Abstract
Different onset rates of insulin-induced hypoglycemia use distinct glucosensors to activate sympathoadrenal counterregulatory responses (CRRs). Glucosensory elements in the portal-mesenteric veins are dispensable with faster rates when brain elements predominate, but are essential for responses to the slower-onset hypoglycemia that is common with insulin therapy. Whether a similar rate-associated divergence exists within more expansive brain networks is unknown. Hindbrain catecholamine neurons distribute glycemia-related information throughout the forebrain. We tested in male rats whether catecholaminergic neurons that project to the medial and ventromedial hypothalamus are required for sympathoadrenal CRRs to rapid- and slow-onset hypoglycemia and whether these neurons are differentially engaged as onset rates change. Using a catecholamine-specific neurotoxin and hyperinsulinemic-hypoglycemic clamps, we found that sympathoadrenal CRRs to slow- but not rapid-onset hypoglycemia require hypothalamus-projecting catecholaminergic neurons, the majority of which originate in the ventrolateral medulla. As determined with Fos, these neurons are differentially activated by the two onset rates. We conclude that 1) catecholaminergic projections to the hypothalamus provide essential information for activating sympathoadrenal CRRs to slow- but not rapid-onset hypoglycemia, 2) hypoglycemia onset rates have a major impact on the hypothalamic mechanisms that enable sympathoadrenal CRRs, and 3) hypoglycemia-related sensory information activates hindbrain catecholaminergic neurons in a rate-dependent manner.
Introduction
Insulin therapies still cannot mimic healthy insulin secretion in all circumstances, and so iatrogenic hypoglycemia remains the main obstacle to maintaining tight glycemic control in type 1 and later-stage type 2 diabetes (1). Normal counterregulatory responses (CRRs) to hypoglycemia are compromised in type 1 diabetes (2). Repeated bouts of insulin-induced hypoglycemia (IIH) suppress sympathoadrenal responses to subsequent hypoglycemia, which in turn leads to a maladaptive cycle of repeated bouts of hypoglycemia. Therefore, compromised endocrine counterregulation combined with hypoglycemia unawareness significantly complicates insulin therapies.
Sympathoadrenal and glucocorticoid CRRs to hypoglycemia are mediated by sets of glucosensing elements in the hypothalamus, hindbrain, and portal-mesenteric veins (PMV) (3–6). PMV-derived glucosensory information reaches the nucleus of the solitary tract (NTS) by way of the spinal cord (7). Each set of glucosensory elements provides information to a distributed neural network to enable CRRs (7). The primacy of brain or peripheral glucosensors for sympathoadrenal CRRs is determined by the rate of fall in glycemia rather than their glycemic thresholds. Brain glucosensing predominates during rapid-onset hypoglycemia, whereas PMV glucosensing is essential with slow-onset hypoglycemia (8). Establishing how these distributed glucosensing elements integrate information to enable sympathoadrenal CRRs to hypoglycemia is important because evidence suggests that the onset of hypoglycemia with conventional insulin therapy in humans is probably slower than is seen with the intravenous administrations often used experimentally (9,10).
Hindbrain glucosensors engage sets of catecholaminergic neurons responsible for feeding and glucocorticoid responses to glycemic challenges (11–14). Some of these provide a dense catecholaminergic input to the hypothalamus that originates in certain hindbrain nuclei (15). Of these, catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus (PVH) are essential for encoding the information that drives ACTH and feeding responses to glycemic perturbations (6,14).
A role for catecholaminergic neurons in regulating sympathoadrenal CRRs is less clear. Beverly et al. have shown that hypoglycemia augments norepinephrine release from catecholaminergic terminals into the ventromedial hypothalamus (16). Furthermore, ventromedial hypothalamic glucose infusions suppress local norepinephrine release and sympathoadrenal CRRs to rapid-onset hypoglycemia (16,17). These results reveal important interactions between glucosensing mechanisms and catecholaminergic inputs to the ventromedial hypothalamus. However, the necessity of these catecholaminergic neurons has never been tested directly using controlled hyperinsulinemic-hypoglycemic clamps. The fact that sympathoadrenal responses to rapid- and slow-onset hypoglycemia are mediated by different sets of glucosensors (8) raises the possibility that catecholaminergic systems are differentially involved as the rate of fall of blood glucose changes. Here we use immunotoxic lesions to determine if catecholaminergic neurons projecting to the PVH and ventromedial hypothalamus are required during hypoglycemia and, if so, whether they are differentially engaged by rapid- and slow-onset hypoglycemia.
Research Design and Methods
Animals
Individually housed adult male Wistar rats (n = 26; body weight 289 ± 4.4 g at the time of the first surgery) were maintained on a 12:12 h light/dark schedule (lights on 0600 h clock time) in temperature-controlled rooms with continuous access to water and chow (Teklad rodent diet 8604) except where stated. Animals were acclimatized for at least 10 days before surgeries. All procedures were approved by the local institutional animal care and use committee.
Immunotoxic PVH Lesions
Catecholaminergic projections to the ventromedial hypothalamus were lesioned with a dopamine-β-hydroxylase (DBH) antibody-saporin conjugate (DSAP; Advanced Targeting Systems, San Diego, CA). Isoflurane anesthetized rats were secured in a stereotaxic frame (David Kopf Instruments). Either 42 ng/200 nL of DSAP (n = 14) or equimolar amounts of control mouse IgG-saporin conjugate (MIgG-SAP; n = 12) were injected bilaterally into the PVH (14,18).
DSAP mediates the entry of saporin (a cytotoxin) only into neurons expressing DBH on the inner membrane of exocytosed vesicles, thereby selectively killing catecholaminergic neurons with terminals proximal to the injection (12,19). Catecholaminergic neurons with extensive collaterals are lesioned even if DSAP affects only a subset of their terminals. For PVH injections, this includes projections to the paraventricular nucleus of the thalamus, supraoptic nucleus, and bed nuclei of the stria terminalis, but not the amygdala (18–20). The pattern of DBH innervation seen in these extrahypothalamic areas after DSAP lesions in this study was consistent with these findings. We have previously reported that DSAP lesions have no effect on food intake or body weights for at least 14 days postsurgery (18).
Catheterizations
At least 1 week later, all animals were anesthetized (ketamine/xylazine/acepromazine) and catheterized in the right carotid artery for sampling (Clay-Adams PE-50) and dual left jugular vein (Silastic ID, 0.025 cm) for insulin/glucose infusions. All catheters were guided subcutaneously and exteriorized dorsally at the neck. A Covance harness (Instech Inc., Plymouth Meeting, PA) was placed around the torso to secure and protect exposed catheters. All animals were allowed 7 days to regain body weight.
Hyperinsulinemic-Hypoglycemic Clamps
The evening before the experiment, all food, but not water, was removed, and cannulae were connected to a dual-channel swivel via a tethering system (Instech). The next morning jugular catheters were connected to insulin and glucose infusion pumps (Razel Scientific Instruments, Inc., Stamford, CT). Animals were then left undisturbed for 30 min. Arterial samples (300 µL) were drawn at minutes −60 and 0 and four times during the clamps for glucose and catecholamine determinations. Sampled blood was replaced throughout the experiment from donor animals. At minute −60, 25 mU·kg·min insulin (Humulin R, human insulin, Lilly, Indianapolis, IN) and variable-rate glucose infusions were initiated. Euglycemic clamps were established for 60 min, during which blood was sampled every 15 min to assure the integrity of the clamp. At minute 0, glucose infusion rates were reduced to achieve deep hypoglycemia (∼2.5 mmol/L) either by minute 20 (rapid onset; blood glucose sampled every 5 min) or minute 70 (slow onset; blood glucose sampled every 10 min). In each case, hypoglycemia was maintained for a further 60 min to minutes 80 or 130, respectively, with blood glucose sampled every 10 min. Animals were then rapidly anesthetized with catheter-delivered tribromoethanol. Perfusions, brain removal, postfixation, cryoprotection, and sectioning were as described previously (21).
Analytical Procedures
Plasma glucose concentrations were determined using a glucose oxidase/fixed enzyme analyzer (YSI, Yellow Springs, OH). Plasma epinephrine and norepinephrine concentrations were determined by single-isotope derivative radioenzymatic assays (22) as previously described (23). All determinations were made in single-run assays.
Immunohistochemistry
Frozen coronal sections (30 μm, 1:5 series) were cut through levels 20–29 (for DBH and thionin only) and 58–73 of the Swanson rat brain atlas (24). Four series were retained for immunocytochemistry; the fifth was thionin stained for cytoarchitectonics. Hindbrain sections were processed immunocytochemically to assess the effect of the clamps and DSAP lesions on three variables in the ventrolateral medulla (VLM) and dorsomedial medulla (NTS; area postrema [AP], dorsal motor nucleus of the vagus [DMX]): 1) the number of DBH- and phenylethanolamine N-methyltransferase (PNMT)-expressing neurons, 2) the numbers of Fos-expressing neurons, and 3) the numbers of neurons exhibiting DBH/Fos colocalization. Determining PNMT/Fos colocalization was compromised because both antibodies were raised in rabbits.
Sections were incubated with various combinations of three primary antibodies: a mouse monoclonal anti-DBH antibody (1:10K; MAB308 [14]), a rabbit polyclonal anti-Fos antibody (1:10K, AB-5, Calbiochem, San Diego, CA [25]), and a rabbit polyclonal anti-PNMT antibody (1:16K; Martha Bohn, Northwestern University [26]). The DBH and Fos antibodies were mixed together for colocalization studies. Specific primary antibody binding was detected with donkey anti-mouse IgG secondary antibody conjugated to Alexa 488 (1:500 Invitrogen) for DBH or PNMT and donkey anti-rabbit IgG conjugated to CY3 (1:500 Jackson ImmunoResearch Laboratories, West Grove, PA) for Fos.
Image Acquisition and Analysis
All images were acquired with a Ziess AxioImager Z1 microscope (Carl Zeiss MicroImaging, Inc., Thorwood, NY) using either epifluorescence and a Hamamatsu Orca ER camera/Volocity acquisition module (version 6.0, PerkinElmer, Waltham, MA) or a LSM700 with Zeiss Zen software. The numbers of neurons labeled with the various markers were counted manually within anatomically defined regions on unmanipulated epifluorescence images. To assign anatomical boundaries of the A1, A1/C1, and C1 regions of the VLM, we referenced sagittal plane coordinates from Geerling et al. (27). These were matched to Swanson (24) so that the A1 region was located on atlas levels 72 and 73, A1/C1 on levels 69–71, and C1 on levels 61–68. Cell counts in the NTS and AP were performed with reference to adjacent Nissl sections and levels 69–70 of Swanson (24). All data are reported as the number of labeled cells per section rather than the total number per region (25).
Data Analysis
Results are expressed as mean ± SEM. Comparisons between groups were made using one-way repeated measures ANOVA and Tukey post hoc test for hormone results or two-way ANOVA and Bonferroni post hoc test for the neuron counts (Excel, Mac 2004, Microsoft; Prism, Mac version 4, GraphPad Software). The probability of significance is considered as P < 0.05.
Results
Effects of MIgG-SAP or DSAP Injections Into the PVH on Catecholaminergic Neurons
Catecholaminergic Innervation of the Hypothalamus
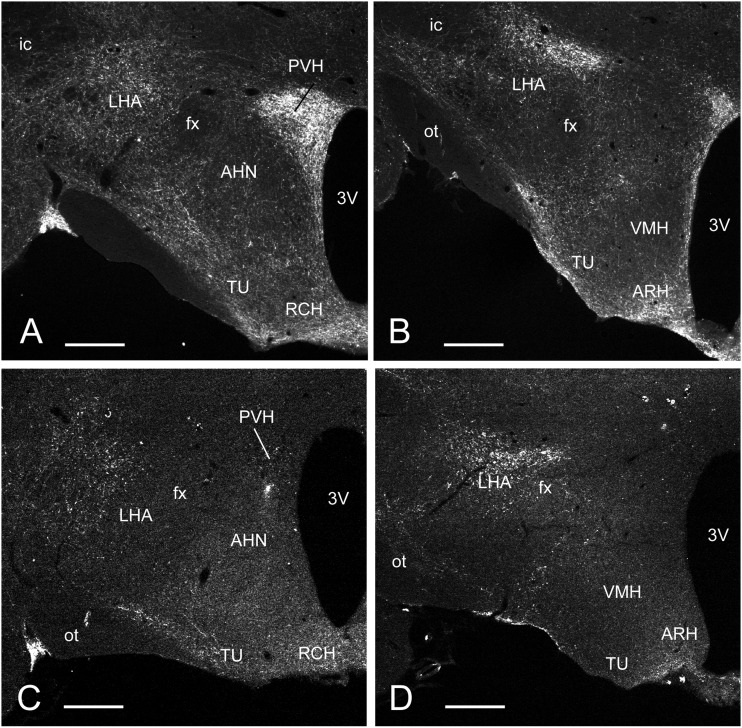
MIgG-SAP injected (intact) animals showed robust catecholaminergic (DBH) innervation of the PVH and lateral hypothalamic area, particularly lateral to the fornix (Fig. 1A). More caudally, the tuberal nucleus, arcuate nucleus, and those parts of the lateral hypothalamic area laterally adjacent to the ventromedial nucleus (VMH) (Fig. 1B) were also heavily innervated. The VMH, however, was noticeably devoid of DBH. Virtually all hypothalamic DBH medial to the fornix was eliminated in DSAP-injected animals (Fig. 1C and D). All lesioned animals exhibited this degree of denervation (n = 14); no data were included from any partially lesioned animals.
Figure 1.
Fluorescence photomicrographs of coronal sections through the hypothalamus of rats showing catecholaminergic innervation revealed with DBH immunocytochemistry. Animals were injected in the PVH with either a MIgG-SAP (A and B) or a DSAP (C and D). Sections correspond to levels 26 (A and C) and 28 (B and D) of Swanson (24). Note the profound loss of catecholaminergic innervation in the mediobasal hypothalamus of the animal injected with DSAP (C and D) compared with that injected with MIgG-SAP (A and B). 3V, third ventricle; AHN, anterior hypothalamic nucleus; ARH, arcuate nucleus; fx, fornix; ic, internal capsule; LHA, lateral hypothalamic area; ot, optic tract; RCH, retrochiasmatic area; TU, tuberal nucleus; VMH, ventromedial nucleus of the hypothalamus. Scale bar = 500 μm.
DBH and PNMT Immunoreactivity in the Hindbrain
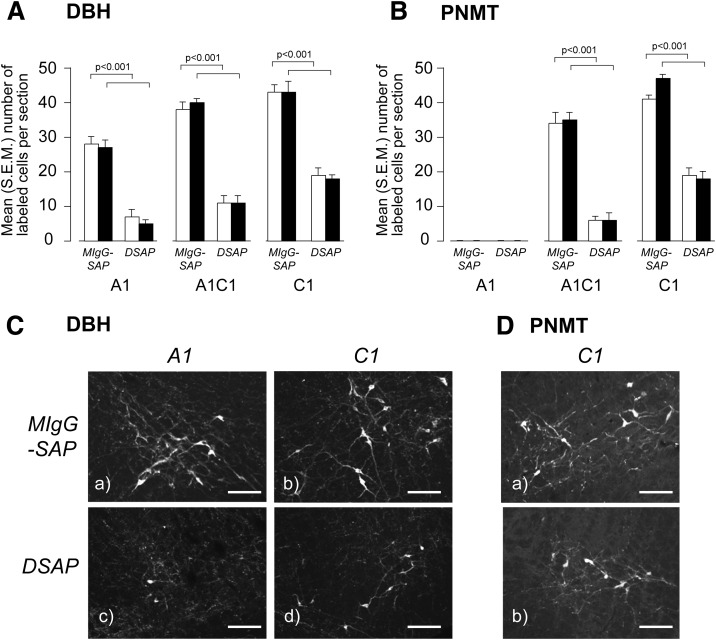
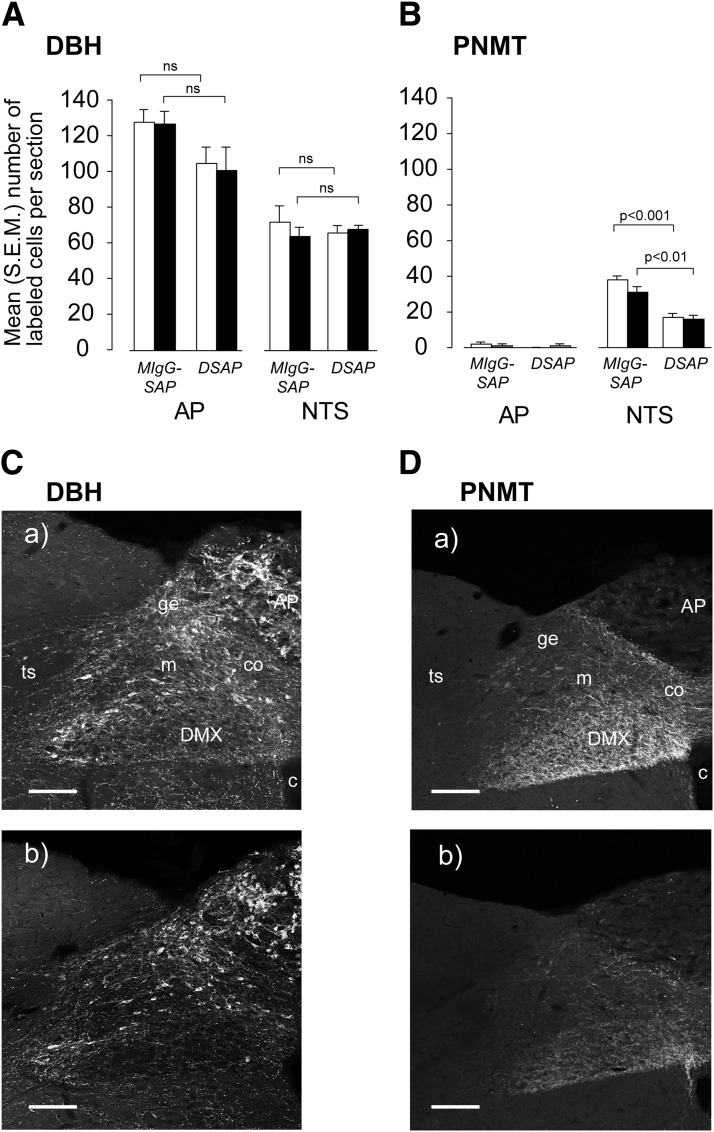
Lesioned animals showed a highly significant 60–80% loss of noradrenergic/adrenergic (DBH) (Fig. 2A and C) and adrenergic (PNMT) (Fig. 2B and D) neurons in the A1, A1/C1, and C1 regions of the VLM compared with intact animals. Lesions had no significant effect on total numbers of DBH neurons in the A2/C2 NTS cell groups and the AP (Fig. 3A and C). There was significant loss of PNMT neurons in the A2/C2 (Fig. 3B and D). PNMT was virtually undetectable in the AP (Fig. 3B and D). Importantly, there was no significant main effect of the lesions on the numbers of DBH and PNMT neurons in any hindbrain region between rapid- and slow-onset IIH.
Figure 2.
Animals injected in the PVH with a DSAP show a significant loss of DBH (A and C) and PNMT (B and D) immunoreactive neurons in the A1, A1/C1, and C1 regions of the VLM compared with animals injected with a MIgG-SAP. Animals were exposed to either rapid- (open bars) or slow-onset (solid bars) hyperinsulinemic-hypoglycemic clamp. Results in A and B are expressed as mean (SEM) number of labeled cells per section. C and D show DBH and PNMT in the A1 (DBH only) and C1 regions of the VLM. Scale bar = 100 μm.
Figure 3.
Animals injected in the PVH with a DSAP show a significant loss of DBH (A and Cb) and PNMT (B and Db) immunoreactive neurons in the AP and NTS compared with animals injected with a MIgG-SAP (Ca and Da). Animals were exposed to either rapid-onset (open bars) or slow-onset (solid bars) hyperinsulinemic-hypoglycemic clamp. Results in A and B are expressed as mean (SEM) number of labeled cells per section. ns, not significant; c, central canal, spinal cord/medulla; co, commisural part of the NTS; ge, gelatinous part of the NTS; m, medial part of the NTS; ts, solitary tract. Scale bar = 200 μm.
Hyperinsulinemic-Hypoglycemic Clamps
Arterial Plasma Glucose Concentrations
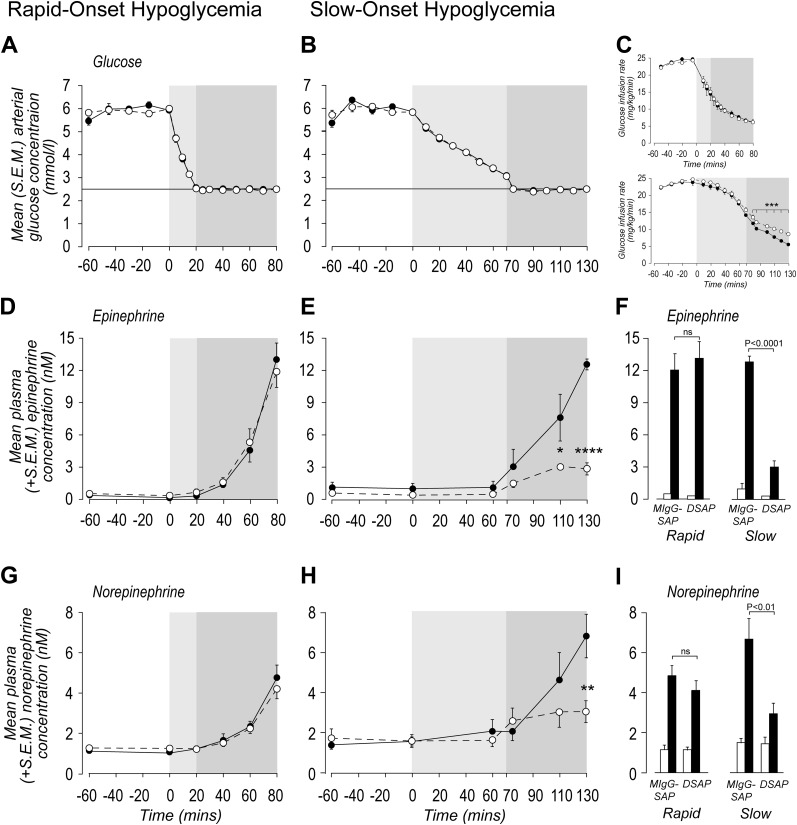
Insulin infusions were initiated at −60 min, and euglycemia was established between −60 and 0 min. At that point, plasma arterial glucose concentrations fell from 5.91 ± 0.01 mmol/L (rapid-onset IIH clamps) (Fig. 4A) or 6.10 ± 0.09 mmol/L (slow-onset clamps) (Fig. 4B) to achieve glycemic nadirs of 2.49 ± 0.01 mmol/L by 20 min (rapid-onset IIH) or 2.47 ± 0.01 mmol/L by 75 min (slow-onset IIH). There were no significant differences between arterial glucose concentrations at 0 min in any experimental group. Hypoglycemia was maintained in both rate groups for a further 60 min. By design, arterial glucose concentrations were carefully matched throughout rapid- and slow-onset clamps.
Figure 4.
Sympathoadrenal hormone responses to rapid- and slow-onset IIH. Panels A and B show the mean (SEM) arterial glucose concentrations in animals injected in the PVH with either a MIgG-SAP (solid circles) or a DSAP (open circles) and exposed to a rapid- or slow-onset hyperinsulinemic-hypoglycemic clamp. C: Corresponding glucose infusion rates. Plasma epinephrine (D–F) and norepinephrine (G–I) responses to the hyperinsulinemic-hypoglycemic clamps. F and I show the plasma epinephrine and norepinephrine concentrations at 0 min (open bars) and after 60 min (solid bars) of hypoglycemia. In all panels except F and I, the light gray area denotes the onset duration of hypoglycemia and the dark gray area denotes the duration of hypoglycemia (2.5 mmol/L). *P < 0.02; **P < 0.001; ***P < 0.0002; ****P < 0.0001; ns, not significant.
Glucose Infusion Rates During Rapid- and Slow-Onset Hypoglycemic-Hyperinsulinemic Clamps
Glucose infusion rates at the onset of the clamps were not significantly different between intact and lesioned animals for both rapid- and slow-onset IIH (Fig. 4C). Consistent with their sympathoadrenal CRRs, glucose infusions over the last 60 min were not significantly different between lesioned and intact animals during rapid-onset IIH, but infusion rates required to maintain hypoglycemia in slow-onset animals was significantly greater from 75 min onward in lesioned animals compared with intact controls.
Sympathoadrenal Responses to Rapid-Onset Hypoglycemia
DSAP lesions had no effect on plasma epinephrine and norepinephrine concentrations at 0 min (Fig. 4D, F, G, and I). Plasma epinephrine and norepinephrine concentrations significantly increased in response to IIH in intact and lesioned animals, and there was no significant difference in plasma epinephrine or norepinephrine concentrations between groups after 60 min of IIH.
Sympathoadrenal Responses to Slow-Onset Hypoglycemia
DSAP lesions had no effect on plasma epinephrine and norepinephrine concentrations at 0 min (Fig. 4E, F, H, and I). But there was a significant suppression of sympathoadrenal CRRs after 40 min (epinephrine only) and 60 min (norepinephrine and epinephrine) of IIH in lesioned animals.
Fos Activation and Its Colocalization with Catecholaminergic Neurons in the VLM and Dorsal Medulla
VLM
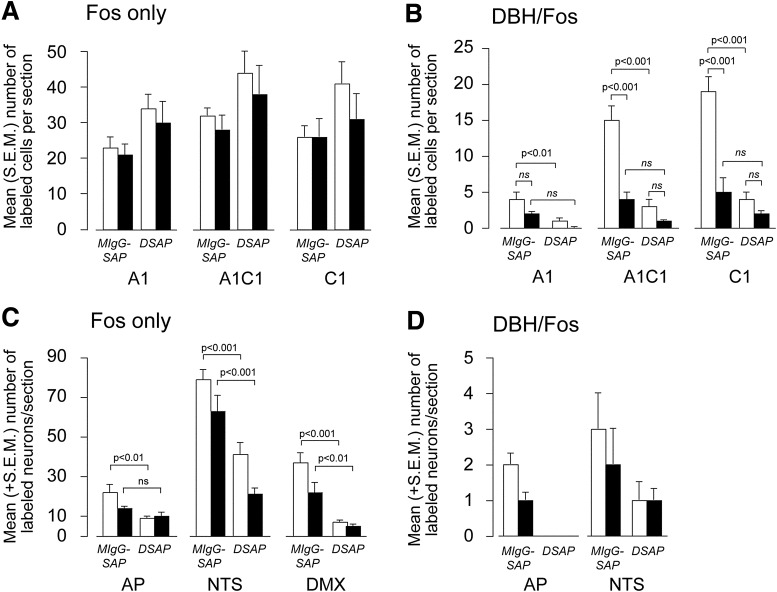
Fos nuclei were prominent in the A1, A1/C1, and C1 regions of intact and lesioned animals following rapid- and slow-onset hypoglycemia (Figs. 5A and 6). However, there were no significant interactions between hypoglycemia onset rates and lesions or any main effects of lesions on the Fos responses to rapid- or slow-onset IIH in any part of the VLM (Fig. 5A).
Figure 5.
Mean (SEM) numbers of Fos (A and C) and Fos/DBH (B and D) colocalized neurons per section in the A1, A1/C1, and C1 regions of the VLM (A and B) and dorsal medulla (C and D). Animals were injected in the PVH with either a MIgG-SAP or a DSAP and exposed to a rapid-onset (open bars) or slow-onset (solid bars) hyperinsulinemic-hypoglycemic clamp. ns, not significant.
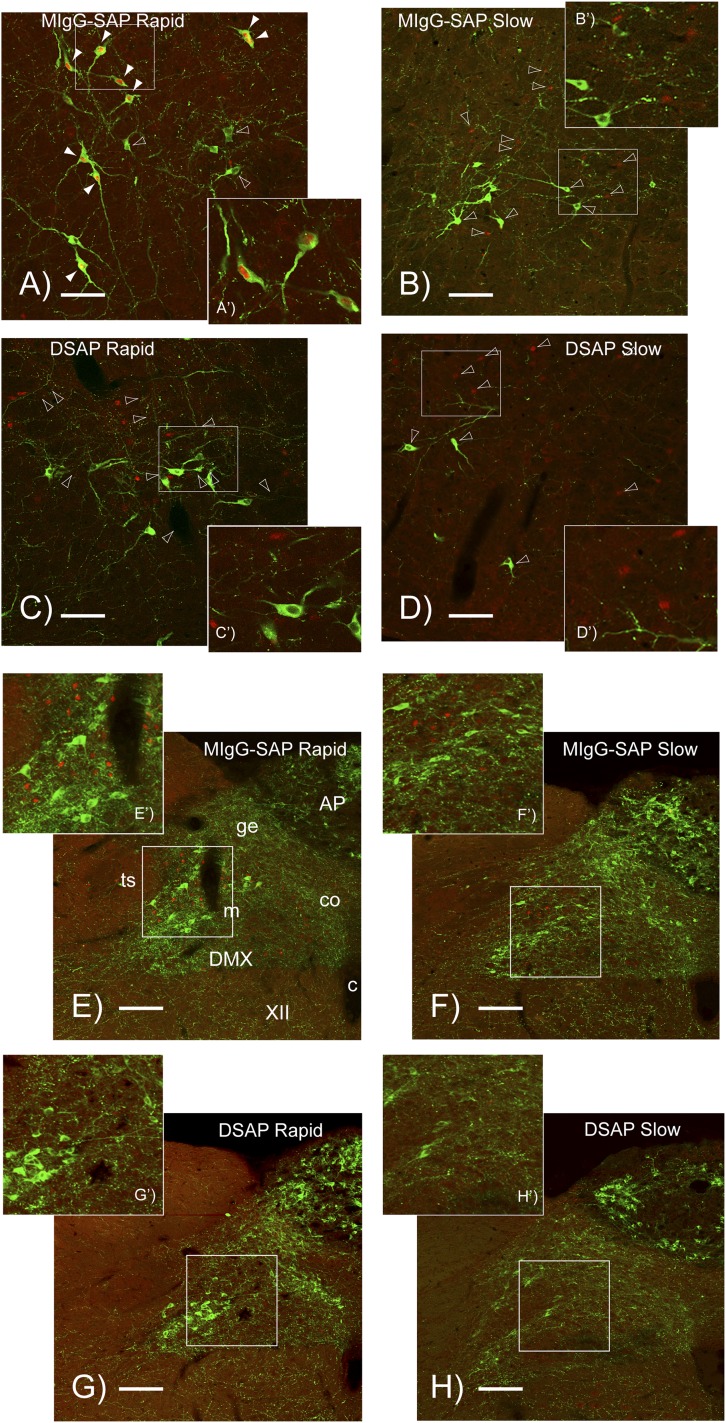
Figure 6.
Confocal photomicrographs showing DBH (green fluorophore) and Fos (red fluorophore) immunoreactive elements in the C1 region of the VLM (A–D) and dorsal medulla (E–H) of animals injected in the PVH with either a MIgG-SAP or a DSAP. Animals were exposed to either rapid- or slow-onset hyperinsulinemic-hypoglycemic clamp. The white rectangle in each main panel is the region shown at higher magnification in the accompanying panel. The arrowheads in A–D indicate representative DBH/Fos double-labeled neurons (solid) and DBH or Fos singly labeled neurons (open). The brightness and contrast of each panel was adjusted uniformly to achieve balance across the entire panel using combinations of the brightness/contrast and curves (γ-correction) tools in Adobe Photoshop CS3 (Adobe Inc.) Please note that brightness/contrast manipulations were only used for the photomicrographs and were not performed on images from which the data used to derive the quantitative results shown in Figs. 2, 3, and 5 were obtained. None of the manipulations changed the nature of the results. c, central canal, spinal cord/medulla; co, commisural part of the NTS; ge, gelatinous part of the NTS; m, medial part of the NTS; ts, solitary tract; XII, hypoglossal nucleus. Scale bars = 100 μm (A–D); scale bars = 200 μm (E–H).
There were significant interactions between the IIH onset rate and the numbers of DBH/Fos colocalized neurons (Figs. 5B and 6) in the A1/C1 (F1,22 = 11.27; P < 0.001) and C1 regions (F1,22 = 18.17; P < 0.001). In all regions, numbers were markedly suppressed in animals exposed to slow-onset, compared with rapid-onset, IIH. However, this effect was absent in the A1 region (F1,22 = 0.404) where there were substantially fewer DBH/Fos colocalized neurons than in the more rostral A1/C1 and C1 parts of the VLM.
There were significant main effects of lesions on the numbers of DBH/Fos colocalized neurons (Figs. 5B and 6A–D) in the A1 (F1,22 = 5.942; P < 0.01), A1/C1 (F1,22 = 26.15; P < 0.001) and C1 (F1,22 = 29.72; P < 0.001) regions in animals with rapid-onset IIH. However, these effects were absent following slow-onset IIH.
Dorsal Medulla
Intact animals exposed to rapid- or slow-onset IIH had substantial numbers of Fos neurons in the NTS but fewer in the AP and DMX (Fig. 5C). The majority of Fos neurons in the NTS were located in its medial part, just medial to the solitary tract (Fig. 6E–H). There were no significant interactions between the IIH onset rate and the number of Fos neurons in the AP, NTS, or DMX. However, there were significant main effects of lesions on the number of Fos neurons in all three dorsal medullary regions (AP, F1,21 = 12.69, P < 0.01 rapid only; NTS, F1,21 = 10.64, P < 0.001 rapid and slow; DMX, F1,21 = 7.03, P < 0.001 rapid and P < 0.01 slow). Very few DBH/Fos neurons were seen in the NTS and AP of animals in any treatment group (Figs. 5D and 6D–H), and no comparisons between treatment groups reached statistical significance (Fig. 5D).
Discussion
We have previously shown that PMV glucosensing mechanisms are essential for generating sympathoadrenal CRRs to slow-onset, but not rapid-onset, IIH (8), where brain glucosensors play a more prominent role. Thus sensory information from slow- and rapid-onset hypoglycemia is processed differentially, irrespective of the fact that both rates engage sympathoadrenal responses by the time glycemia reaches 2.5 mmol/L. Sensory information from the PMV is conveyed to the hindbrain by spinal afferents (23,28) where it drives neuronal activation in the NTS (29,30). However, where this information goes after leaving the NTS to drive sympathoadrenal responses remains unknown.
Two results now show that as development rates of IIH decrease, not only are different glucosensing elements involved, but distinct brain mechanisms are engaged to activate sympathoadrenal CRRs. First, catecholaminergic neurons with projections to the medial hypothalamus are required for sympathoadrenal responses to slow-onset, but not rapid-onset, IIH. Second, the development rate of IIH markedly alters the activation state of catecholaminergic neurons in the VLM, meaning that how these particular neurons are affected by IIH is determined by its development rate.
Glucosensing mechanisms in the ventromedial hypothalamus are closely associated with endocrine CRRs. They do this using a multicomponent complex consisting of, at least, glucosensing neurons (31), contributions from GABAergic and catecholaminergic inputs (16,32), and possibly tanycytes (33,34). Importantly, our lesions almost certainly eliminate the neurons that release the microdialyzed norepinephrine measured in these studies (16). Glucosensing neurons have been identified in the PVH (35), but their role in sympathoadrenal counterregulation is unknown. Furthermore, is not known whether catecholamine neurons themselves function as classic glucosensors.
Ventromedial hypothalamic glucosensing components interact so that IIH increases norepinephrine release while decreasing γ-aminobutyric acid (GABA) release from local terminals (16,36), thereby activating sympathoadrenal CRRs. Conversely, maintaining ventromedial hypothalamic glucose concentrations locally during IIH blocks all of these responses (37). Altered ventromedial hypothalamic GABA and norepinephrine release therefore requires a fall in ambient glucose that is detected by local glucosensing mechanisms.
A variety of pharmacological manipulations show that glucose-sensitive GABA release into the ventromedial hypothalamus is modulated by adrenergic receptors (38) and by agents that open those ATP-sensitive potassium channels that contribute directly to glucosensing (39). Thus Zhu et al. (36) suggest that GABA neurons that are responsive to local glucosensing can restrain endocrine CRRs. This inhibition is removed during IIH. Changes in ambient ventromedial hypothalamic glucose therefore can modulate synaptic norepinephrine and GABA release in a way that influences sympathoadrenal and glucagon CRRs (16,32).
Despite contributions of norepinephrine-dependent mechanisms to glucosensing in the ventromedial hypothalamus, we now show that catecholaminergic inputs to this region are surprisingly not required for sympathoadrenal CRRs to rapid-onset IIH. During rapid-onset IIH, glucosensing mechanisms remain fully capable of mediating sympathoadrenal CRRs in the absence of catecholaminergic inputs. One explanation for this effect is that despite the significant rise in ventromedial hypothalamic norepinephrine in these circumstances (16,40), local glucosensing mechanisms by themselves provide adequate reductions in GABA release without a catecholaminergic component. Presumably, increased norepinephrine release in the PVH and ventromedial hypothalamus contributes to other processes that occur during rapid-onset IIH, notably ACTH release (14,41) and feeding (42).
Sensory information derived from rapid- and slow-onset IIH not only engages different glucosensing elements, but we now show that it must be processed by the brain in a rate-dependent manner. This is clearly evident in the VLM where rapid-onset IIH produces approximately three times more catecholaminergic/Fos neurons than slow-onset IIH. These differences are region specific because less than 4% of Fos-labeled neurons in the NTS after rapid-onset IIH are catecholaminergic compared with ∼60% in the more rostral parts of the VLM. However, the very limited Fos activation in our catecholaminergic NTS neurons contrasts with that found after higher doses of 2-deoxy-D-glucose (2DG) (11) and most likely reflects the different stimulus characteristics of IIH and 2DG. In contrast to DBH/Fos-labeled neurons, we found no differences in the numbers of Fos-labeled neurons in any region examined following either IIH development rate. That the development rate did not affect the number of Fos neurons in the NTS is consistent with our previous results where we also used hyperinsulinemic clamps to control the development rate of hypoglycemia (30).
Despite the fact that forebrain-projecting catecholamine neurons are only required to activate sympathoadrenal CRRs to slow-onset IIH, many more DBH/Fos neurons were seen after rapid-onset IIH. This inverse and seemingly paradoxical relationship between the numbers of Fos-labeled catecholamine neurons in the VLM and their apparent relative importance as development rates of IIH change reveals complex functional interactions between catecholaminergic systems and sympathoadrenal CRRs to IIH. Three points require consideration.
First, in addition to sympathoadrenal CRRs, IIH also generates neuroendocrine, autonomic, and behavioral responses, including ACTH release and feeding (14,43). During IIH, catecholamine neurons are required to drive ACTH (13,14), feeding (as implied from 2DG-induced feeding), and sympathoadrenal CRRs (12 and present study). Therefore, at least some VLM Fos-labeled catecholamine neurons must subserve events other than sympathoadrenal CRRs. That there is a degree of structural separation between these systems is shown by the fact that feeding and sympathoadrenal CRRs are differentially affected by recurrent IIH (44) and that PVH/ventromedial DSAP lesions that do not affect CRRs following rapid-onset IIH (present study) completely eliminate ACTH responses to bolus intravenous insulin injections (14) and feeding responses to 2DG (12). Feeding and ACTH responses are likely driven, at least partly, by the large numbers of Fos-labeled catecholaminergic VLM neurons with forebrain projections (based on the number of double-labeled neurons remaining in rapid-onset animals with DSAP lesions). From the numbers of VLM DBH neurons remaining after forebrain DSAP lesions, almost 60% of these project to the PVH/ventromedial hypothalamus. Importantly, these numbers are virtually identical in animals exposed to different development rates of IIH, meaning that differences in CRRs cannot be explained by varying lesion efficacies. Collectively, these results show that sympathoadrenal CRRs, ACTH, and feeding are driven by somewhat separate catecholamine-containing neural networks even though each network receives information from glucosensing elements.
Second, the amount of Fos accumulated by a neuron after a stimulus is not tightly coupled to changes in its action potential frequency. Indeed, increased neuronal firing can occur with no Fos accumulation (45), meaning that the processes driving action potentials do not themselves increase Fos. Instead, c-fos transcription requires receptor activation and elevated intracellular calcium (46). Slow-onset IIH clearly requires forebrain-projecting catecholaminergic neurons. Yet these neurons accumulate significantly less Fos than after rapid-onset IIH. This suggests that the different glucosensing mechanisms engaged by slow- and rapid-onset IIH differentially activate intracellular signaling processes in catecholaminergic neurons, which in turn generates different amounts of Fos. We do not know whether the IIH rate-dependent engagement of those intracellular signals responsible for Fos expression also alters their firing rates in a way that directly impacts sympathoadrenal CRRs.
Third, because our goal was to determine whether the rate of IIH development, rather than IIH itself, affected Fos activation, the experimental design did not require hyperinsulinemic-euglycemic clamps. This means that we cannot completely rule out that at least some neuronal activation derives from hyperinsulinemia rather than IIH. However, hyperinsulinemic-euglycemic clamps result in significantly less NTS Fos expression compared with hypoglycemia (47). Our findings showing that the two development rates of IIH produced different amounts of DBH/Fos colocalization despite the same insulin dose argues against hyperinsulinemia being a significant factor. Moreover, the fact that the longer insulin exposures experienced by the slow-onset IIH animals produced the same overall number of Fos-labeled neurons as rapid-onset IIH, but less colocalization, shows that colocalization was independent of the duration of insulin exposure.
In terms of potential mechanisms, Beverly and colleagues noted that IIH produces the same magnitude fall in ventromedial hypothalamic glucose as seen after an overnight fast (48). Yet we show that sympathoadrenal CRR only occurs after IIH, not an overnight fast; all our clamped animals were fasted overnight yet had no sympathoadrenal response before IIH. Since ambient ventromedial glucose falls much quicker during IIH than fasting, the rate of fall in local glucose must be an important contributory factor to mechanisms stimulating endocrine CRRs (40). Fasting reduces ambient ventromedial hypothalamic glucose to that seen during IIH yet does not increase sympathoadrenal CRRs. Therefore mechanisms additional to those operating during fasting must be required to enable slowly falling ventromedial hypothalamic glucose to activate CRRs during IIH. We now show that catecholamine inputs are well placed to contribute in this manner during slow-onset IIH, perhaps through interactions with local GABAergic terminals. Because the vast majority of VMH neurons are glutamatergic, it seems highly unlikely that the cell bodies of these GABA neurons are located within the VMH (49).
Our results therefore support a mechanism where a catecholaminergic-facilitated fall in GABA release is required during slow-onset, but not rapid-onset, IIH to activate CRRs. This mechanism may also explain why the slow reduction in ambient glucose seen after fasting (48) is insufficient to drive CRRs, because there is no concomitant increase in catecholaminergic activity to facilitate reduced GABA release. Because of the importance of PMV glucosensing mechanisms to slow-onset IIH (8), it is tempting to speculate that at least part of the information encoded by ascending catecholamine neurons derives from glucosensing elements in the PMV.
In summary, we have previously used PMV denervation to show that slow- and rapid-onset IIH use different glucosensing elements to drive sympathoadrenal responses (8). We now demonstrate that compromising a brain network component—PVH/ventromedial hypothalamus–projecting catecholamine neurons—generates the same outcome as denervating the PMV. The mechanisms used by ventromedial hypothalamic glucosensing and its downstream processes during IIH are therefore determined by the rate of fall of blood glucose. In this way, slow-onset, but not rapid-onset, IIH requires information encoded catecholaminergic neurons projecting to the ventromedial hypothalamic glucosensing elements where we suggest they interact with local GABAergic terminals to maintain sympathoadrenal CRRs. Finally, our Fos results show that glucosensing information is processed in a rate-dependent manner by the brain and that the outcomes of this processing are then distributed by somewhat different neural networks. The fact that slow-onset IIH may prevail during type 1 diabetes–associated insulin therapy (9,10) shows the importance of fully understanding the functional organization of these glucose-engaged neural networks.
Article Information
Funding. This work was supported by a research grant from the JDRF (1-2008-710) and NS029728 from the National Institute of Neurological Disorders and Stroke/National Institutes of Health to A.G.W.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. The study was designed by all three authors. The experiments were performed by A.J.J., and the results were analyzed by A.J.J. and A.G.W. The manuscript was written by A.J.J. and A.G.W., with input from C.M.D. A.G.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, FL, 25–29 June 2010 and at Neuroscience 2012 from the Society for Neuroscience, New Orleans, LA, 13–17 October 2012.
Footnotes
References
- 1.Cryer PE. Hypoglycemia in type 1 diabetes mellitus. Endocrinol Metab Clin North Am 2010;39:641–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeldtke RD, Boden G. Epinephrine secretion, hypoglycemia unawareness, and diabetic autonomic neuropathy. Ann Intern Med 1994;120:512–517 [DOI] [PubMed] [Google Scholar]
- 3.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes 2004;53:2521–2528 [DOI] [PubMed] [Google Scholar]
- 4.Donovan CM. Portal vein glucose sensing. Diabetes Nutr Metab 2002;15:308–312; discussion 313–314 [PubMed] [Google Scholar]
- 5.Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes 1997;46:1521–1525 [DOI] [PubMed] [Google Scholar]
- 6.Ritter S, Li AJ, Wang Q, Dinh TT. Minireview: The value of looking backward: the essential role of the hindbrain in counterregulatory responses to glucose deficit. Endocrinology 2011;152:4019–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol 2010;31:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saberi M, Bohland M, Donovan CM. The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of portal-superior mesenteric vein glucose sensing. Diabetes 2008;57:1380–1386 [DOI] [PubMed] [Google Scholar]
- 9.Kovatchev BP, Clarke WL, Breton M, Brayman K, McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther 2005;7:849–862 [DOI] [PubMed] [Google Scholar]
- 10.Bolli GB, Gottesman IS, Cryer PE, Gerich JE. Glucose counterregulation during prolonged hypoglycemia in normal humans. Am J Physiol 1984;247:E206–E214 [DOI] [PubMed] [Google Scholar]
- 11.Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res 1998;805:41–54 [DOI] [PubMed] [Google Scholar]
- 12.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol 2001;432:197–216 [DOI] [PubMed] [Google Scholar]
- 13.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology 2003;144:1357–1367 [DOI] [PubMed] [Google Scholar]
- 14.Khan AM, Kaminski KL, Sanchez-Watts G, et al. MAP kinases couple hindbrain-derived catecholamine signals to hypothalamic adrenocortical control mechanisms during glycemia-related challenges. J Neurosci 2011;31:18479–18491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol 1975;163:467–505 [DOI] [PubMed] [Google Scholar]
- 16.Beverly JL, De Vries MG, Bouman SD, Arseneau LM. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Am J Physiol Regul Integr Comp Physiol 2001;280:R563–R569 [DOI] [PubMed] [Google Scholar]
- 17.de Vries MG, Lawson MA, Beverly JL. Hypoglycemia-induced noradrenergic activation in the VMH is a result of decreased ambient glucose. Am J Physiol Regul Integr Comp Physiol 2005;289:R977–R981 [DOI] [PubMed] [Google Scholar]
- 18.Kaminski KL, Watts AG. Intact catecholamine inputs to the forebrain are required for appropriate regulation of corticotrophin-releasing hormone and vasopressin gene expression by corticosterone in the rat paraventricular nucleus. J Neuroendocrinol 2012;24:1517–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritter S, Dinh TT, Bugarith K, Salter DM. Chemical Dissection of Brain Glucoregulatory Circuitry. In Molecular Neurosurgery With Targeted Toxins. Wiley RG, Lappi DA, Eds. Totowa, Humana Press Inc., 2005, p. 181–218 [Google Scholar]
- 20.Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J Neurosci 2006;26:11442–11453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan AM, Ponzio TA, Sanchez-Watts G, Stanley BG, Hatton GI, Watts AG. Catecholaminergic control of mitogen-activated protein kinase signaling in paraventricular neuroendocrine neurons in vivo and in vitro: a proposed role during glycemic challenges. J Neurosci 2007;27:7344–7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peuler JD, Johnson GA. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci 1977;21:625–636 [DOI] [PubMed] [Google Scholar]
- 23.Fujita S, Bohland M, Sanchez-Watts G, Watts AG, Donovan CM. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol Endocrinol Metab 2007;293:E96–E101 [DOI] [PubMed] [Google Scholar]
- 24.Swanson LW. Brain Maps: Structure of the Rat Brain. 3rd ed. San Diego, Academic Press, 2004 [Google Scholar]
- 25.Watts AG, Sanchez-Watts G. Rapid and preferential activation of Fos protein in hypocretin/orexin neurons following the reversal of dehydration-anorexia. J Comp Neurol 2007;502:768–782 [DOI] [PubMed] [Google Scholar]
- 26.Bohn MC, Dreyfus CF, Friedman WJ, Markey KA. Glucocorticoid effects on phenylethanolamine N-methyltransferase (PNMT) in explants of embryonic rat medulla oblongata. Brain Res 1987;465:257–266 [DOI] [PubMed] [Google Scholar]
- 27.Geerling JC, Shin J-W, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol 2010;518:1460–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita S, Donovan CM. Celiac-superior mesenteric ganglionectomy, but not vagotomy, suppresses the sympathoadrenal response to insulin-induced hypoglycemia. Diabetes 2005;54:3258–3264 [DOI] [PubMed] [Google Scholar]
- 29.Adachi A, Shimizu N, Oomura Y, Kobáshi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett 1984;46:215–218 [DOI] [PubMed] [Google Scholar]
- 30.Bohland M, Matveyenko AV, Saberi M, Khan AM, Watts AG, Donovan CM. Activation of hindbrain neurons is mediated by portal-mesenteric vein glucosensors during slow-onset hypoglycemia. Diabetes 2014;63:2866–2875 [DOI] [PMC free article] [PubMed]
- 31.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 2004;53:549–559 [DOI] [PubMed] [Google Scholar]
- 32.Chan O, Paranjape S, Czyzyk D, et al. Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Diabetes 2011;60:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders NM, Dunn-Meynell AA, Levin BE. Third ventricular alloxan reversibly impairs glucose counterregulatory responses. Diabetes 2004;53:1230–1236 [DOI] [PubMed] [Google Scholar]
- 34.Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 2011;152:2552–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melnick IV, Price CJ, Colmers WF. Glucosensing in parvocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci 2011;34:272–282 [DOI] [PubMed] [Google Scholar]
- 36.Zhu W, Czyzyk D, Paranjape SA, et al. Glucose prevents the fall in ventromedial hypothalamic GABA that is required for full activation of glucose counterregulatory responses during hypoglycemia. Am J Physiol Endocrinol Metab 2010;298:E971–E977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 1997;99:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szepietowska B, Zhu W, Chan O, Horblitt A, Dziura J, Sherwin RS. Modulation of β-adrenergic receptors in the ventromedial hypothalamus influences counterregulatory responses to hypoglycemia. Diabetes 2011;60:3154–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan O, Lawson M, Zhu W, Beverly JL, Sherwin RS. ATP-sensitive K(+) channels regulate the release of GABA in the ventromedial hypothalamus during hypoglycemia. Diabetes 2007;56:1120–1126 [DOI] [PubMed] [Google Scholar]
- 40.Barnes MB, Lawson MA, Beverly JL. Rate of fall in blood glucose and recurrent hypoglycemia affect glucose dynamics and noradrenergic activation in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 2011;301:R1815–R1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacák K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 2001;22:502–548 [DOI] [PubMed] [Google Scholar]
- 42.Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Res Bull 1988;21:905–912 [DOI] [PubMed] [Google Scholar]
- 43.Grossman SP. The role of glucose, insulin and glucagon in the regulation of food intake and body weight. Neurosci Biobehav Rev 1986;10:295–315 [DOI] [PubMed] [Google Scholar]
- 44.Sanders NM, Figlewicz DP, Taborsky GJ, Jr, Wilkinson CW, Daumen W, Levin BE. Feeding and neuroendocrine responses after recurrent insulin-induced hypoglycemia. Physiol Behav 2006;87:700–706 [DOI] [PubMed] [Google Scholar]
- 45.Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci 1994;14:4825–4830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watts AG, Khan AM, Sanchez-Watts G, Salter D, Neuner CM. Activation in neural networks controlling ingestive behaviors: what does it mean, and how do we map and measure it? Physiol Behav 2006;89:501–510 [DOI] [PubMed] [Google Scholar]
- 47.Ao Y, Wu S, Go VL, Toy N, Yang H. Maintaining euglycemia prevents insulin-induced Fos expression in brain autonomic regulatory circuits. Pancreas 2005;31:142–147 [DOI] [PubMed] [Google Scholar]
- 48.de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes 2003;52:2767–2773 [DOI] [PubMed] [Google Scholar]
- 49.Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol 2002;448:217–229 [DOI] [PubMed] [Google Scholar]