Glucose-sensitive afferent nerve fibres in the hepatic branch of the vagus nerve in the guinea-pig. J Physiol 1982;332:315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward SM, Bayguinov J, Won K-J, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol 2003;465:121–135 [DOI] [PubMed] [Google Scholar]
- 41.Berthoud HR. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol 2004;280:827–835 [DOI] [PubMed] [Google Scholar]
- 42.Goehler LE, Sternini C. Calcitonin gene-related peptide innervation of the rat hepatobiliary system. Peptides 1996;17:209–217 [DOI] [PubMed] [Google Scholar]
- 43.Vallet PG, Baertschi AJ. Spinal afferents for peripheral osmoreceptors in the rat. Brain Res 1982;239:271–274 [DOI] [PubMed] [Google Scholar]
- 44.Schmitt M. Influences of hepatic portal receptors on hypothalamic feeding and satiety centers. Am J Physiol 1973;225:1089–1095 [DOI] [PubMed] [Google Scholar]
- 45.Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 2011;152:2552–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdakov D, Karnani MM, Gonzalez A. Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol Behav 2013;121:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donovan CM, Hamilton-Wessler M, Halter JB, Bergman RN. Primacy of liver glucosensors in the sympathetic response to progressive hypoglycemia. Proc Natl Acad Sci USA 1994;91:2863–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frizzell RT, Jones EM, Davis SN, et al. Counterregulation during hypoglycemia is directed by widespread brain regions. Diabetes 1993;42:1253–1261 [DOI] [PubMed] [Google Scholar]
- 49.Watts AG, Khan AM, Sanchez-Watts G, Salter D, Neuner CM. Activation in neural networks controlling ingestive behaviors: what does it mean, and how do we map and measure it? Physiol Behav 2006;89:501–510 [DOI] [PubMed] [Google Scholar]
- 50.Kovatchev BP, Clarke WL, Breton M, Brayman K, McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther 2005;7:849–862 [DOI] [PubMed] [Google Scholar]