Abstract
Acetylcholine regulates hormone secretion from the pancreatic islet and is thus crucial for glucose homeostasis. Little is known, however, about acetylcholine (cholinergic) signaling in the human islet. We recently reported that in the human islet, acetylcholine is primarily a paracrine signal released from α-cells rather than primarily a neural signal as in rodent islets. In this study, we demonstrate that the effects acetylcholine produces in the human islet are different and more complex than expected from studies conducted on cell lines and rodent islets. We found that endogenous acetylcholine not only stimulates the insulin-secreting β-cell via the muscarinic acetylcholine receptors M3 and M5, but also the somatostatin-secreting δ-cell via M1 receptors. Because somatostatin is a strong inhibitor of insulin secretion, we hypothesized that cholinergic input to the δ-cell indirectly regulates β-cell function. Indeed, when all muscarinic signaling was blocked, somatostatin secretion decreased and insulin secretion unexpectedly increased, suggesting a reduced inhibitory input to β-cells. Endogenous cholinergic signaling therefore provides direct stimulatory and indirect inhibitory input to β-cells to regulate insulin secretion from the human islet.
Introduction
Acetylcholine, a classical neurotransmitter that also functions as a nonneuronal paracrine signal, activates muscarinic receptors that play a key role in maintaining many metabolic functions, including glucose homeostasis. There is strong evidence that cholinergic mechanisms are important for function and survival of the endocrine pancreas, the islet of Langerhans (1). Activation of muscarinic receptors leads to improved insulin secretion from pancreatic islets (2–7). Because the muscarinic M3 receptor has been shown to play a critical role in maintaining blood glucose homeostasis in mouse models, approaches aimed at enhancing signaling through β-cell M3 receptors have been proposed as selective pharmacologic intervention points in the treatment of diabetes (5,8).
It is not always possible, however, to extrapolate structural or functional information from rodent studies to the human situation (9–15). Indeed, recent findings indicate that in the human islet acetylcholine is primarily a paracrine signal released from the glucagon-producing α-cell rather than primarily a neuronal signal as in the mouse islet (16). In light of these striking species differences, it is likely that muscarinic signaling affects human islet function in ways not predicted by studies in rodents. There is evidence that genetic variations in the M3 receptor are associated with early-onset type 2 diabetes and the acute insulin response in Pima Indians (17), but few studies have investigated cholinergic mechanisms in human islets (16,18–20). Thus, the cellular responses activated by acetylcholine in human islets remain mostly unknown, in particular those produced in endocrine cells other than β-cells.
To understand the effects of acetylcholine in the human islet, we systematically investigated the molecular and functional expression of muscarinic receptors. Using immunohistochemistry, RT-PCR, Western blots, and functional recordings of cytoplasmic free Ca2+ concentration ([Ca2+]i) and hormone secretion, we identified muscarinic receptors in the different endocrine cells of the islet. Our results indicate that human β-cells express the muscarinic receptors M3 and M5, whereas human δ-cells express M1. Activation of these receptors by endogenously released acetylcholine regulates hormone secretion in a complex manner. Endogenous acetylcholine not only stimulates β-cell function directly by activating M3 and M5 receptors, but also recruits δ-cells by activating M1 receptors and somatostatin secretion, which in turn inhibits β-cell function. Our results suggest that in the human islet, endogenous acetylcholine provides direct stimulatory as well as indirect inhibitory input to β-cells to regulate insulin secretion.
Research Design and Methods
Pancreatic Islets
We obtained human pancreatic islets from deceased donors from the Human Islet Cell Processing Facility at the Diabetes Research Institute, University of Miami Miller School of Medicine, or Integrated Islet Distribution Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and the Juvenile Diabetes Research Foundation.
Insulin, Glucagon, and Somatostatin Secretion
A high-capacity, automated perifusion system was used to dynamically measure hormone secretion from pancreatic islets (BioRep Perifusion V2.0.0; BioRep, Miami, FL). A low-pulsatility peristaltic pump pushed HEPES-buffered solution (in mmol: 125 NaCl, 5.9 KCl, 2.56 CaCl2, 1 MgCl2, 25 HEPES, and 0.1% BSA [pH 7.4]; perifusion rate of 100 µL/min) through a column containing 100 pancreatic islets immobilized in Bio-Gel P-4 Gel (Bio-Rad, Hercules, CA). Unless otherwise stated, glucose concentration was adjusted to 3 mmol/L for all experiments.
Stimuli were applied with the perifusion buffer. The perifusate was collected in an automatic fraction collector designed for a 96-well plate format. The columns containing the islets and perifusion solutions were kept at 37°C, and the perifusate in the collecting plate was kept at <4°C. Perifusates were collected every minute. Insulin and glucagon release in the perifusate was determined with the human or mouse Endocrine LINCOplex Kit following the manufacturer’s instructions (Linco Research, St. Charles, MO). Somatostatin secretion was determined using a fluorescent somatostatin enzyme-linked immunoassay kit (Phoenix Pharmaceuticals, Inc., Burlingame, CA). Thapsigargin was purchased from Invitrogen (Carlsbad, CA), the muscarinic toxins MT3 and MT7 from Peptide Institute, Inc. (Osaka, Japan), and all other drugs from Tocris Bioscience (Ellisville, MO).
To stimulate muscarinic receptors, we used acetylcholine at a concentration of 10 μmol/L because it was the lowest concentration at which we reliably stimulated hormone secretion. We did not use higher concentrations (e.g., 100 μmol/L) to avoid saturating responses that could hamper pharmacological studies with antagonists and modulators. Also, our studies suggest that the local concentration of acetylcholine may be in the range of 10 μmol/L because inhibiting acetylcholine degradation with physostigmine produced insulin responses that were similar to those elicited by 10 μmol/L acetylcholine (16).
Working with human islet preparations, we could not afford to conduct pilot studies to establish thorough concentration-response relationships for all drugs tested. We used concentrations that are approximately an order of magnitude higher than published affinity constants of agonists and antagonists for human muscarinic receptors. Because many of the drugs show a narrow range of specificity, we took care not to use concentrations that start affecting other receptors or produce unspecific effects.
Determination of Cytoplasmic Free Intracellular Calcium Concentration
Imaging of intracellular calcium concentration ([Ca2+]i) was performed as described elsewhere (21). Islets or dispersed islet cells were immersed in HEPES-buffered solution. Glucose was added to give a final concentration of 3 mmol/L. Islets or dispersed islet cells were incubated in Fura-2-acetoxymethyl (2 μmol/L; 1 h) and placed in a closed small-volume imaging chamber (Warner Instruments, Hamden, CT). Stimuli were applied with the bathing solution. Islets loaded with Fura-2 were alternatively excited at 340 and 380 nm with a monochromator light source (Cairn Reseach Optoscan Monochromator; Cairn Research Ltd., Faversham, U.K.). Images were acquired with a Hamamatsu camera (Hamamatsu Corporation, Hamamatsu City, Japan) attached to a Zeiss Axiovert 200 microscope (Carl Zeiss, Jena, Germany). Changes in the 340/380 fluorescence emission ratio were analyzed over time in individual islets and dispersed cells using MetaFluor imaging software. Peak changes in the fluorescence ratio were measured to compare response profiles between endocrine cells.
To identify endocrine cells, we transduced whole human islets with adenoviral constructs that allow islet expression of either GFP or tdTomato under the control of rat insulin promoter-1 (−410/+1 bp), rat glucagon promoter (−775/+7 bp), or human somatostatin promoter (−719/+258 bp), thus color-coding β-, α-, and δ-cells, respectively. The rat insulin promoter-1/GFP cassette was described earlier (22). The glucagon promoter (−773/+35 bp) was amplified by PCR from genomic rat DNA using 5′-TCCTTCTGTTGAATGGCCAG-3′ as the upstream primer and 5′-TTTGAGTGTGTTCTGCGCC-3′ as the downstream primer. A HindIII site was created by exchanging G for an A at position +7 bp, which allowed generation of pGlcg.GFP and pGlcg.tdTomato. The human somatostatin promoter was subcloned from pLightSwitch.SST (SwitchGear Genomics, Menlo Park, CA). The tdTomato cDNA was subcloned from pRSET.tdTomato. The respective expression cassettes consisting of promoter-GFP-bGHpA or promoter-tdTomato-bGHpA were first subcloned into pENTR1A entry vectors (Invitrogen), and adenoviruses were generated by using the ViraPower Adenoviral Expression System (Invitrogen). Human whole islets were also transfected with an adenoviral construct driving expression of DsRed2 under the control of the somatostatin promoter (pAAV-fSST-RFP). pAAV-fSST-RFP was a gift from Edward Callaway (Addgene plasmid #22913). Three to 5 days after virus transduction, we dispersed islets into single cells and imaged [Ca2+]i.
By using identified islet cells and [Ca2+]i imaging, we were able to establish a stimulation protocol that differentiates among the different endocrine cells (Supplementary Fig. 1). Responses to KCl depolarization were unique to endocrine cells and served to tell them apart from other contaminating cells from the pancreas (e.g., acinar cells). β-Cells typically responded to KCl depolarization and acetylcholine, but not to kainate or γ-aminobutyric acid (GABA). α-Cells responded to KCl depolarization and kainate, but not to acetylcholine or GABA. In addition to responding to KCl depolarization, kainate, and acetylcholine, δ-cells were the only ones that responded to GABA with increases in [Ca2+]i. We exposed dispersed cells to these stimuli in random order at the end of the experiment to avoid altering the responses to muscarinic drugs. Using this protocol, it was no longer necessary to transfect cells to identify α- and β-cells, and, indeed, for all results shown on dispersed α- and β-cells, we did not use virus transduction. However, we still had to use virus transduction to find the very scarce δ-cells.
To unmask activities of M2 and M4 receptors that do not couple to increases in [Ca2+]i and do not increase hormone secretion, we challenged [Ca2+]i and hormone responses to acetylcholine or changes in glucose concentration with receptor-specific antagonists.
Immunohistochemistry
Blocks of human pancreas (0.5 cm3) were fixed in 4% paraformaldehyde for 4 h, cryoprotected in sucrose, and cut on a cryostat (20 µm). Pancreatic biopsies from healthy donors were obtained from the Human Islet Cell Processing Facility at the Diabetes Research Institute, University of Miami Miller School of Medicine. After a rinse with PBS–Triton X-100 (0.3%), sections were incubated in blocking solution (PBS–Triton X-100 and Universal Blocker Reagent; Biogenex, San Ramon, CA). Thereafter, sections were incubated for 24 h (20°C) with primary antibodies diluted in blocking solution. Antibodies used included rabbit antibody to muscarinic M1 receptor (AB5164; Millipore) (23), rabbit antibody to CHRM1 (HPA014101; Sigma-Aldrich; validated by the Human Protein Atlas), rabbit antibody to muscarinic M2 receptor (AB5166; Millipore) (24), rabbit antibody to muscarinic M3 receptor (AB9453; Millipore, discontinued), rabbit antibody to CHRM3 (HPA024106; Sigma-Aldrich; validated by the Human Protein Atlas), mouse antibody to M4 (MAB1578; Millipore) (25,26), rabbit antibody to muscarinic M5 receptor (AB9454; Millipore, discontinued) (27), rabbit antibody to CHRM5 (HPA013172; Sigma-Aldrich; validated by the Human Protein Atlas), rat antibody to somatostatin (MAB354; Millipore), mouse antibody to glucagon (G2654-5; Sigma-Aldrich), and guinea pig antibody to insulin (A0564; DakoCytomation). Antibodies against the same receptor produced similar staining patterns, indicating specificity. Immunostaining was visualized by using Alexa Fluor–conjugated secondary antibodies (1:500 in PBS; 12 h at 20°C; Invitrogen). Cell nuclei were stained with DAPI. Slides were mounted with ProLong Anti Fade (Invitrogen). In control experiments, we incubated primary antibodies with corresponding control peptide at a ratio of 50 μg antigenic peptide/1 μg antibody at room temperature for 5 h.
Confocal images (pinhole = airy 1) of randomly selected islets (two to three islets per section, minimum three sections per human specimen) were acquired on a confocal laser-scanning microscope (Leica SP5; Leica Microsystems) with a 63× objective (HCX PL APO 63×/1.4 NA Oil λ blue) at 1,024 × 1,024 pixel resolution.
Determination of Somatostatin Secretion With Biosensor Cells
We examined somatostatin secretion using Chem-1 cells expressing the somatostatin receptor 3 coupled to the promiscuous G protein Gα15 to increase [Ca2+]i via the inositol triphosphate signaling cascade (Millipore) (Supplementary Fig. 2). Somatostatin biosensors reliably responded to low concentrations of somatostatin (threshold ≈10 nmol/L), making them highly sensitive somatostatin detectors. Somatostatin biosensor responses were measured using [Ca2+]i imaging. We loaded somatostatin biosensors with the [Ca2+]i indicator Fura-2 and plated them on cover slips in a perfusion chamber. Individual human islets were placed on top of this layer of biosensor cells. Somatostatin secretion was examined in biosensors cells located immediately downstream of the islet in recordings lasting at least 20 min to be able to detect rhythmic behavior. Somatostatin secretion was examined by inspecting recordings for increases in [Ca2+]i in biosensor cells. Changes in the amount of secreted somatostatin were quantified by measuring the area under the curve of [Ca2+]i responses in the biosensor cells during defined time intervals. We expressed data as average traces (± SEM) of the [Ca2+]i responses of the biosensor cells.
We established that biosensor cells responded only to somatostatin and not to other substances. Biosensor cells for somatostatin did not respond to changes in glucose from 3 to 11 mmol/L, from 11 to 3 mmol/L, or to neurotransmitters such as acetylcholine (10 μmol/L), GABA (100 μmol/L), serotonin (10 μmol/L), muscimol (100 μmol/L), kainate (100 μmol/L), epinephrine (10 μmol/L), or KCl (30 mmol/L). These stimuli did not themselves either elicit biosensor responses or alter the ability of biosensors to respond to somatostatin. We conducted these controls by [Ca2+]i imaging of biosensor cells plated at the same density and in the absence of islets. Crucially, the antagonist cyclosomatostatin (10 μmol/L) completely blocked somatostatin receptors on the somatostatin biosensors and eliminated responses generated from islets.
Western Blotting
Immunoblot analysis was carried out by standard methods with the antibodies used for immunohistochemistry (1:600). Human islet samples were run in parallel with whole normal brain lysate (NB820-59177; Novus Biologicals), which gave bands at similar molecular weights. For unknown reasons, M3 receptor bands for islet lysates were visible at a higher molecular weight than those for brain lysates.
RT-PCR
RNA was extracted from human brain or human islets using RiboPure Kit (Ambion, Austin, TX), and cDNA was prepared using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). PCR reactions were run in duplicate using TaqMan gene expression assays (Applied Biosystems) in a StepOnePlus Real-Time PCR System (Applied Biosystems). Relative quantification of gene expression was done based on the equation relative quantification = 2−ΔCt × 10,000, where ΔCt is the difference between the threshold cycle (Ct) value (number of cycles at which amplification for a gene reaches a threshold) of the target gene and the threshold cycle value of the ubiquitous housekeeping gene glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analyses
For statistical comparisons, we used the Student t test. Throughout the manuscript, we presented data as average ± SEM.
Results
Acetylcholine Elicits Diverse Responses in Endocrine Cells of the Human Islet
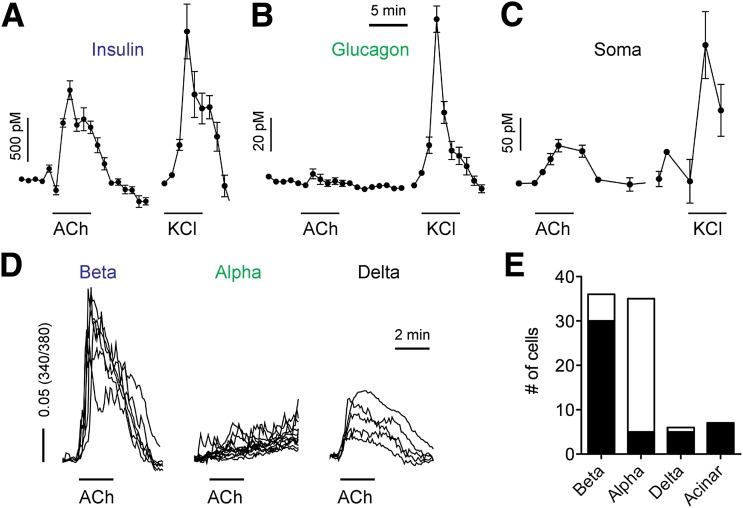
We examined hormone secretion from human islets and found that applying acetylcholine (10 μmol/L) stimulated secretion of insulin and somatostatin, but not that of glucagon (Fig. 1A–C). We further performed [Ca2+]i imaging in identified single isolated human islet cells. Acetylcholine elicited [Ca2+]i responses in β- and δ-cells but not in α-cells (Fig. 1D and E). These results indicate that acetylcholine activates receptors that couple to increases in [Ca2+]i and hormone secretion in β- and δ-cells. Our results further show that acetylcholine did not stimulate glucagon secretion, either directly or indirectly via other cells within the islet. Importantly, given that somatostatin strongly inhibits insulin and glucagon secretion (28,29), acetylcholine may affect hormonal output from the islet indirectly by activating somatostatin secretion.
Figure 1.
Acetylcholine stimulates β- and δ-cells, but not α-cells, in human islets. Acetylcholine (ACh; 10 μmol/L) increased insulin (A) and somatostatin (Soma; C), but not glucagon (B), secretion. Responses to KCl depolarization (KCl; 30 mmol/L) are used as reference (n = 3 islet preparations). Horizontal lines in all graphs denote drug application. D: Traces of [Ca2+]i responses to ACh and KCl depolarization (30 mmol/L) in β-, α-, and δ-cells. E: Quantification of results as in D showing the fractions of endocrine and exocrine cells responding to ACh (black portion of the bar; results pooled from n = 4 islet preparations).
Human β-Cells Express Functional M3 Receptors
We next sought to identify the muscarinic receptors mediating the effects of acetylcholine in the human islet. Acetylcholine acts on five different muscarinic receptors termed M1–5, which differ in signaling pathways and pharmacological properties (30). In general, activation of M1, M3, and M5 receptors increases [Ca2+]i via phospholipase C, whereas activation of M2 and M4 receptors leads to a reduction in cAMP levels. To obtain comprehensive evidence for the presence of the different muscarinic receptors, we studied their molecular and functional expression in human islets using a variety of approaches.
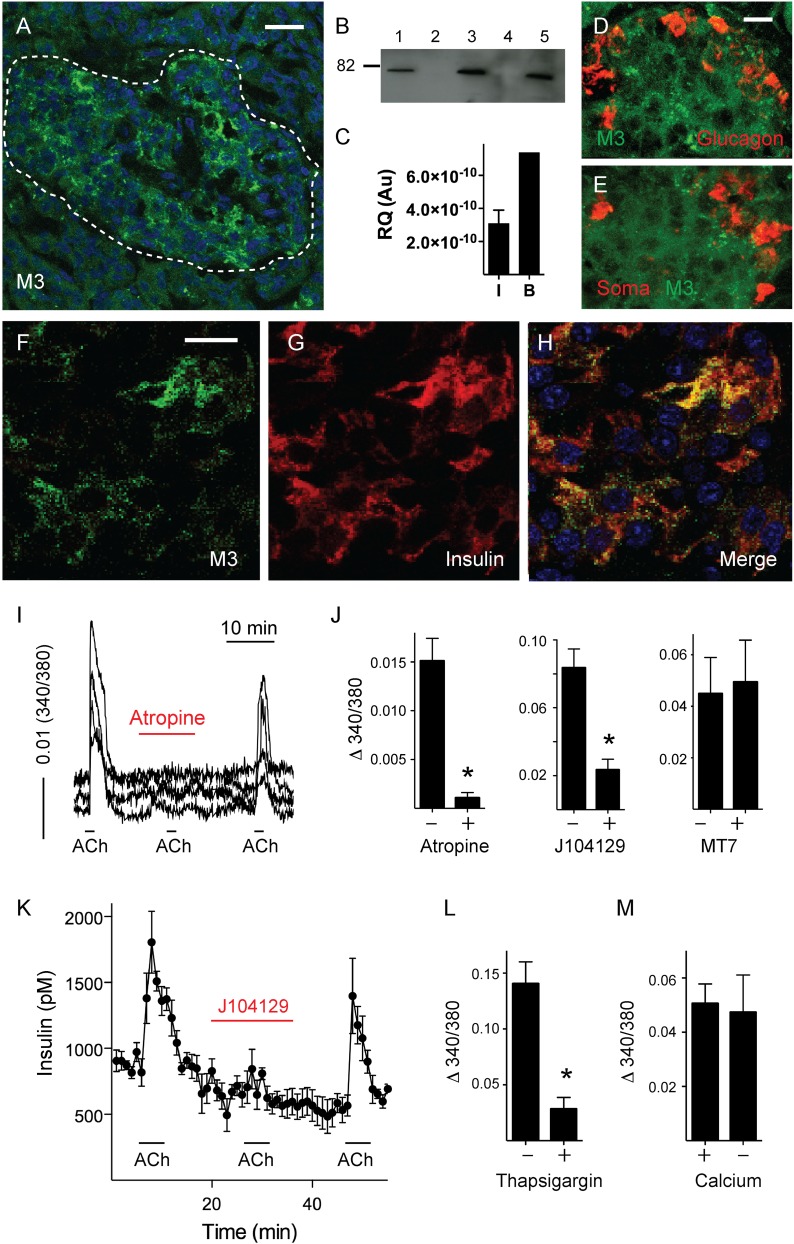
We first focused on the expression of M3 receptors because they play a major role in mouse β-cells. Immunohistochemistry, Western blotting, and RT-PCR experiments showed that M3 receptors were present in human islets (Fig. 2). M3 receptor expression was exclusively localized to β-cells (Fig. 2D–H). Application of acetylcholine induced increases in [Ca2+]i in β-cells that were blocked by atropine (∼95% reduction), indicating that the effects of acetylcholine were mediated by muscarinic receptors, not nicotinic receptors (Fig. 2I and J). The M3 receptor–specific antagonist J104129 (50 μmol) (31), but not the M1 receptor–specific antagonist MT7 (20 nmol/L) (32), reduced [Ca2+]i responses to acetylcholine (Fig. 2J). [Ca2+]i responses to acetylcholine in β-cells decreased when intracellular Ca2+ stores were depleted with thapsigargin (1 μmol/L) and were not affected by the absence of extracellular [Ca2+], indicating that acetylcholine activated signaling pathways leading to Ca2+ release from intracellular stores (Fig. 2L and M). Insulin secretion stimulated by acetylcholine was also inhibited in the presence of J104129 (Fig. 2K), which is in line with previous results (16). We conclude that β-cells in human islets express M3 receptors. These receptors are not present in α- or δ-cells.
Figure 2.
Human β-cells express functional M3 muscarinic receptors. M3 muscarinic receptors were present in human islets as detected by confocal microscopy of immunostained human pancreatic sections (A), Western blotting of lysates from five human islet preparations (B), and RT-PCR in human islets (I; n = 5) and brain (B) as a control (C). Molecular weight markers were run in parallel (shown is the 82-kDa marker). Scale bar, 20 μm. Confocal images of human pancreatic sections showing islets immunostained for M3 receptor (D–F, green), glucagon (D, red), somatostatin (Soma; E, red), or insulin (G, red). Scale bars, 10 μm (in D applies to E and in F to G and H). I: Traces of [Ca2+]i responses in β-cells showing that responses to acetylcholine (ACh; 10 μmol/L) were inhibited in the presence of atropine (10 μmol/L). J: Quantification of results as in I shows that peak responses to ACh (Δ 340/380) were inhibited by atropine and the M3 receptor–specific antagonist J104129 (50 nmol/L), but not by the M1 receptor–specific antagonist MT7 (20 nmol/L) (n = 4 islet preparations; Student t test, P < 0.05). K: Perifusion assay of insulin secretion showing that increases in insulin secretion induced by ACh were inhibited by J104129 (50 nmol/L). Quantification of [Ca2+]i responses to ACh in the presence of thapsigargin (L) and in nominal 0 [Ca2+] (M) (n = 16 cells from three preparations; Student t test; P < 0.05). Au, arbitrary unit; RQ, relative quantification. Asterisks denote significance.
Human β-Cells Express Functional M5 Receptors
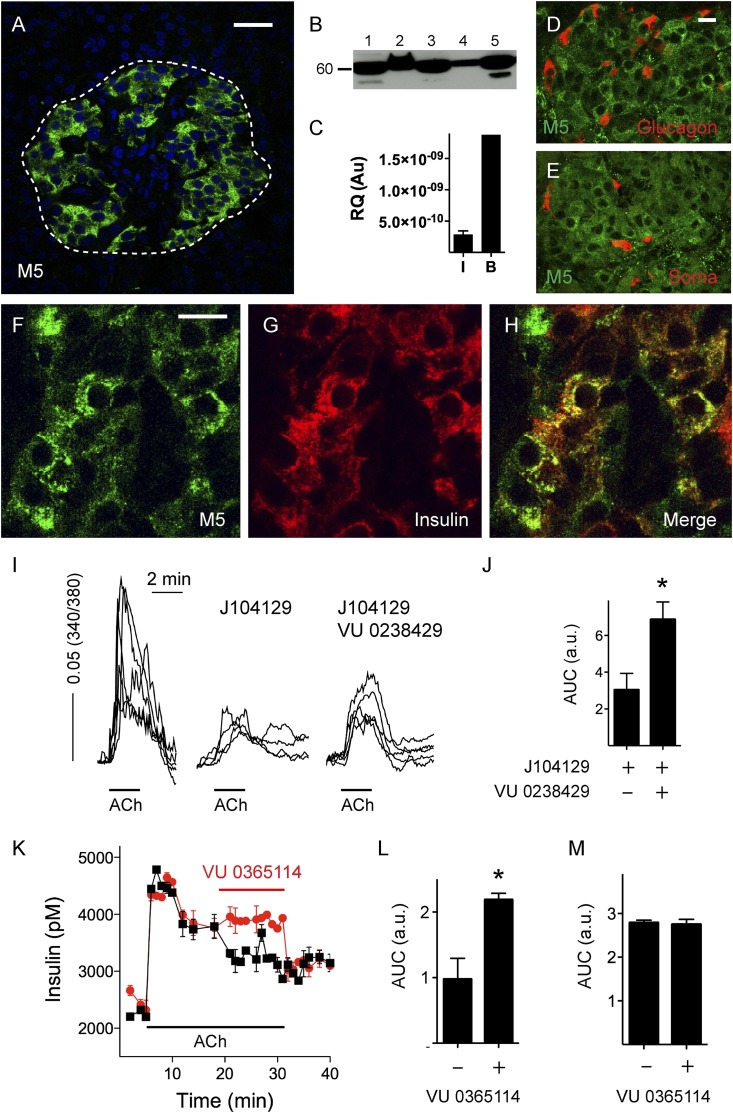
Immunohistochemical, Western blotting, and RT-PCR results further indicated that the muscarinic receptor M5 was strongly expressed in human islets (Fig. 3). Like M3 receptors, M5 receptors were localized to β-cells (Fig. 3D–H). Because there are no specific agonists or antagonists for M5 receptors, we relied on positive allosteric modulators to reveal the presence of functional M5 receptors (33,34). As described above, the M3 receptor antagonist J104129 inhibited [Ca2+]i responses in β-cells to acetylcholine, but not completely (Fig. 2J and K). We found that the remaining [Ca2+]i response after blocking M3 receptors with J104129 was increased in the presence of the M5 receptor–specific allosteric modulator VU 0238429 (10 μmol/L; Fig. 3I and J) (33). Similarly, insulin responses to acetylcholine could be amplified by modulating the M5 receptor with VU 0365114 (10 μmol/L), another allosteric modulator of M5 receptors (Fig. 3K and L) (34). VU 0365114 (10 μmol/L) further increased insulin secretion at basal glucose concentration (3 mmol/L) in the absence of exogenous acetylcholine, indicating that endogenous acetylcholine stimulated M5 receptors in β-cells. The insulin response to VU 0365114 was blocked by atropine, and VU 0365114 did not alter somatostatin secretion in response to acetylcholine or at basal glucose concentration (Fig. 3M). These results show that human β-cells, but not δ-cells, express functional M5 receptors.
Figure 3.
Human β-cells express functional M5 muscarinic receptors. M5 muscarinic receptors were present in human islets as detected by confocal microscopy of immunostained human pancreatic sections (A), Western blotting of lysates from five human islet preparations (B), and RT-PCR in human islets (I; n = 5) and brain (B) as a control (C). Molecular weight markers were run in parallel (shown is the 60-kDa marker). Scale bar, 20 μm. Confocal images of human pancreatic sections showing islets immunostained for M5 receptor (D–F, green), glucagon (D, red), somatostatin (Soma; E, red), or insulin (G, red). Scale bars, 10 μm (in D applies to E and in F to G and H). I: Traces of [Ca2+]i responses in β-cells showing responses to acetylcholine (ACh) alone (10 μmol/L, left), in the presence of J104129 (50 nmol/L, middle), and in the presence of J104129 and the M5 receptor modulator VU 0238429 (10 μmol/L, right). J: Quantification of results as in I shows that peak responses to ACh (Δ 340/380) in the presence of J104129 were amplified by VU 0238429 (n = 9 cells from three preparations; Student t test, P < 0.05). K: Perifusion assay of insulin secretion showing that increases in insulin secretion induced by ACh were amplified by VU 0365114, an allosteric modulator of M5 receptors (10 μmol/L). L: Quantification of results as in K shows that VU 0365114 increased insulin secretion stimulated by ACh (n = 4 preparations; Student t test, P < 0.05). M: Quantification of perifusion assays of somatostatin secretion shows that VU 0365114 did not alter somatostatin secretion stimulated by ACh (n = 3 preparations). Au/a.u., arbitrary unit; AUC, area under the curve; RQ, relative quantification. Asterisks denote significance.
Human δ-Cells Express Functional M1 Receptors
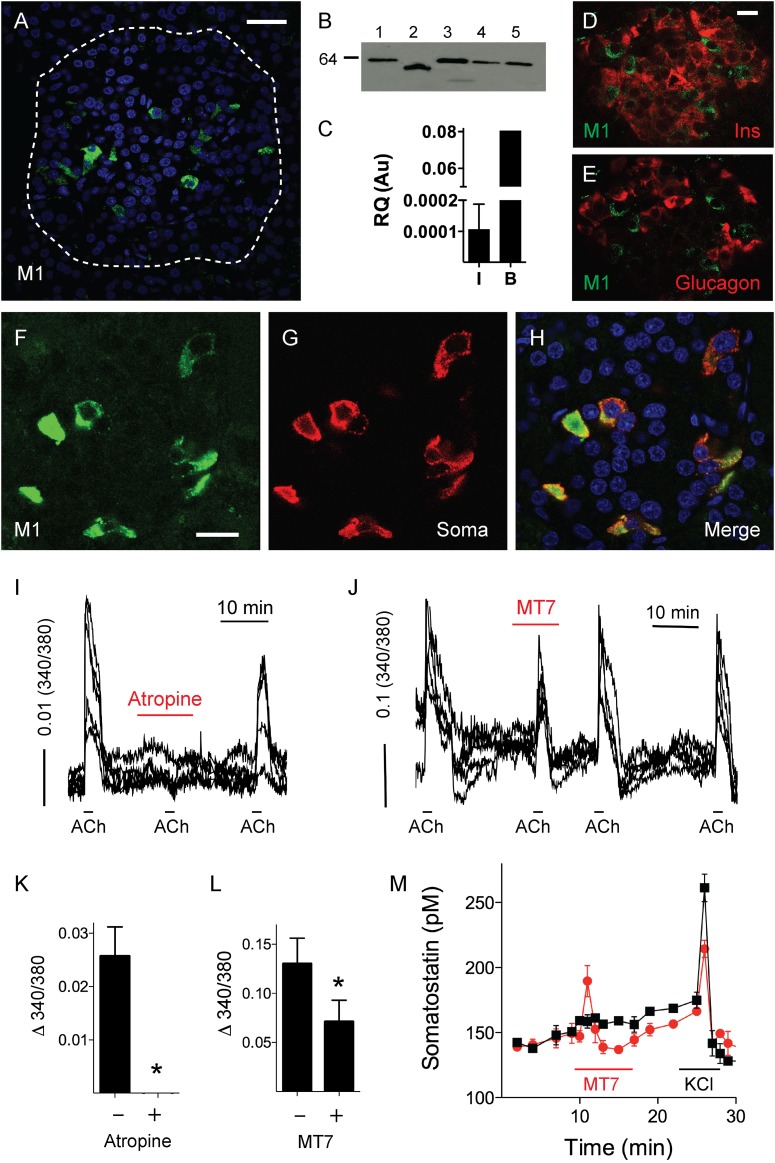
Immunohistochemistry, Western blotting, and RT-PCR experiments revealed that M1 receptors are expressed in human islets (Fig. 4). In contrast to the expression pattern of M3 and M5 receptors, few cells were immunostained for M1 receptors (Fig. 4A). These cells were somatostatin-secreting δ-cells, and no other cell type within the human islet expressed M1 receptors (Fig. 4D–H). When we inspected δ-cells with [Ca2+]i imaging, we found that responses to acetylcholine were abolished by atropine, indicating that responses were mediated by muscarinic receptors (Fig. 4I and K). The M1-specific antagonist MT7 (20 nmol/L) (32) inhibited [Ca2+]i responses elicited by acetylcholine (Fig. 4J and L). These responses were only partially inhibited by MT7, most likely because this toxin fails to elicit a complete inhibition of the receptor (35). Somatostatin secretion was also affected by application of MT7, with an initial, short-lived increase followed by a prolonged inhibition of secretion (Fig. 4M). These changes in somatostatin secretion occurred in the absence of exogenously applied acetylcholine, indicating that endogenously released acetylcholine was activating M1 receptors in δ-cells at basal glucose concentration (3 mmol/L).
Figure 4.
Human δ-cells express functional M1 muscarinic receptors. M1 muscarinic receptors were present in human islets as detected by confocal microscopy of immunostained human pancreatic sections (A), Western blotting of lysates from five human islet preparations (B), and RT-PCR in human islets (I; n = 5) and brain (B) as a control (C). Molecular weight markers were run in parallel (shown is the 64-kDa marker). Scale bar, 20 μm. Confocal images of human pancreatic sections showing islets immunostained for M1 receptor (D–F, green), insulin (Ins; D, red), glucagon (E, red), or somatostatin (Soma; G, red). Scale bars, 10 μm (in D applies to E and in F to G and H). Traces of [Ca2+]i responses in δ-cells showing that responses to acetylcholine (ACh; 10 μmol/L) were inhibited in the presence of atropine (10 μmol/L; I) and the M1 receptor–specific antagonist MT7 (20 nmol/L; J). Quantification of results as in I and J shows that peak responses to ACh (Δ 340/380) were inhibited by atropine (K) and MT7 (L) (n > 12 cells from three preparations; Student t test, P < 0.05). M: Perifusion assay of somatostatin secretion showing that basal somatostatin secretion at 3 mmol/L glucose concentration was inhibited by MT7 (20 nmol/L; n = 3 preparations). Au, arbitrary unit; RQ, relative quantification. Asterisks denote significance.
Expression of M2 and M4 Receptors in Human Islets
M2 and M4 receptors were elusive to molecular and functional characterization. Although both were detected in RT-PCR experiments, M2 receptor immunostaining was absent in the human islet, and immunostaining for M4 receptors gave inconsistent results. Furthermore, we could not determine any changes in hormone secretion or [Ca2+]i using the M2 antagonist AF-DX116 (100 nmol/L) (36) and the M4 antagonist MT3 (10 nmol/L) (37) in the absence or presence of acetylcholine or at different glucose concentrations.
Direct and Indirect Effects of Endogenous Acetylcholine on Insulin Secretion
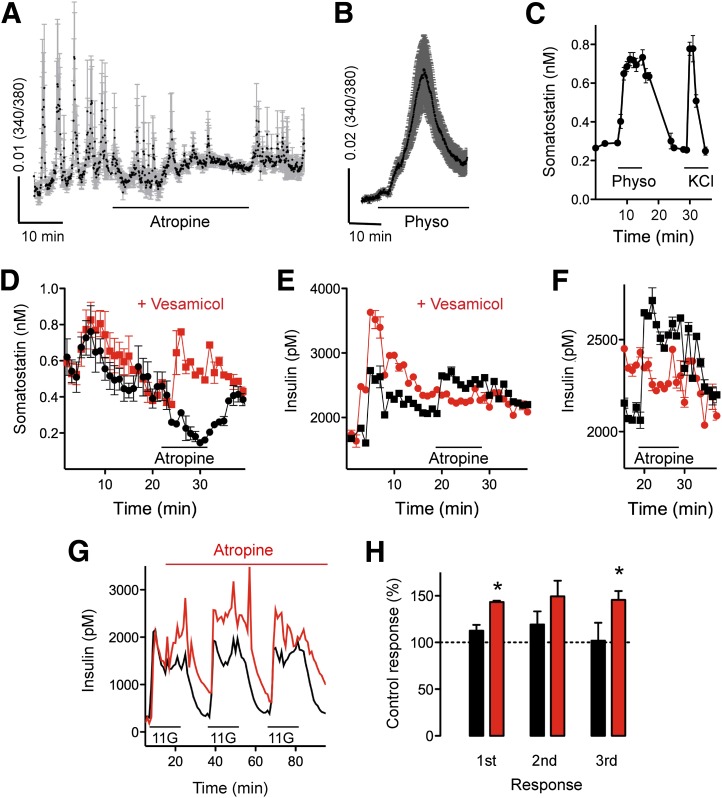
Because β- and δ-cells express different muscarinic receptors, we sought to understand how endogenously released acetylcholine affects hormone output from the human islet. Using biosensor cells to detect somatostatin secretion in real time (Supplementary Fig. 2), we found that somatostatin secretion was inhibited by atropine (10 μmol/L) and stimulated by blocking acetylcholine degradation with physostigmine (30 μmol/L), indicating that somatostatin secretion was strongly dependent on endogenous cholinergic input (Fig. 5A and B). Perifusion assays of hormone secretion showed similar effects on somatostatin secretion in the presence of atropine and physostigmine at basal glucose concentration (3 mmol/L; Fig. 5C and D). Preincubation with vesamicol (10 μmol/L), a blocker of vesicular acetylcholine transporter that depletes α-cells of releasable acetylcholine (16), abolished the inhibition of somatostatin secretion by atropine (Fig. 5D), suggesting that acetylcholine released from α-cells stimulates somatostatin release.
Figure 5.
Effects of endogenous acetylcholine on hormone secretion from the human islet. A: Pulsatile secretion of somatostatin as measured by somatostatin biosensor cells was inhibited by atropine (10 μmol/L) at 1 mmol/L glucose concentration (representative of four experiments from three islet preparations). B: Somatostatin secretion as measured by somatostatin biosensor cells increased in the presence of the cholinesterase inhibitor physostigmine (Physo; 30 μmol/L) at 3 mmol/L glucose concentration (representative of four experiments from three islet preparations). Perifusion assays showing increases in somatostatin secretion in the presence of Physo (30 μmol/L) or KCl (30 mmol/L; n = 3 preparations) (C) and decreases in somatostatin secretion in the presence of atropine (10 μmol/L; n = 4 preparations) (D). E and F: Perifusion assay of insulin secretion showing an increase in insulin secretion in the presence of atropine (10 μmol/L; n = 3 preparations). F is a closeup of E. In D–F, the red trace shows secretion in the presence of vesamicol, a blocker of vesicular acetylcholine transporter (10 μmol/L). Glucose concentration was changed from 3 to 11 mmol/L at the beginning of the experiment. G: Perifusion assay showing that atropine (10 μmol/L) amplified insulin responses to repeated increases in glucose concentration to 11 mmol/L (11G). A control experiment with untreated islets was run in parallel (black trace). Representative of four experiments. H: Quantification of results as in G shows significant increases in insulin secretion in the presence of atropine (n = 4 preparations; one sample t test to compare the actual mean to a theoretical mean of 100%, P < 0.05). Asterisks denote significance.
Because somatostatin is a strong inhibitor of insulin secretion (28,29), we investigated if endogenous acetylcholine could affect insulin secretion indirectly via δ-cells. Applying the broad muscarinic antagonist atropine (10 μmol/L) to block cholinergic input increased insulin secretion at basal glucose concentration (3 mmol/L; Fig. 5E and F), suggesting that under basal conditions, the net effect of endogenous acetylcholine is a decrease in insulin secretion. This inhibition likely involves the δ-cell and somatostatin secretion because direct endogenous cholinergic input to the β-cell would stimulate insulin secretion. Because preincubation with vesamicol abolished atropine-induced increases in insulin secretion and decreases in somatostatin secretion (Fig. 5D–F), it is likely that acetylcholine derived from α-cells inhibits insulin secretion by stimulating somatostatin secretion.
In vivo, α-cells become activated at periods of ∼10 min (38). To imitate this pattern in vitro and to periodically stimulate acetylcholine secretion from α-cells, we used a protocol in which glucose concentrations were varied every 10 min from low (3 mmol/L) to high concentrations (11 mmol/L) (16). Under these circumstances, applying the M3 receptor–specific antagonist J104129 (50 nmol/L) decreased the amount of secreted insulin, confirming previous results (Supplementary Fig. 3) (16). However, in the presence of the broad muscarinic antagonist atropine (10 μmol/L), insulin secretion increased (Fig. 5G and H). These results indicate that endogenously released acetylcholine stimulates insulin secretion directly by activating M3 receptors in β-cells and inhibits insulin secretion indirectly by activating M1 receptors and somatostatin secretion from δ-cells.
Discussion
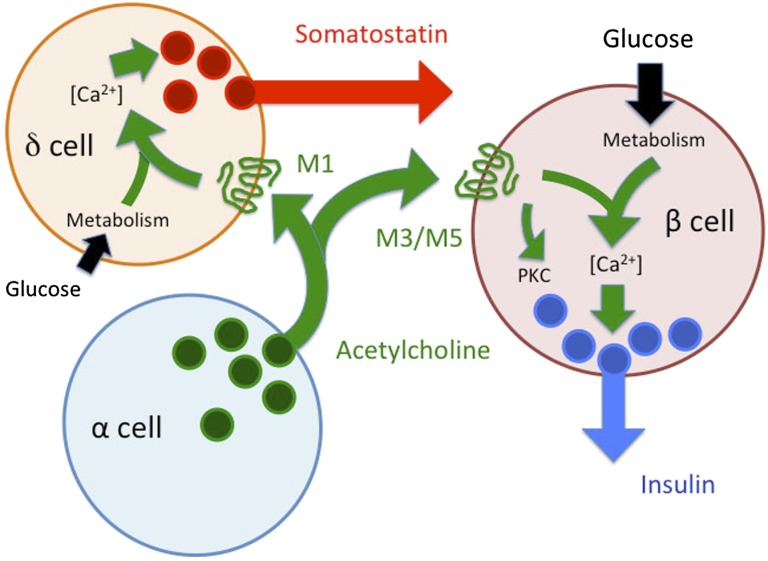
We have demonstrated that acetylcholine activates M3 and M5 muscarinic receptors in β-cells and M1 muscarinic receptors in δ-cells to modulate hormone secretion from the human pancreatic islet (Fig. 6). δ-Cells and somatostatin secretion, in particular, are strongly influenced by endogenously released acetylcholine. We previously showed that endogenous acetylcholine primes β-cells to respond optimally to subsequent increases in glucose concentration (16). In this study, we show that endogenously released acetylcholine activates δ-cells to secrete somatostatin, a major inhibitor of insulin secretion. Thus, intraislet cholinergic signaling provides positive and negative signaling pathways regulating insulin secretion. The net effects of endogenous acetylcholine on insulin secretion likely depend on the spatial and temporal patterns of acetylcholine secretion from neural or paracrine sources as well as on the biophysical properties of the different muscarinic receptors. The presence of multiple muscarinic receptors on different endocrine cells in the human islets has important implications for the use of cholinergic agents to intervene in diabetes.
Figure 6.
Proposed model for paracrine cholinergic signaling in the human islet. Acetylcholine is released from α-cells and activates M1 receptors on δ-cells and M3 and M5 receptors on β-cells. Acetylcholine stimulates insulin secretion directly, but at the same time provides inhibition via somatostatin secretion from δ-cells. The net effect of acetylcholine on insulin secretion likely depends on the proximity of the different cells, the pharmacological properties of the different receptors, and glucose concentration. Not to be neglected is additional input from cholinergic innervation. PKC, protein kinase C.
Our results reveal that the expression pattern of muscarinic receptors in the human islet is different from what has been described in cell lines and rodent islets (3,39–41). While M3 receptors play a major role in both human and mouse β-cells, we now show that human β-cells also express M5 receptors, which are barely detectable in rodent islets (3,39). In the human islet, M1 receptors were confined to δ-cells and not expressed in β-cells, as studies on rodent islets had suggested (3,39,42). Also, in contrast to what has been reported for rodent α-cells, human α-cells did not express muscarinic receptors that couple to increases in [Ca2+]i or glucagon secretion (43–45). None of the muscarinic agonists and antagonists tested affected α-cell responses or glucagon secretion, even under conditions that would unmask receptors such as M2 and M4 that are coupled to decreases in cell activity. Thus, the effects of acetylcholine in human islets could not have been predicted from studies in rodents.
To understand how acetylcholine may influence hormone secretion from the human islet, it is important to consider human islet cell architecture, the position of the different endocrine cell types with respect to one another, and circulatory flow. Endocrine cells of different types intermingle more in the human islet, such that most β-cells face α-cells, δ-cells, or both (10). This close association enables paracrine interactions that may not be possible in the mouse islet (46). An additional consequence of human islet cytoarchitecture is that blood flows through regions with heterogeneous cell populations. This rules out a hierarchy in the sequence in which the different endocrine cells are perfused. Thus, endocrine cells in the human islet are positioned to be able to influence mutually via paracrine signaling using the interstitial space or the vascular route. Given the cellular arrangement in the human islet, paracrine signaling molecules released by any endocrine cells can readily reach the other cell types. This is likely the case for acetylcholine, which we recently found to be secreted as a paracrine signal within the human islet (16).
In light of these findings, models for islet cholinergic signaling now should feature cholinergic input primarily from endocrine α-cells in human islets and be distinguished from cholinergic input in mouse islets, which originates mainly from parasympathetic innervation (16,47,48). The local paracrine source of acetylcholine makes sense in the context of a cellular arrangement that promotes paracrine interactions. In human islets, most β-cells are directly exposed to α-cell secretions, whereas in mouse islets, paracrine signals derived from α-cells would affect only a minority of β-cells. It is also likely that in the human islet, paracrine acetylcholine readily reaches δ-cells. This proximity is important because acetylcholine is rapidly degraded in the extracellular space. Thus, within the human islet, paracrine acetylcholine can act on endocrine cells of different types and activate a combination of diverse muscarinic receptors. Given the variety of receptors, target cells, and sources for acetylcholine, it is not surprising that the effects of endogenous cholinergic signaling could not be mimicked by exogenous acetylcholine. Indeed, applying exogenous acetylcholine activated both insulin and somatostatin secretion, but blocking endogenous cholinergic signaling with atropine paradoxically increased insulin secretion.
What is the possible role of paracrine acetylcholine in the control of insulin secretion during fluctuations in plasma glucose concentrations? Because α-cells are activated at glucose concentrations that inhibit insulin secretion, it seems improbable that acetylcholine derived from α-cells affects β-cell function. It is important, however, to keep in mind that acetylcholine activates signaling cascades that last for tens of minutes (49). Thus, even if secreted exclusively during glucose nadirs, acetylcholine can potentiate β-cell responses to forthcoming rises in glucose concentration. An alternative proposal is that acetylcholine is released independently of glucagon in response to Ca2+ entry via L-type Ca2+ channels at elevated glucose (50). That acetylcholine and glucagon do not appear to be stored in the same granules suggest that independent release is possible (16). Not to be neglected is a conceivable contribution from a subgroup of δ-cells, which also express vesicular acetylcholine transporter and therefore should be capable of vesicular release of acetylcholine (16). A cholinergic input from δ-cells would potentiate insulin secretion while β-cells are being stimulated at elevated glucose.
Given the multiple sources for acetylcholine and the various muscarinic receptors, the net effects of cholinergic signaling in the islet will be the sum of many activities that may be constantly fine-tuned under different physiological conditions. The different muscarinic receptors have different desensitization, internalization, and downregulation properties that may affect the net effects of acetylcholine in the islet (51,52). A selective downregulation of M1 receptors in δ-cells after prolonged exposure to acetylcholine, for instance, could shift the balance to a point at which cholinergic signaling now promotes insulin secretion. It is also likely that changes in glucose concentration or diabetic conditions affect cholinergic signaling. Because the circumstances in the human islet will be very different from those in rodent models and because experiments are more difficult to conduct in human beings, addressing these possibilities experimentally may require establishing research models in which mice are transplanted with human islets (53,54).
More than 10 years ago, Gilon and Henquin (1) thoroughly reviewed an impressive amount of data on cholinergic mechanisms in the islet. In view of recent findings, however, it seems that we are just starting to understand cholinergic signaling in the human islet. Despite decades of research, the identity of muscarinic receptors mediating the effects of acetylcholine had remained elusive. The current study represents an effort to determine the role of muscarinic receptors that couple to an increase in activity in islet endocrine cells, namely M1, M3, and M5 receptors. Although still elusive in our hands, the possibility of negative regulation involving M2 or M4 receptors is intriguing and warrants exploring further the role of M2 and M4 receptors.
Supplementary Material
Article Information
Acknowledgments. The authors thank Kevin Johnson, Lily Barash, and Yuan Liu for technical assistance.
Funding. This work was supported by the Diabetes Research Institute Foundation, National Institutes of Health grants R56-DK-084321 (to A.C.) and R01-DK-084321 (to A.C.), the Juvenile Diabetes Research Foundation, the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Diabetes Association, the Erling-Persson Family Foundation, the Skandia Insurance Company Ltd., Strategic Research Programme in Diabetes at Karolinska Institutet, the Berth von Kantzow Foundation, Virtual Biodiversity Research and Access Network for Taxonomy (FP7-2288933), the Knut and Alice Wallenberg Foundation, funds from Karolinska Institutet, Diabetes Research and Wellness Foundation, the Stichting af Jochnick Foundation, and the World Class University program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-2008-000-10105-0).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M. designed experiments, researched data, and edited the manuscript. R.R.-D. researched data and reviewed and edited the manuscript. A.F. and M.C.J.-S. researched data. P.-O.B. and A.C. conceived and designed experiments, analyzed data, and wrote the manuscript. A.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-1371/-/DC1.
References
- 1.Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 2001;22:565–604 [DOI] [PubMed] [Google Scholar]
- 2.Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 2000;43:393–410 [DOI] [PubMed] [Google Scholar]
- 3.Duttaroy A, Zimliki CL, Gautam D, Cui Y, Mears D, Wess J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes 2004;53:1714–1720 [DOI] [PubMed] [Google Scholar]
- 4.Zawalich WS, Zawalich KC, Tesz GJ, et al. Effects of muscarinic receptor type 3 knockout on mouse islet secretory responses. Biochem Biophys Res Commun 2004;315:872–876 [DOI] [PubMed] [Google Scholar]
- 5.Gautam D, Han SJ, Hamdan FF, et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 2006;3:449–461 [DOI] [PubMed] [Google Scholar]
- 6.Henquin JC, Nenquin M. The muscarinic receptor subtype in mouse pancreatic B-cells. FEBS Lett 1988;236:89–92 [DOI] [PubMed] [Google Scholar]
- 7.Boschero AC, Szpak-Glasman M, Carneiro EM, et al. Oxotremorine-m potentiation of glucose-induced insulin release from rat islets involves M3 muscarinic receptors. Am J Physiol 1995;268:E336–E342 [DOI] [PubMed] [Google Scholar]
- 8.Gautam D, Han SJ, Duttaroy A, et al. Role of the M3 muscarinic acetylcholine receptor in beta-cell function and glucose homeostasis. Diabetes Obes Metab 2007;9(Suppl. 2):158–169 [DOI] [PubMed] [Google Scholar]
- 9.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–1097 [DOI] [PubMed] [Google Scholar]
- 10.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun M, Ramracheya R, Johnson PR, Rorsman P. Exocytotic properties of human pancreatic beta-cells. Ann N Y Acad Sci 2009;1152:187–193 [DOI] [PubMed] [Google Scholar]
- 12.Braun M, Ramracheya R, Amisten S, et al. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia 2009;52:1566–1578 [DOI] [PubMed] [Google Scholar]
- 13.Braun M, Ramracheya R, Bengtsson M, et al. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes 2010;59:1694–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosco D, Armanet M, Morel P, et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 2010;59:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacques-Silva MC, Correa-Medina M, Cabrera O, et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci U S A 2010;107:6465–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Diaz R, Dando R, Jacques-Silva MC, et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med 2011;17:888–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Traurig M, Ma L, et al. CHRM3 gene variation is associated with decreased acute insulin secretion and increased risk for early-onset type 2 diabetes in Pima Indians. Diabetes 2006;55:3625–3629 [DOI] [PubMed] [Google Scholar]
- 18.Cabrera O, Jacques-Silva MC, Berman DM, et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell Transplant 2008;16:1039–1048 [PMC free article] [PubMed] [Google Scholar]
- 19.Rojas E, Carroll PB, Ricordi C, Boschero AC, Stojilkovic SS, Atwater I. Control of cytosolic free calcium in cultured human pancreatic beta-cells occurs by external calcium-dependent and independent mechanisms. Endocrinology 1994;134:1771–1781 [DOI] [PubMed] [Google Scholar]
- 20.Shi CL, Täljedal IB, Nordin A, Andersson A. Human islets transplanted to nude mice: in vitro insulin release from retrieved grafts. Ups J Med Sci 2000;105:193–206 [PubMed] [Google Scholar]
- 21.Cabrera O, Jacques-Silva MC, Speier S, et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab 2008;7:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leibiger B, Moede T, Schwarz T, et al. Short-term regulation of insulin gene transcription by glucose. Proc Natl Acad Sci U S A 1998;95:9307–9312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AI, Stormann TM, Brann MR. Bacterial expression of human muscarinic receptor fusion proteins and generation of subtype-specific antisera. FEBS Lett 1990;275:65–69 [DOI] [PubMed] [Google Scholar]
- 24.Disney AA, Aoki C. Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin-immunoreactive neurons. J Comp Neurol 2008;507:1748–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AI, Edmunds SM, Hersch SM, Wiley RG, Heilman CJ. Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol 1995;351:339–356 [DOI] [PubMed] [Google Scholar]
- 26.Yamada ES, Dmitrieva N, Keyser KT, Lindstrom JM, Hersh LB, Marshak DW. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. J Comp Neurol 2003;461:76–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strang CE, Renna JM, Amthor FR, Keyser KT. Muscarinic acetylcholine receptor localization and activation effects on ganglion response properties. Invest Ophthalmol Vis Sci 2010;51:2778–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauge-Evans AC, King AJ, Carmignac D, et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 2009;58:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunicardi FC, Kleinman R, Moldovan S, et al. Immunoneutralization of somatostatin, insulin, and glucagon causes alterations in islet cell secretion in the isolated perfused human pancreas. Pancreas 2001;23:302–308 [DOI] [PubMed] [Google Scholar]
- 30.Eglen RM. Overview of muscarinic receptor subtypes. Handb Exp Pharmacol 2012;208:3–28 [DOI] [PubMed] [Google Scholar]
- 31.Mitsuya M, Mase T, Tsuchiya Y, et al. J-104129, a novel muscarinic M3 receptor antagonist with high selectivity for M3 over M2 receptors. Bioorg Med Chem 1999;7:2555–2567 [DOI] [PubMed] [Google Scholar]
- 32.Bradley KN, Rowan EG, Harvey AL. Effects of muscarinic toxins MT2 and MT7, from green mamba venom, on m1, m3 and m5 muscarinic receptors expressed in Chinese Hamster Ovary cells. Toxicon 2003;41:207–215 [DOI] [PubMed] [Google Scholar]
- 33.Bridges TM, Marlo JE, Niswender CM, et al. Discovery of the first highly M5-preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5-trifluoromethoxy N-benzyl isatins. J Med Chem 2009;52:3445–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bridges TM, Kennedy JP, Cho HP, et al. Chemical lead optimization of a pan G(q) mAChR M(1), M(3), M(5) positive allosteric modulator (PAM) lead. Part I: Development of the first highly selective M(5) PAM. Bioorg Med Chem Lett 2010;20:558–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onali P, Adem A, Karlsson E, Olianas MC. The pharmacological action of MT-7. Life Sci 2005;76:1547–1552 [DOI] [PubMed] [Google Scholar]
- 36.Billard W, Binch H, 3rd, Crosby G, McQuade RD. Identification of the primary muscarinic autoreceptor subtype in rat striatum as m2 through a correlation of in vivo microdialysis and in vitro receptor binding data. J Pharmacol Exp Ther 1995;273:273–279 [PubMed] [Google Scholar]
- 37.Olianas MC, Ingianni A, Maullu C, Adem A, Karlsson E, Onali P. Selectivity profile of muscarinic toxin 3 in functional assays of cloned and native receptors. J Pharmacol Exp Ther 1999;288:164–170 [PubMed] [Google Scholar]
- 38.Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med 1979;301:1023–1027 [DOI] [PubMed] [Google Scholar]
- 39.Iismaa TP, Kerr EA, Wilson JR, Carpenter L, Sims N, Biden TJ. Quantitative and functional characterization of muscarinic receptor subtypes in insulin-secreting cell lines and rat pancreatic islets. Diabetes 2000;49:392–398 [DOI] [PubMed] [Google Scholar]
- 40.Miguel JC, Abdel-Wahab YH, Mathias PC, Flatt PR. Muscarinic receptor subtypes mediate stimulatory and paradoxical inhibitory effects on an insulin-secreting beta cell line. Biochim Biophys Acta 2002;1569:45–50 [DOI] [PubMed] [Google Scholar]
- 41.Grill V, Ostenson CG. Muscarinic receptors in pancreatic islets of the rat. Demonstration and dependence on long-term glucose environment. Biochim Biophys Acta 1983;756:159–162 [DOI] [PubMed] [Google Scholar]
- 42.Balakrishnan S, Mathew J, Antony S, Paulose CS. Muscarinic M(1), M(3) receptors function in the brainstem of streptozotocin induced diabetic rats: their role in insulin secretion from the pancreatic islets as a function of age. Eur J Pharmacol 2009;608:14–22 [DOI] [PubMed] [Google Scholar]
- 43.Kimura H, Katagiri K, Ohno T, et al. Effect of acetylcholine and new cholinergic derivative on amylase output, insulin, glucagon, and somatostatin secretions from perfused isolated rat pancreas. Horm Metab Res 1982;14:356–360 [DOI] [PubMed] [Google Scholar]
- 44.Ahrén B, Lundquist I. Secretin potentiates cholinergically induced glucagon secretion in the mouse. Acta Physiol Scand 1986;128:575–578 [DOI] [PubMed] [Google Scholar]
- 45.Winzell MS, Ahrén B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther 2007;116:437–448 [DOI] [PubMed] [Google Scholar]
- 46.Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol 2013;24:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 2011;14:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Diaz R, Speier S, Molano RD, et al. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc Natl Acad Sci U S A 2012;109:21456–21461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zawalich WS, Zawalich KC, Rasmussen H. Cholinergic agonists prime the beta-cell to glucose stimulation. Endocrinology 1989;125:2400–2406 [DOI] [PubMed] [Google Scholar]
- 50.Rorsman P, Braun M, Zhang Q. Regulation of calcium in pancreatic α- and β-cells in health and disease. Cell Calcium 2012;51:300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thangaraju A, Sawyer GW. Comparison of the kinetics and extent of muscarinic M1-M5 receptor internalization, recycling and downregulation in Chinese hamster ovary cells. Eur J Pharmacol 2011;650:534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nathanson NM. Synthesis, trafficking, and localization of muscarinic acetylcholine receptors. Pharmacol Ther 2008;119:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ichii H, Inverardi L, Pileggi A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant 2005;5:1635–1645 [DOI] [PubMed] [Google Scholar]
- 54.Greiner DL, Brehm MA, Hosur V, Harlan DM, Powers AC, Shultz LD. Humanized mice for the study of type 1 and type 2 diabetes. Ann N Y Acad Sci 2011;1245:55–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.