Abstract
We present the case of a 54-year-old man with a brown-red nodule on the hand that had been present since early adulthood. Histology of the excisional biopsy revealed hyperplasia and proliferation of eccrine, apocrine, lipomatous, and vascular structures. These findings were most characteristic of the entity known as eccrine angiomatous hamartoma (EAH), an uncommon tumor that may present variable clinical and histological features. In addition, this particular case exhibited a prominent component of arterio-venous malformation that distinguishes it from other EAHs described in the literature and adds to the spectrum of histologic findings that can be seen with this entity.
Case report
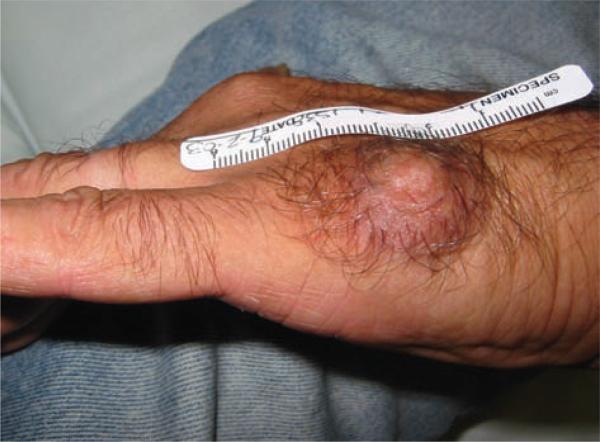
A 54-year-old army veteran was referred to our clinic for surgical excision of a bump on his hand that had been present since the age of 19. The red-brown nodule on the lateral aspect of the left hand measured approximately 3 cm across with 1 cm elevation off the skin and was non-tender on palpation (Fig. 1). The epidermis and regional hair growth appeared grossly unremarkable. The patient denied any history of trauma to that site, but did notice occasional sweating and occasional pain when he accidentally bumped the lesion against a hard object, such as a door or a piece of furniture. The patient said that approximately 15 years earlier, his previous dermatologist, long since retired, had shaved off the top of the lesion. The patient was not aware of the diagnosis, but noted that after healing, the lesion reassumed its original appearance with no discernable increase in size over the past decade. An excisional biopsy was obtained, and the histologic findings are discussed below. One year after excision, the patient reported no recurrence of the lesion.
Fig. 1.
Clinical presentation of a red-brown nodule on the left hand.
Microscopic findings
The epidermis showed mild focal acanthosis covered with compact, laminated hyperkeratosis. No significant papillomatosis was present. In the dermis, there was an ill-defined multinodular mass composed of a combined proliferation of eccrine, vascular adipose, and neural elements (Fig. 2). These components exhibited an intimate association, although their relative proportions varied from area to area. The sweat gland component showed distinct hyperplasia of mature eccrine glands associated with varying qualitative changes including ductal dilatation, cystic changes, and occasional intraluminal papillary fronds (Fig. 3A,B). Focal areas displayed changes consistent with apoeccrine differentiation, with protruding columnar epithelial cells exhibiting decapitation-type secretion (Fig. 3C,D). Some of the lumina contained eosinophilic proteinaceous material. Throughout the lesion there was also proliferation of blood vessels ranging from capillaries to small arteries and venules, as well as larger vessels with hybrid features of arterioles and venules (Fig. 4A,B). Some vessels showed prominent uneven intimal proliferation and had poorly developed elastic internal membranes but prominent muscular walls. The latter findings were particularly prominent in certain areas and were consistent with arterio-venous malformations (Fig. 4C,D). Another major component of the tumor was mature adipose tissue, which was extensively infiltrated throughout the vascular and sweat gland components and not confined to the subcutis. Scattered hyperplastic nerve fascicles were also present throughout the lesion (Fig. 5). The remainder of the connective tissue showed changes from condensed collagenous to loose, slightly ‘immature’ stroma with few elastic fibers (Fig. 5). No increase or aberrations of hair follicles or erector pilar muscles were identified. No appreciable inflammatory infiltrate was observed. The lesion did not display any characteristic signs of malignancy. Movat's pentachrome stain highlighted various components of the lesion, particularly the vascular structures and elastic fibers (Figs 2–5).
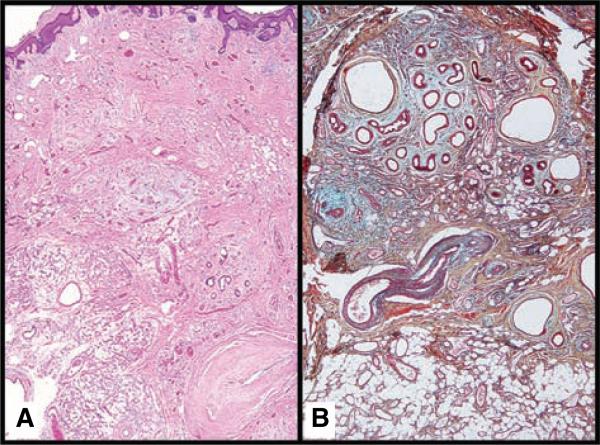
Fig. 2.
Eccrine angiomatous hamartoma (EAH). (A) A panoramic view of EAH shows an ill-defined dermal mass (H&E × 2). (B) Movat's pentachrome outlines intermixed proliferations of sweat glands, vascular structures and mature adipose tissue (× 4).
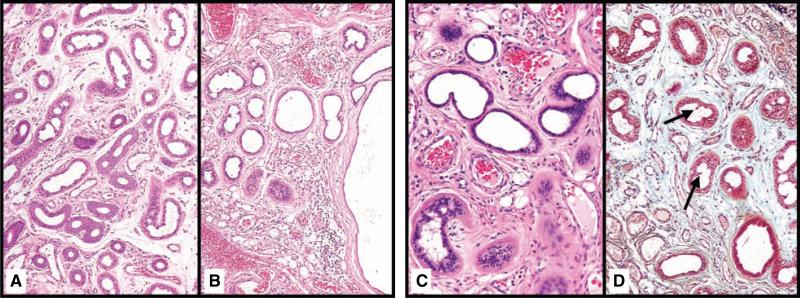
Fig. 3.
Eccrine and apoeccrine structures in eccrine angiomatous hamartoma. Eccrine structures exhibited both quantitative (A) and qualitative (B) changes within an immature edematous stroma (H&E 10). Focal areas exhibited apocrine-like decapitation secretion with intraluminal papillary fronds (black arrows) on H&E (C) and Movat's (D) ( 10).
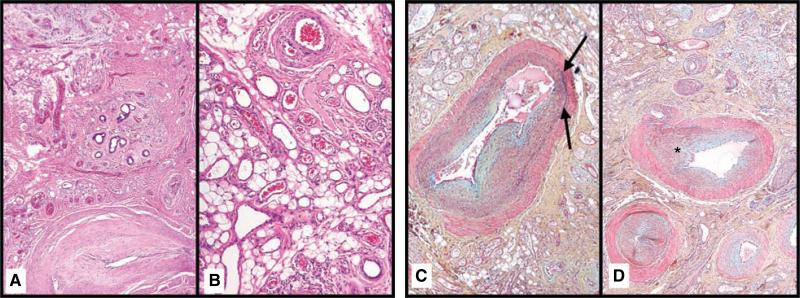
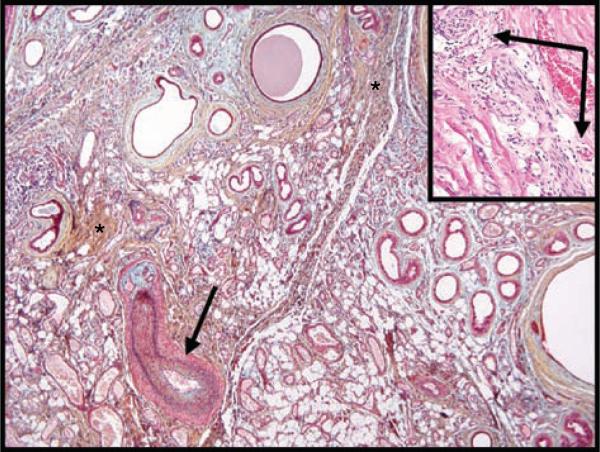
Fig. 4.
Various vascular structures are present, including arteriovenous malformations. (A) A large vessel adjacent to a sweat gland proliferation exhibits features of both an arteriole and venule (H&E 10). (B) A delicate capillary proliferation is admixed with mature adipose tissue (H&E ×). (C) Large vessels with incomplete internal elastic membranes (black arrows) and irregular smooth muscle walls are present (Movat's × 10). (D) Mpvant's stain highlights small arterioles with intimal proliferation (asterisk) (×10).
Fig. 5.
This histologic variant of eccrine angiomatous hamartoma also exhibits nerve hyperplasia. The stroma exhibits both areas of condensed collagen (black asterisk) and areas of loose, immature stroma with diminished elastic fibers. An arrow indicates a vessel with features of both an arteriole and a venule (Movat's 4). The inset shows hyperplastic nerve fibers in association with a capillary proliferation (H&E × 20).
Discussion
The term ‘hamartoma’ describes the non-neoplastic proliferation of cells and tissue components that normally occur in an affected area. An entity fitting the description of eccrine angiomatous hamartoma (EAH) was described as far back as 1859 by the German physician Dr Karl von Lotzbeck, although the term ‘eccrine angiomatous hamartoma’ was not proposed until 1968.1
A recent review found almost 70 cases of EAH published in the world literature.2 The median presentation of EAH occurs around 10 years of age (range 2 months to 73 years). More than 80% of lesions are solitary and occur on the extremities. There is no apparent gender preference. Associated hyperhidrosis, hypertrichosis, or pain can provide a valuable diagnostic clue, although these symptoms are not present in all cases of EAH. The clinical presentation varies from a patch to a plaque to a nodule, and the color can range from red to yellow to brown to blue. In some instances, the lesions have been skin-colored. Epidermal changes are usually minimal, but ulceration or a verrucous epidermis have been reported. Clinically, the differential diagnosis of EAH includes vascular lesions such as venous malformations, blue rubber bleb nevus, glomus tumor, and tufted angioma. A smooth muscle hamartoma or Becker's nevus would also be a consideration. Simple excision of the lesion is usually curative, although it should be noted that in some instances, the size of the tumor has required amputation to alleviate pain.3
Histologic features of EAH include hyperplastic eccrine structures, increased proliferation of vascular structures, and variable presentations of increased lymphatic, smooth muscle, and pilar structures.1,2 Apocrine glands have been described before in EAH.4 In addition, lipomatous changes have also been previously reported.4,5 Limited data exist regarding the immunohistochemical staining of EAH. Not surprisingly, markers associated with normal eccrine structures, including carcinoembryonic antigen, S100, and CD44 also stain EAH.4 Sulica et al.4 noted that CD34 also strongly stained the surrounding stroma, but not the pericytes or vascular endothelium.
Our case fulfills all previously described histologic criteria of EAH, but in addition it also contains unique features not yet been documented in the literature. Specifically, it contained a mixture of variously sized blood vessels with hybrid features of arterioles and venules. These elements were composed of enlarged vessels with unevenly thickened muscular walls and partially developed internal elastic membranes. They also showed intimal proliferation with partial obliteration of the lumina. Other vessels had less-prominent muscular walls without internal elastic membranes and dilated lumina. Lobules of smaller vessels and capillaries were intimately admixed with these structures. All of these features were characteristic of arterio-venous malformations.
Other noteworthy findings in this case included the wide spectrum of changes in the sweat gland units, ranging from ductular and cystic dilation, intraluminal papillary frond formation to partial apocrine differentiation. All of these changes were compatible with hamartomatous malformation, but were beyond what is usually seen in ordinary sweat gland hyper-plasia. Additional components supporting the hamartomatous nature of the lesion included the presence of immature connective tissue matrix with focal myxomatous changes and the altered distribution of the elastic fibers. In some areas, the lipomatous component was particularly prominent with mature adipocytes. Nowhere in the lesion was any evidence of cytologic atypia or appreciable mitotic activity. All of the tissue constituents were clearly discernible by the use of Movat's penta-chrome stain, and the use of immunohistochemical studies was not deemed necessary.
In 1955, the late Dr Henry Z. Movat was a graduate student in experimental pathology at Queens University in Canada when he published a pentachromic staining technique that allowed distinct visualization of all elements of connective tissue in a single section.6 Using Movat's pentachrome stain, elastic fibers stain black, collagen stains yellow, ground substance and mucin stain blue, fibrin stains an intense red, and muscle stains a lighter red. This simple stain, in the age of immunohistochemistry, still remains a particularly useful and cost-effective method for rapid assessment of the various connective tissue components in routine histology.
The differential diagnosis of EAH includes an entity known as sudoriparous angioma, so much so that many authors have considered these terms to be essentially synonymous.2,7 Other authors have noted that sudoriparous angioma lacks hyperplasia of eccrine structures and has larger-caliber vessels than EAH.2 Both entities likely represent related hamartomatous proliferations. However, our case is distinctly different from the previously described cases of sudoriparous angioma, because it contained not only abnormal sweat glands and large caliber vessels, but hyperplastic eccrine elements and small vessels with arterio-venous malformations as well. The absence of angiomatous hyperplasia in eccrine nevus, a rare entity characterized by groupings of normal to enlarged eccrine structures, distinguishes it from EAH.
In conclusion, we report a previously undescribed variant of EAH with a prominent component of an arterio-venous malformation, corroborating the hamartomatous nature of this peculiar lesion.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Hyman AB, Harris H, Brownstein MH. Eccrine angiomatous hamartoma. NY State J Med. 1968;68:2803. [PubMed] [Google Scholar]
- 2.Pelle MT, Pride HB, Tyler WB. Eccrine angiomatous hamartoma. J Am Acad Dermatol. 2002;47(3):429. doi: 10.1067/mjd.2002.121030. [DOI] [PubMed] [Google Scholar]
- 3.Gabrielsen TO, Elgjo K, Sommerschild H. Eccrine angiomatous hamartoma of the finger leading to amputation. Clin Exp Dermatol. 1991;16(1):44. doi: 10.1111/j.1365-2230.1991.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 4.Sulica RL, Kao GF, Sulica VI, Penneys NS. Eccrine angiomatous hamartoma (nevus): immunohistochemical findings and review of the literature. J Cutan Pathol. 1994;21(1):71. doi: 10.1111/j.1600-0560.1994.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 5.Donati P, Amantea A, Balus L. Eccrine angiomatous hamartoma: a lipomatous variant. J Cutan Pathol. 1989;16(4):227. doi: 10.1111/j.1600-0560.1989.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 6.Movat HZ. Demonstration of all connective tissue elements in a single section; pentachrome stains. AMA Arch Pathol. 1955;60(3):289. [PubMed] [Google Scholar]
- 7.Domonkos AN, Suarez LS. Sudoriparous angioma. Arch Dermatol. 1967;96:552. [PubMed] [Google Scholar]