Abstract
Glucagon-like peptide 1 (GLP-1) acutely recruits muscle microvasculature, increases muscle delivery of insulin, and enhances muscle use of glucose, independent of its effect on insulin secretion. To examine whether GLP-1 modulates muscle microvascular and metabolic insulin responses in the setting of insulin resistance, we assessed muscle microvascular blood volume (MBV), flow velocity, and blood flow in control insulin-sensitive rats and rats made insulin-resistant acutely (systemic lipid infusion) or chronically (high-fat diet [HFD]) before and after a euglycemic-hyperinsulinemic clamp (3 mU/kg/min) with or without superimposed systemic GLP-1 infusion. Insulin significantly recruited muscle microvasculature and addition of GLP-1 further expanded muscle MBV and increased insulin-mediated glucose disposal. GLP-1 infusion potently recruited muscle microvasculature in the presence of either acute or chronic insulin resistance by increasing muscle MBV. This was associated with an increased muscle delivery of insulin and muscle interstitial oxygen saturation. Muscle insulin sensitivity was completely restored in the presence of systemic lipid infusion and significantly improved in rats fed an HFD. We conclude that GLP-1 infusion potently expands muscle microvascular surface area and improves insulin’s metabolic action in the insulin-resistant states. This may contribute to improved glycemic control seen in diabetic patients receiving incretin-based therapy.
INTRODUCTION
Skeletal muscle is a major target organ of insulin action and responsible for ∼80–90% of insulin-stimulated whole-body glucose disposal (1,2). To exert its action in muscle, insulin must first be delivered to the microvasculature nourishing the myocytes and then transported through the vascular endothelium into the muscle interstitium, two rate-limiting steps in overall insulin action (3–5). Recent evidence has confirmed a pivotal role of muscle microvasculature in the determination of muscle insulin action, as it provides the endothelial exchange surface area and changes in muscle microvascular perfusion profoundly affect insulin delivery to and action in muscle (5,6). We and others have shown that expansion of muscle microvascular blood volume (MBV), as induced by exercise, angiotensin II type 1 receptor blockade, and adiponectin increases muscle delivery and action of insulin (7–9). On the contrary, microvascular decruitment induced by angiotensin II type 2 receptor blockade exerts the opposite effects (10).
Insulin facilitates its own delivery to muscle by both recruiting muscle microvasculature, thereby increasing microvascular exchange surface area, and enhancing its own trans-endothelial transport (3,4). These actions contribute up to 40% of insulin-mediated glucose disposal in rats during insulin clamp (11). Insulin resistance is clearly present in the muscle microcirculation in humans or animal models of obesity and/or diabetes, which is directly coupled with metabolic insulin resistance (12,13). As such, microvascular endothelium has emerged as an attractive therapeutic target for diabetes prevention and/or management, as expansion of muscle microvascular exchange surface area and improvement of microvascular insulin sensitivity afford the potential to increase muscle delivery and action of insulin with resultant improvement in glycemic control (14,15).
Glucagon-like peptide 1 (GLP-1), an incretin secreted in response to nutrient intake, stimulates glucose-dependent insulin secretion, inhibits glucagon secretion and regulates postprandial glycemia (16,17). GLP-1 also has many nonpancreatic actions, including a beneficial effect on the endothelium (18). Infusion of GLP-1 improves vasodilator response to acetylcholine in Dahl salt-sensitive rats (19), increases acetylcholine-induced vasodilatation without changing the vasorelaxant response to nitroprusside in healthy humans (20), and ameliorates endothelial dysfunction as evidenced by improved flow-mediated dilatation in patients with type 2 diabetes and stable coronary artery disease (21). These likely resulted from a direct action of GLP-1 on the endothelium as GLP-1 receptors are abundantly expressed on the vascular endothelium (21), and studies using rat arterial rings have shown a direct, dose-dependent vasorelaxant effect of GLP-1 that was abolished by the removal of the endothelium (22). We have recently reported that GLP-1, when infused systemically, increases muscle glucose uptake by recruiting muscle microvasculature and increasing insulin delivery to muscle, likely via a protein kinase A (PKA)-dependent pathway (23,24). These findings plausibly explain the observations that GLP-1 increases muscle glucose uptake independent of its ability to enhance insulin secretion (25–27) and that GLP-1 receptor agonist treatment in patients with type 1 diabetes results in a reduction of insulin doses with improved or unaltered glycemic control (28–30).
In the current study, we measured muscle microvascular and metabolic insulin responses in the presence or absence of GLP-1 infusion in insulin-sensitive and acutely or chronically insulin-resistant rats. Our results indicate that GLP-1 can potently recruit muscle microvasculature, increase muscle delivery of insulin, and improve muscle insulin action in insulin-resistant states.
RESEARCH DESIGN AND METHODS
Animal Preparation and Experimental Protocols
Adult male Sprague-Dawley rats (170–350 g) were purchased from Charles River Laboratories (Wilmington, MA), housed at 22 ± 2°C on a 12-h light/dark cycle, and fed either a low-fat chow diet (LFD; protein 28 kcal%, carbohydrate 60 kcal%, and fat 12 kcal%) for 1–4 weeks or high-fat diet (HFD; protein 20 kcal%, carbohydrate 20 kcal%, and fat 60 kcal%; Research Diets Inc.) for 4 weeks with water access ad libitum. At the end of dietary intervention, rats were then fasted overnight, anesthetized with pentobarbital sodium (50 mg/kg i.p.; Abbott Laboratories, North Chicago, IL), and intubated to maintain a patent airway. They were placed in a supine position on a heating pad to ensure euthermia. The carotid artery and jugular vein were cannulated with polyethylene tubing (PE-50; Fisher Scientific, Newark, DE) for arterial blood pressure monitoring, arterial blood sampling, and various infusions. After a 30–45-min baseline period to ensure hemodynamic stability and a stable level of anesthesia, rats received infusions of insulin, GLP-1, Intralipid + heparin, and/or saline for 30–240 min, as dictated by each study protocol. Hindleg skeletal muscle MBV and microvascular blood flow velocity (MFV) were measured using contrast-enhanced ultrasound (CEU) at various time points as described previously (10,23,31). Muscle microvascular blood flow (MBF) was derived as product of MBV and MFV (i.e., MBF = MBV × MFV). At the end of the study, rats were killed by anesthetic overdose, gastrocnemius muscle was collected for determination of protein kinase B (Akt), extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and endothelial nitric oxide (NO) synthase (eNOS) phosphorylation, and aorta for quantification of PKA phosphorylation, as previously described (10,24,32).
Throughout the study, mean arterial pressure (MAP) was monitored via a sensor connected to the carotid arterial catheter (Harvard Apparatus, Holliston, MA, and ADInstruments, Inc., Colorado Springs, CO). Pentobarbital sodium was infused at a variable rate to maintain steady levels of anesthesia and blood pressure throughout the study. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication number 85-23, revised 1996). The study protocols were approved by the Animal Care and Use Committee of the University of Virginia.
Infusions of Insulin, GLP-1, and Lipid
Insulin, GLP-1, and Intralipid + heparin were infused for various times as detailed in each study protocol. Insulin (Humulin; Eli Lilly and Company) was infused systemically at 3 mU/kg/min with arterial blood glucose determined every 10 min using an Accu-Chek Advantage glucometer (Roche Diagnostics, Indianapolis, IN) and 30% dextrose (30% weight for volume) infused at a variable rate to maintain blood glucose within 10% of basal. The time course and area under the curve (AUC) of the glucose infusion rate (GIR; mg/kg/min) were calculated. GLP-1 (7–36) amide (Bachem Americas, Inc.) was infused continuously at 30 pmol/kg/min. Intralipid (6.6%) plus heparin (60 U/mL) was infused at 5 µl/min. At the rates selected, insulin and GLP-1 each potently recruit muscle microvasculature (8,11,23,24), and Intralipid + heparin abrogates insulin-mediated muscle microvascular and metabolic responses (8,33).
Measurement of Plasma NO Level
Plasma NO levels were measured using 280i Nitric Oxide Analyzer (GE Analytical Instruments), according to the manufacturer’s instructions. In brief, ice-cold ethanol was added into plasma samples at a ratio of 2:1. The mixture was kept at 0°C for 30 min and then centrifuged at 14,000 rpm for 5 min. The supernatant was then used for NO analysis based on a gas-phase chemiluminescent reaction between NO and ozone.
Quantification of Muscle Interstitial Oxygen Saturation
Muscle interstitial oxygen saturation was measured using a fiber-optic oxygen measurement system (OxyMicro; World Precision Instruments), based on the effect of dynamic luminescence quenching by molecular oxygen. In brief, a needle housing the fiber-optic oxygen microsensor was inserted into the hindleg skeletal muscle, and the glass fiber with its oxygen-sensitive tip inside the needle was extended into the muscle by carefully pressing the syringe plunger. Measurements were taken every 10 s, and the values were averaged.
Muscle 125I-Insulin Uptake
125I-Insulin was used to track the muscle uptake of native insulin, as described previously (7,10,23). Five minutes after a bolus injection of 1.5 µCi 125I-insulin (PerkinElmer, Boston, MA), a blood sample was collected, and each rat was then flushed with 120 mL ice-cold saline (10 mL/min) via the carotid artery catheter. Gastrocnemius muscle was dissected from the right hind limb. Protein-bound 125I in blood and muscle samples was precipitated with 30% trichloroacetic acid, and radioactivity was measured using a γ-counter. Fractions of intact 125I-insulin in blood and muscle were calculated as an index of 125I-insulin degradation. Muscle insulin uptake (fmol/g muscle/5 min) = muscle 125I-insulin (disintegrations per minute/g dry weight/5 min)/blood 125I-insulin (disintegrations per minute/mL) × plasma [insulin] (fmol/mL).
Statistical Analysis
All data are presented as mean ± SEM. Statistical analyses were performed with SigmaStat 3.1.1 software (Systat Software, Inc.) using either Student t test or ANOVA with post hoc analysis as appropriate. A P value of <0.05 was considered statistically significant.
RESULTS
Superimposition of GLP-1 Increases Insulin-Mediated Whole-Body Glucose Disposal, Microvascular Recruitment, and Muscle Uptake of Insulin in Control Animals
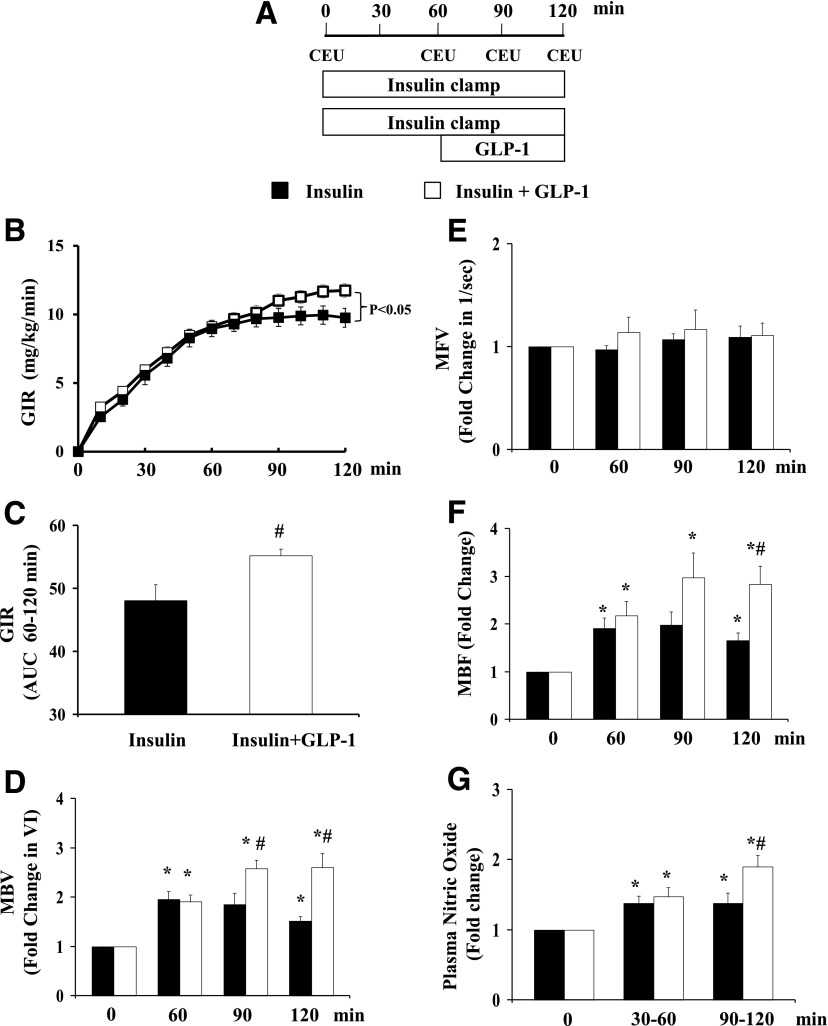
We have previously reported that systemic administration of GLP-1 potently recruits muscle microvasculature and increases muscle delivery of 125I-insulin and glucose uptake (23) in the absence of increases in basal insulin. To examine whether GLP-1–mediated microvascular recruitment modulates insulin’s metabolic actions, we raised plasma insulin (3 mU/kg/min euglycemic insulin clamp) for 120 min after determining baseline MAP and collecting baseline samples for glucose and insulin levels at 0 min with or without a superimposed systemic infusion of GLP-1 (7–36) amide (30 pmol/kg/min) during the last 60 min of the clamp (Fig. 1A). As shown in Table 1, addition of GLP-1 did not alter MAP or plasma glucose or insulin concentrations. However, it significantly increased insulin-stimulated whole-body glucose disposal (Fig. 1B and C). Insulin alone significantly increased muscle MBV and MBF and plasma NO concentrations. Superimposing GLP-1 on insulin infusion further increased muscle MBV and MBF (by ∼40%; P < 0.05, Fig. 1D and F) as well as plasma NO concentrations (by ∼40%; P < 0.05, Fig. 1G). Neither insulin nor GLP-1 affected muscle MFV (Fig. 1E). Thus, their effects on MBF were secondary to an increased MBV only.
Figure 1.
GLP-1 enhances insulin-mediated glucose disposal and muscle microvascular recruitment. Each rat received a 2-h euglycemic insulin clamp (3 mU/kg/min) for 120 min with or without GLP-1 infusion (30 pmol/kg/min) superimposed between 60 and 120 min. CEU measurements were done at 0, 60, 90, and 120 min. A: Study protocol. B: GIR during insulin clamp. C: GIR AUC between 60 and 120 min. D: MBV. E: MFV. F: MBF. G: Plasma NO levels. n = 4–15 each. Compared with 0 min, *P < 0.05; compared with insulin, #P < 0.05. VI, video intensity.
Table 1.
Changes in MAP, plasma glucose, and insulin concentrations during insulin ± GLP-1 infusions
| 0 min | 30 min | 60 min | 90 min | 120 min | |
|---|---|---|---|---|---|
| MAP (mmHg) | |||||
| Insulin | 106 ± 2 | 110 ± 3 | 109 ± 2 | 103 ± 2 | 111 ± 3 |
| Insulin + GLP-1 | 108 ± 7 | 108 ± 3 | 110 ± 3 | 100 ± 4 | 104 ± 10 |
| Plasma glucose (mmol/L) | |||||
| Insulin | 5.4 ± 0.2 | 4.7 ± 0.1 | 5.3 ± 0.1 | 5.5 ± 0.3 | 5.5 ± 0.2 |
| Insulin + GLP-1 | 4.8 ± 0.3 | 4.3 ± 0.5 | 4.5 ± 0.6 | 4.6 ± 0.4 | 5.0 ± 0.4 |
| Plasma insulin (pmol/L) | |||||
| Insulin | 87 ± 22 | 335 ± 12* | 339 ± 11* | 453 ± 63* | 448 ± 63* |
| Insulin + GLP-1 | 78 ± 17 | 325 ± 58* | 374 ± 49* | 322 ± 39* | 337 ± 69* |
N = 4–9 each.
Compared with 0 min, P < 0.05 (ANOVA).
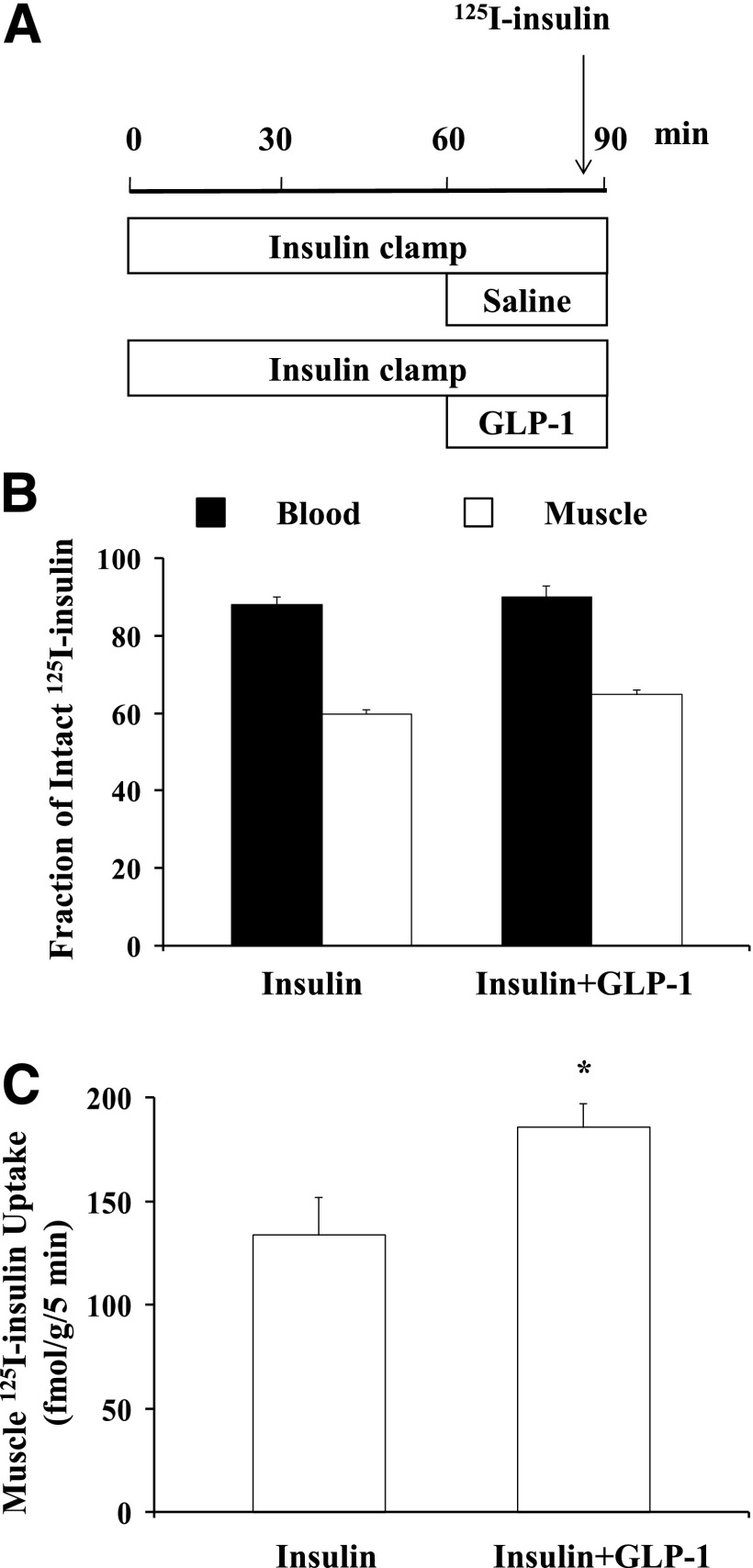
As muscle insulin action closely correlates with muscle interstitium insulin concentrations (34,35), and microvascular recruitment is associated with increased muscle delivery of insulin (3,4), we next examined whether the increase in insulin-mediated whole-body glucose disposal was secondary to GLP-1–induced muscle delivery of insulin. We used 125I-insulin to trace native insulin movement in vivo during insulin clamp in the presence or absence of a 30-min GLP-1 coinfusion. Addition of GLP-1 did not alter 125I-insulin degradation in either blood or muscle but increased muscle insulin uptake by ∼40% (P < 0.05; Fig. 2).
Figure 2.
GLP-1 increases muscle 125I-insulin uptake. Each rat received a 90-min euglycemic insulin clamp (3 mU/kg/min) with or without simultaneous GLP-1 infusion (30 pmol/kg/min) for the last 30 min. A bolus intravenous injection of 125I-insulin (1.5 µCi) was given 5 min before the end of insulin ± GLP-1 infusions. Blood and skeletal muscle were collected for determination of intact 125I-insulin. A: Study protocol. B: Fraction of intact 125I-insulin in blood and muscle. C: Muscle insulin uptake. n = 5 each. Compared with insulin alone, *P < 0.05.
GLP-1 Recruits Muscle Microvasculature in the Insulin-Resistant Rats
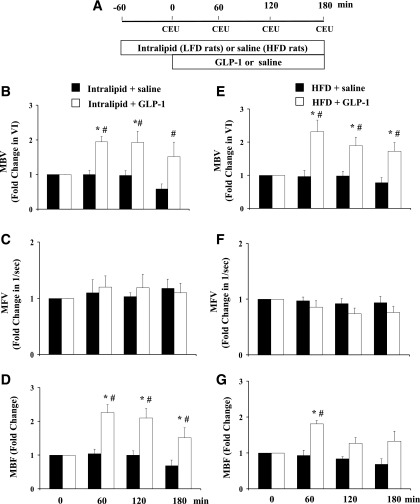
The above findings, coupled with the knowledge that GLP-1 acts via an insulin receptor-independent but NO-dependent mechanism, led us to examine whether GLP-1 was able to recruit muscle microvasculature in the presence of insulin resistance. We created both an acute and a chronic insulin-resistant rodent model by subjecting rats to either a 240-min Intralipid + heparin infusion or 4 weeks of HFD feeding. Each group of these rats then received a 3-h systemic infusion of GLP-1 at 30 pmol/kg/min or equal volume of saline. We have shown previously that GLP-1 at this dose potently recruits muscle microvasculature in insulin-sensitive rats (23,24). As shown in Fig. 3, saline infusion did not alter microvascular parameters, but GLP-1 infusion significantly enhanced microvascular perfusion by increasing muscle MBV (P < 0.05 for all) without affecting muscle MFV in the presence of either acute or chronic insulin resistance.
Figure 3.
GLP-1 recruits muscle microvasculature in the presence of acute and chronic insulin resistance. A: Infusion protocol. Each rat received a 3-h infusion of GLP-1 (30 pmol/kg/min) or equal volume of saline. B–D: GLP-1–mediated changes in muscle microvascular parameters in the presence of acute insulin resistance induced by 4 h of lipid infusion. n = 4–5. E–G: GLP-1–mediated changes in muscle microvascular parameters in the presence of chronic insulin resistance induced by 4 weeks of HFD feeding. n = 4–7. Compared with 0 min, *P < 0.05; compared with control (Intralipid or HFD only), #P < 0.05. VI, video intensity.
GLP-1 Recruits Muscle Microvasculature and Abolishes Metabolic Insulin Resistance During Lipid Infusion
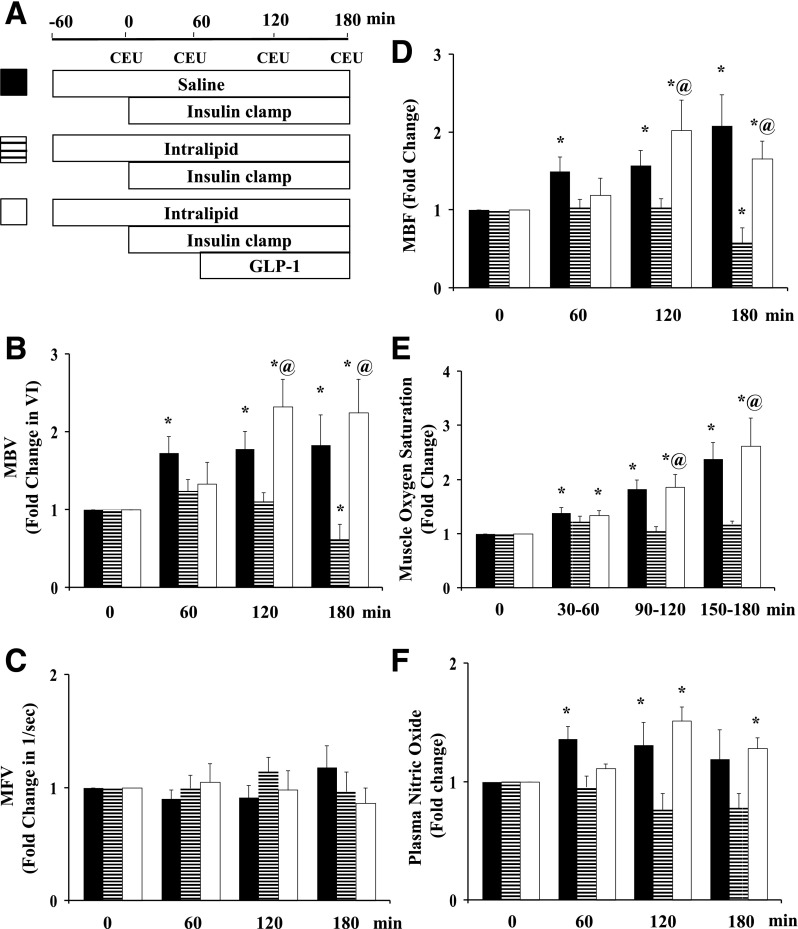
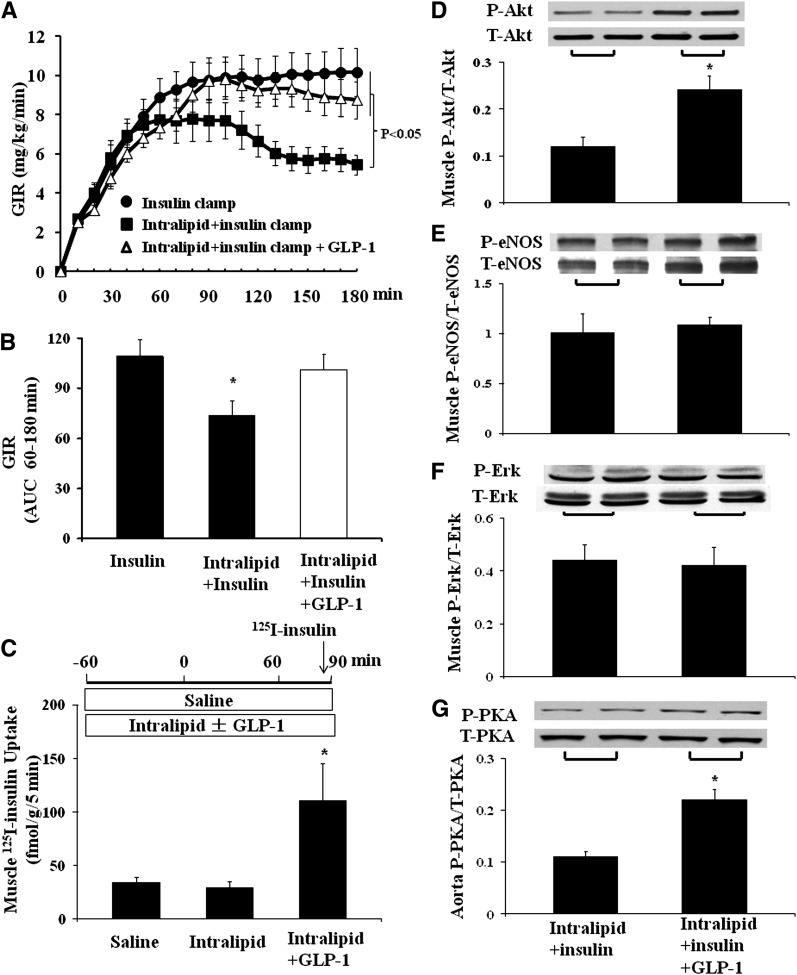
To further examine whether this GLP-1–induced increase in muscle MBV and MBF in the acute insulin-resistant state modulates insulin’s microvascular and metabolic actions, we performed insulin clamps for 3 h with or without simultaneous GLP-1 infusion for the last 2 h in rats receiving a 4-h systemic lipid infusion. As shown in Fig. 4, systemic lipid infusion fully inhibited insulin-mediated microvascular recruitment, NO generation, and increase in muscle oxygen saturation. Toward the end of the study, insulin even decreased muscle MBV in the presence of lipid infusion. Adding the GLP-1 infusion restored MBV, MBF, plasma NO, and muscle oxygen saturation back to the levels seen in rats given insulin alone.
Figure 4.
Lipid infusion abrogates insulin-mediated but not GLP-1 + insulin–mediated muscle microvascular perfusion. A: Infusion protocol. Each rat received a systemic infusion of either saline or Intralipid + heparin for 240 min with a euglycemic insulin clamp (3 mU/kg/min) superimposed in the last 3 h and either saline or GLP-1 (30 pmol/kg/min) for the last 2 h. B: MBV. C: MFV. D: MBF; n = 10 each. E: Muscle oxygen saturation. n = 5–6. F: Plasma NO levels. n = 4–8. Compared with respective baseline (0 min), *P < 0.05; compared with Intralipid + insulin, @P < 0.05. VI, video intensity.
The acute vascular insulin resistance induced by lipid infusion was associated with a marked metabolic insulin resistance, as evidenced by a significantly lower insulin-stimulated whole-body glucose disposal (Fig. 5A and B). Intralipid infusion slightly decreased muscle uptake of insulin (34.5 ± 5.5 vs. 29 ± 6 fmol/g/5 min, saline vs. lipid; P = 0.5), but addition of GLP-1 robustly increased muscle uptake of 125I-insulin in the presence of lipid infusion (to 110 ± 34.5 fmol/g/5 min; P = 0.007; Fig. 5C). Addition of GLP-1 significantly increased insulin-mediated Akt, but not eNOS or ERK1/2, phosphorylation in muscle (Fig. 5D–F). Furthermore, PKA phosphorylation was increased significantly in rat aorta after GLP-1 infusion (Fig. 5G).
Figure 5.
GLP-1 restores muscle metabolic insulin sensitivity during lipid infusion. Each rat received a systemic infusion of either saline or lipid for 240 min with a euglycemic insulin clamp (3 mU/kg/min) superimposed in the last 3 h and either saline or GLP-1 (30 pmol/kg/min) for the last 2 h. A: Time course of GIR (n = 10–15/group). B: GIR AUC from 60–180 min; compared with insulin alone, *P < 0.05. C: Muscle insulin uptake; n = 5–11 each. Compared with saline or Intralipid group, *P < 0.05. D: Muscle Akt phosphorylation; compared with Intralipid + insulin, *P < 0.01. E: Muscle eNOS phosphorylation. F: Muscle ERK1/2 phosphorylation. G: Aorta PKA phosphorylation; compared with Intralipid + insulin, *P < 0.01. n = 4–8/group for D–G. P, phosphorylated; T, total.
GLP-1 Recruits Muscle Microvasculature and Improves Metabolic Insulin Responses in HFD Rats
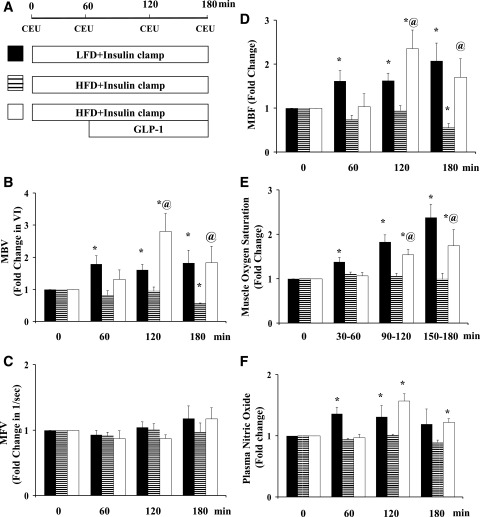
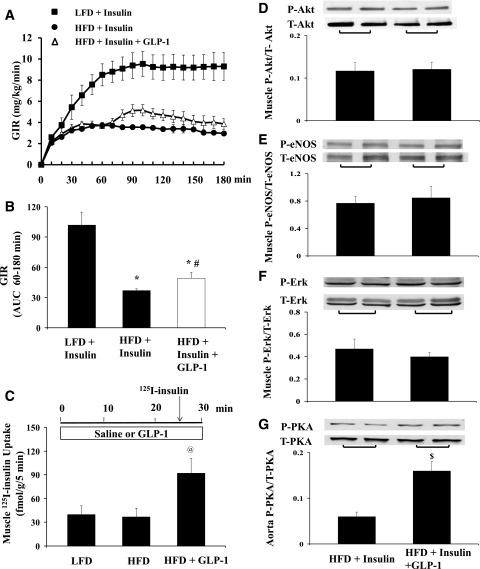
Finally, we examined GLP-1’s effects on insulin-mediated microvascular and metabolic responses in the setting of chronic insulin resistance. Rats fed either an LFD or HFD for 4 weeks were fasted overnight and then given a 3-h insulin clamp with or without GLP-1 for the last 2 h. Fig. 6 shows the changes in muscle MBV, MFV, and MBF, muscle oxygen saturation, and plasma NO levels. HFD clearly abolished both insulin-mediated muscle microvascular recruitment and NO production and enhancement in muscle oxygenation. Adding GLP-1 increased both MBV and MBF. This was also associated with significantly increased muscle oxygen saturation and plasma NO levels. Interestingly, unlike the full restoration of insulin-stimulated whole-body glucose disposal seen in rats receiving systemic lipid infusion, GLP-1 infusion in HFD rats conferred a much smaller albeit statistically significant (∼30%; P < 0.04) increase in insulin-mediated glucose disposal (Fig. 7A and B). Though muscle uptake of 125I-insulin was significantly higher in rats receiving GLP-1 (Fig. 7C), insulin-stimulated muscle Akt, eNOS, or ERK1/2 phosphorylation did not change in these rats (Fig. 7D–F). Similar to rats receiving Intralipid, GLP-1 infusion also significantly increased aortic PKA phosphorylation in HFD rats (Fig. 7G).
Figure 6.
HFD feeding abolishes insulin-mediated but not GLP-1 + insulin–mediated muscle microvascular perfusion. Each rat was fed an LFD or HFD for 4 weeks. A: Infusion protocol. B: MBV. C: MFV. D: MBF; n = 9 to 10/group. E: Muscle oxygen saturation; n = 4–8/group. F: Plasma NO levels; n = 4–8/group. Compared with respective baseline (0 min), *P < 0.05; compared with HFD + Insulin, @P < 0.05. VI, video intensity.
Figure 7.
GLP-1 infusion increases muscle insulin delivery and improves muscle metabolic response to insulin in HFD-fed rats. Each rat was fed either an LFD or HFD for 4 weeks. A: Time course of GIR. B: GIR AUC (60–180 min); n = 8–10/group. C: Muscle 125I-insulin uptake; n = 6–11/group. D: Muscle Akt phosphorylation. E: Muscle eNOS phosphorylation. F: Muscle ERK1/2 phosphorylation. G: Aorta PKA phosphorylation. n = 6–8/group for D–G. Compared with LFD + Insulin, *P < 0.05; compared with HFD + Insulin, #P < 0.05, $P < 0.01; compared with LFD or HFD, @P < 0.05. P, phosphorylated; T, total.
DISCUSSION
Novel findings of the current study include that acute GLP-1 infusion: 1) effectively recruits muscle microvasculature and increases muscle insulin delivery in the presence of both acute and chronic insulin resistance; 2) completely restores muscle metabolic insulin action in the presence of acute insulin resistance induced by lipid infusion; 3) significantly but much less effectively improves muscle metabolic insulin response in the presence of chronic insulin resistance; and 4) effectively increases muscle interstitial oxygenation in both acute and chronic insulin-resistant states. Our results thus clearly demonstrate that the microvascular actions of GLP-1 are preserved in the insulin-resistant states studied, and this could improve both glycemic control and other muscle functions.
We and others have shown that muscle microvasculature plays a significant role in regulating muscle insulin action, and interventions that expand muscle’s endothelial exchange surface area increase muscle insulin uptake and insulin action (3–5). The current findings that GLP-1 acutely increases muscle MBV and insulin uptake in the presence of either acute or chronic insulin resistance are of potential clinical significance. Indeed, both lipid infusion and HFD feeding caused significant insulin resistance, as evidenced by abolished microvascular and decreased metabolic insulin responses. As the microvascular actions of insulin contribute up to 40% of insulin-mediated whole-body glucose disposal during the insulin clamp, and GLP-1 clearly acts via an insulin receptor independent pathway to recruit muscle microvasculature, it is very much likely that these actions contribute to the glycemic control seen in patients receiving incretin-based therapies.
Evidence thus far confirms that both insulin and GLP-1 recruit muscle microvasculature via an NO-dependent mechanism, as each is able to stimulate eNOS phosphorylation and NO production in vitro in cultured endothelial cells and increase plasma NO levels in vivo, and inhibition of eNOS activity completely abolishes insulin- or GLP-1–mediated microvascular perfusion (11,23). However, it appears that they induce this NO-dependent microvascular recruitment via entirely different cellular pathways. Our results clearly show that insulin effect is abolished by both acute and chronic insulin resistance, while GLP-1 effect is preserved. While insulin acts via its receptors to activate the phosphatidylinositol 3-kinase (PI3-kinase)/Akt/eNOS pathway to generate NO and recruit muscle microvasculature, in our prior study, we showed that GLP-1 acts on its own receptors to increase NO production and recruit muscle microvasculature likely via a PKA-dependent pathway (23,24). That GLP-1 infusion potently increased PKA phosphorylation in rats receiving either acute lipid infusion or 4 weeks of HFD feeding strongly suggests that the GLP-1 receptor/PKA/eNOS pathway in the microvasculature maintains its normal response to GLP-1 in the insulin-resistant states. Thus, clinically, GLP-1 may enhance insulin action in patients with diabetes by removing the vascular barrier effect induced by endothelial insulin resistance.
In the current study, we quantified GLP-1 effect on microcirculation for 180 min and GLP-1–induced increase in MBF appeared to be lower at the end of the study in the insulin-resistant rats. Though GLP-1 receptor desensitization has been observed in insulin-secreting cells in vitro (36,37), this does not appear to be the case with endothelial cells, as exendin-4–induced eNOS phosphorylation remains elevated after 48 h (38). The seeming downward trend in MBF with time was likely secondary to a slight decrease in MBF in the control group (lipid study) or a combination of a slight decrease of MBF in the control group and of MFV in the GLP-1 group (HFD study).
Similar to a prior report (33), we in the current study observed that insulin not only failed to recruit muscle microvasculature, but it also actually paradoxically decreased muscle MBV in the presence of systemic lipid infusion or in rats fed an HFD. This decrement is consistent with the concept of selective resistance to insulin’s vasodilatory action via the PI3-kinase/Akt/eNOS pathway, while its vasoconstrictive effect via the mitogen-activated protein kinase pathway is spared, leading to increased endothelin-1 production and decreased NO production/bioavailability (14,39–41). Indeed, we did not observe a significant difference in insulin-stimulated ERK1/2 phosphorylation between the insulin-sensitive and -resistant animals, and a prior study has shown that endothelin receptor type 1 antagonism is capable of preventing the decline in MBV observed in response to combined lipid and insulin infusion (33).
Though both lipid infusion and the HFD induced significant metabolic insulin resistance, it appears that lipid infusion induced acute metabolic insulin resistance mainly by affecting vascular insulin sensitivity and HFD feeding provoked both microvascular and myocytic insulin resistance. Prior reports have shown that microvascular insulin resistance is clearly present 1.5 h after systemic lipid infusion (8,33), but extension of lipid infusion to 5 h significantly increases myocytic diacylglycerol concentration and protein kinase Cθ activation and reduces insulin-mediated insulin receptor substrate-1 tyrosine phosphorylation and insulin receptor substrate-1–associated PI3-kinase activity in muscle (42,43). As such, we elected to begin the insulin clamp 1 h after and GLP-1 infusion 2 h after beginning the lipid infusion to test whether GLP-1–mediated microvascular recruitment could restore muscle metabolic insulin sensitivity.
The current findings that GLP-1 infusion completely restores insulin’s vascular and metabolic actions are reminiscent of our experience with administrating the angiotensin II type 1 receptor blocker losartan, which also potently recruits muscle microvasculature, increases muscle delivery of insulin, and restores insulin’s metabolic effect during lipid infusion (8). Together, these findings do strongly suggest that metabolic insulin resistance induced by lipid infusion, at least within the first several hours, mainly stems from microvascular insulin resistance.
We were intrigued to see that despite potently recruiting muscle microvasculature and increasing muscle insulin uptake, GLP-1 infusion only increased the GIRs by 30% during insulin clamp in rats fed an HFD (Fig. 7). This is not surprising, as acute GLP-1 infusion likely only bypasses the vascular insulin resistance without affecting myocyte insulin resistance. Indeed, insulin-mediated Akt phosphorylation in muscle was comparable between the two groups. The lack of a significant increase in muscle eNOS phosphorylation after GLP-1 treatment in the insulin-resistant states, despite a marked increase in plasma NO levels and muscle microvascular recruitment, is not surprising, as muscle expresses much fewer GLP-1 receptors than blood vessels (44) and further suggests that GLP-1–induced NO generation is of vascular origin.
We did not analyze phosphorylation of the insulin receptor substrates, PI3-kinase activity, and glucose transporter 4 translocation in the current study, as we have demonstrated that GLP-1 improves insulin-mediated glucose disposal, the last step of insulin’s metabolic signaling pathway. Whether chronic activation of the GLP-1 receptors, as seen clinically in patients on incretin-based therapies, improves muscle’s metabolic insulin sensitivity remains to be defined.
Another major function of the microvasculature is to supply tissue with oxygen to meet metabolic needs. We have previously demonstrated that expansion of the muscle microvasculature induced by insulin and losartan markedly increases muscle interstitial oxygen saturation in healthy, insulin-sensitive rats (10). Our current findings that both acute and chronic insulin resistance abolishes insulin-mediated increases in muscle interstitial oxygen saturation while GLP-1 enhances muscle oxygenation in the insulin-resistant states are of particular clinical and pathophysiological interest. Indeed, recent studies have shown that tissue hypoxia may contribute to the pathogenesis of insulin resistance likely via increased tissue inflammation (45). Furthermore, patients with diabetes are prone to tissue dysfunction/organ failure. It remains possible that microvascular insulin resistance may contribute to tissue hypoxia, and incretin-based therapy might have the potential to alleviate the resulting organ dysfunction and damage. This certainly warrants further investigation.
In conclusion, GLP-1 infusion potently expands muscle microvascular surface area, increases muscle delivery and uptake of insulin, and improves insulin’s metabolic action in both acute and chronic insulin-resistant states. This may contribute to improved glycemic control seen clinically in diabetic patients receiving incretin-based therapies and afford potential to improve tissue/organ function and reduce morbidity and mortality associated with diabetes via microvascular recruitment and increased tissue perfusion.
Article Information
Funding. This work was supported by American Diabetes Association grants 1-11-CR-30 and 9-09-NOVO-11 (to Z.L.) and National Institutes of Health grant R01-HL-094722 (to Z.L.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. W.C., X.Z., and Z.L. researched data and wrote the manuscript. E.J.B. contributed to discussion. Z.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Ferrannini E, Simonson DC, Katz LD, et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988;37:79–85 [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 1985;76:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett EJ, Eggleston EM, Inyard AC, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009;52:752–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 2011;301:E252–E263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark MG, Wallis MG, Barrett EJ, et al. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 2003;284:E241–E258 [DOI] [PubMed] [Google Scholar]
- 6.Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 2011;13:294–307 [DOI] [PubMed] [Google Scholar]
- 7.Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 2007;56:2194–2200 [DOI] [PubMed] [Google Scholar]
- 8.Wang N, Chai W, Zhao L, Tao L, Cao W, Liu Z. Losartan increases muscle insulin delivery and rescues insulin’s metabolic action during lipid infusion via microvascular recruitment. Am J Physiol Endocrinol Metab 2013;304:E538–E545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, Chai W, Fu Z, et al. Globular adiponectin enhances muscle insulin action via microvascular recruitment and increased insulin delivery. Circ Res 2013;112:1263–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes 2011;60:2939–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 2003;285:E123–E129 [DOI] [PubMed] [Google Scholar]
- 12.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006;55:1436–1442 [DOI] [PubMed] [Google Scholar]
- 13.Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark ADH, Clark MG. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 2002;51:3492–3498 [DOI] [PubMed] [Google Scholar]
- 14.Liu Z. The vascular endothelium in diabetes and its potential as a therapeutic target. Rev Endocr Metab Disord 2013;14:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett EJ, Liu Z. The endothelial cell: an “early responder” in the development of insulin resistance. Rev Endocr Metab Disord 2013;14:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153–165 [DOI] [PubMed] [Google Scholar]
- 17.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 2007;117:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012;33:187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 2003;21:1125–1135 [DOI] [PubMed] [Google Scholar]
- 20.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab 2007;293:E1289–E1295 [DOI] [PubMed] [Google Scholar]
- 21.Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- 22.Richter G, Feddersen O, Wagner U, Barth P, Göke R, Göke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol 1993;265:L374–L381 [DOI] [PubMed] [Google Scholar]
- 23.Chai W, Dong Z, Wang N, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 2012;61:888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Z, Chai W, Wang W, et al. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab 2013;304:E222–E228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 2009;150:1155–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao T, Parikh P, Bhashyam S, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther 2006;317:1106–1113 [DOI] [PubMed] [Google Scholar]
- 27.Nikolaidis LA, Elahi D, Hentosz T, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 2004;110:955–961 [DOI] [PubMed] [Google Scholar]
- 28.Kielgast U, Krarup T, Holst JJ, Madsbad S. Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual beta-cell function. Diabetes Care 2011;34:1463–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raman VS, Mason KJ, Rodriguez LM, et al. The role of adjunctive exenatide therapy in pediatric type 1 diabetes. Diabetes Care 2010;33:1294–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rother KI, Spain LM, Wesley RA, et al. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care 2009;32:2251–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai W, Wang W, Liu J, et al. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension 2010;55:523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D, Luo Z, Ma S, et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab 2010;12:130–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inyard AC, Chong DG, Klibanov AL, Barrett EJ. Muscle contraction, but not insulin, increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes 2009;58:2457–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest 1994;93:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu JD, Richey JM, Harrison LN, et al. Direct administration of insulin into skeletal muscle reveals that the transport of insulin across the capillary endothelium limits the time course of insulin to activate glucose disposal. Diabetes 2008;57:828–835 [DOI] [PubMed] [Google Scholar]
- 36.Gromada J, Dissing S, Rorsman P. Desensitization of glucagon-like peptide 1 receptors in insulin-secreting beta TC3 cells: role of PKA-independent mechanisms. Br J Pharmacol 1996;118:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baggio LL, Kim J-G, Drucker DJ. Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes 2004;53(Suppl. 3):S205–S214 [DOI] [PubMed] [Google Scholar]
- 38.Erdogdu Ö, Nathanson D, Sjöholm Å, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 2010;325:26–35 [DOI] [PubMed] [Google Scholar]
- 39.Eringa EC, Stehouwer CDA, van Nieuw Amerongen GP, Ouwehand L, Westerhof N, Sipkema P. Vasoconstrictor effects of insulin in skeletal muscle arterioles are mediated by ERK1/2 activation in endothelium. Am J Physiol Heart Circ Physiol 2004;287:H2043–H2048 [DOI] [PubMed] [Google Scholar]
- 40.Eringa EC, Stehouwer CDA, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 2002;56:464–471 [DOI] [PubMed] [Google Scholar]
- 41.Eringa EC, Stehouwer CDA, Walburg K, et al. Physiological concentrations of insulin induce endothelin-dependent vasoconstriction of skeletal muscle resistance arteries in the presence of tumor necrosis factor-alpha dependence on c-Jun N-terminal kinase. Arterioscler Thromb Vasc Biol 2006;26:274–280 [DOI] [PubMed] [Google Scholar]
- 42.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 1999;103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002;277:50230–50236 [DOI] [PubMed] [Google Scholar]
- 44.Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys 2008;478:136–142 [DOI] [PubMed] [Google Scholar]
- 45.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 2007;293:E1118–E1128 [DOI] [PubMed] [Google Scholar]