Abstract
Objective
Apart from cystectomy, few treatment options exist for the management of bacillus Calmette-Guerin refractory non–muscle invasive bladder cancer (NMIBC). We report a multi-institutional experience with sequential intravesical combination chemotherapy using gemcitabine and mitomycin C (MMC) for NMIBC in the treatment of high-risk patients.
Methods
We performed a retrospective review of patients who received 6 weekly treatments with sequential intravesical gemcitabine (1 g) and MMC (40 mg) chemotherapy for NMIBC. Gemcitabine was administered first and retained for 90 minutes and then drained. MMC was then administered directly after and retained for an additional 90 minutes. Forty-seven patients received treatment from 3 academic tertiary referral centers between 2000 and 2010.
Results
Forty-seven patients (median age 70, range 32–85; 36 males, 11 females) who previously failed a median of 2 intravesical treatments were reviewed. Complete response, 1-year, and 2-year recurrence-free survival rates for all patients were 68%, 48%, and 38%, respectively. Median recurrence-free survival for all patients was 9 months (range 1–80). Fourteen of 47 patients (30%) remained free of recurrence with a median time to follow-up of 26 months (range 6–80 mo). Ten patients required cystectomy.
Conclusion
Sequential intravesical combination chemotherapy using gemcitabine and MMC appears to be a useful treatment for patients with high-grade NMIBC as well as those with prior bacillus Calmette-Guerin failure. Further prospective studies are warranted.
Keywords: Bladder cancer, Gemcitabine, Mitomycin, Antineoplastic therapy, Chemotherapy protocols
1. Introduction
The complete response (CR) rate to bacillus Calmette-Guerin (BCG) therapy in patients with high-risk non–muscle invasive bladder cancer (NMIBC) can be as high as 84% [1]; however, it has been estimated that as many as 50% of patients with high-risk disease will suffer from recurrence within 1 year and 90% within 5 years [2]. If CR to therapy is not seen by 6 months after initial BCG treatment and 1 further reinduction course, cystectomy is the treatment of choice [3–5]. Although all conservative (bladder sparing) treatments for BCG-failure patients remain investigational and uncertain, cystectomy remains the gold standard. However, some patients are not candidates for radical surgery because of comorbid medical illness and others refuse to consider the change in their lifestyle that the surgery entails despite being counseled about the risks. Treatment options for patients following recurrence of NMIBC after BCG therapy who refuse cystectomy are limited and the majority are experimental. Options include repeat BCG treatment, an alternate immunotherapy regimen, chemotherapy, or device-assisted therapy (including electromotive intravesical administration of mitomycin C [MMC] and microwave hyperthermia and administration of MMC) [6].
Although multiagent chemotherapy has become the norm in almost all cases of systemic chemotherapy, the practice is not widely used in the treatment of NMIBC. Initial attempts to use combined or alternating intravesical agents such as adriamycin and MMC have been complicated by severe cystitis despite some evidence of enhanced activity [7]. Cystitis associated with adriamycin and MMC administration is thought to be because of their vesicant (tissue irritating) activity which appear to act synergistically. Because of the apparent enhanced activity of multiagent chemotherapies, other combinations of chemotherapeutic agents in which one or both are nonvesicants have recently been evaluated. Two recent studies reported one such combination of immediate sequential gemcitabine (a non-vesicant) followed by MMC. Initial reports found a near 50% recurrence-free rate in 27 BCG-failure patients at 18 months, compared with 20% in 12 similar patients treated with gemcitabine alone [8]. Breyer et al. reported similar findings in patients with prior BCG failure treated with sequential gemcitabine followed by MMC [9]. In their series of 10 patients, 6 (60%) remained disease free at median follow-up of 26 months.
We present combined data from a multi-institutional review of sequential intravesical gemcitabine and MMC (G/MMC) in the treatment of NMIBC.
2. Materials and methods
This study is a multi-institutional retrospective review of all patients treated with sequential intravesical G/MMC for NMIBC at the University of Iowa; the University of California, San Francisco; and the University of Minnesota between 2000 and 2010. Institutional Review Board approval was granted at all institutions. All patients who had received 6 weekly intravesical treatments for NMIBC were included in analysis. The majority of patients had prior BCG-based (BCG or BCG and interferon alfa-2b) intra-vesical immunotherapy failure or did not tolerate immuno-therapy because of toxicity. Patients who received treatment were unfit for cystectomy or initially refused cystectomy.
All patients had a complete transurethral resection followed by the administration of an immediate single dose of chemotherapy prior to initiation of therapy. All patients underwent a 6-week induction regimen followed by a 12-month, once-monthly maintenance regimen if they were deemed to have responded to the induction course and remained disease free based on cystoscopic and cytologic examination. Induction consisted of intravesical gemcitabine followed sequentially by MMC every week for 6 weeks.
Gemcitabine is a nonvesicant chemotherapeutic agent that acts as a deoxycytidine analog, thereby inhibiting deoxyribonucleic acid (DNA) synthesis [10,11]. Gemcitabine is given first because it is likely the better tolerated of the 2 drugs and its mechanism of action is promoted by active DNA synthesis, a step that could be theoretically blocked by MMC. One gram of gemcitabine in 50 ml of sterile water was retained for 90 minutes and then drained. MMC is a vesicant chemotherapeutic agent and acts as a bifunctional or trifunctional alkylating agent. It works by cross-linking to DNA double helix and subsequently causing cell death. MMC dosed at 40 mg in 20 ml of sterile water was instilled and retained for 90 minutes and then drained.
Six weeks after induction with G/MMC, each patient had bladder washings for cytology, random bladder biopsies, or if cystoscopically visible disease was present, a repeat transurethral resection was performed. Those patients with biopsy-proven recurrent disease after an induction course of G/MMC therapy were advised to undergo cystectomy. CR was defined as normal cytology, cystoscopy, and biopsy at 6 weeks after completion of a full course of induction therapy.
Patients with negative biopsies following induction went on to receive maintenance therapy using the same dose of G/MMC once per month for 12 months. Follow-up evaluations consisting of cytology and cystoscopy were performed at 3-month intervals after the first negative postinduction evaluation. Upper tract evaluation with CT urography was performed annually.
Statistical analyses were mainly descriptive (mean, median, and distributions). Kaplan-Meier curves were generated to show the recurrence-free survival (RFS) of various bladder cancer cohorts and log-rank testing was utilized for curve comparisons. Statistical analyses were performed using Statistical Analysis System (SAS) version 9.2.
3. Results
3.1. Demographics
A total of 52 patients were treated with sequential G/MMC among the 3 institutions. Five of these patients received greater than 6 weekly treatments and were therefore excluded from analyses. The median age at treatment was 70 years (range 32–86). Patient characteristics are shown in Table 1. Ten patients were BCG naive prior to treatment and all were on high-dose immunosuppression (7 for prior transplant and 3 for rheumatologic disease). In total, 10 patients underwent cystectomy after recurrence. Two patients died from meta-static disease. Two years after treatment with G/MMC, and 4 months after undergoing a cystectomy, a man died from rapidly progressing metastatic disease. Another man died 1 year after immediately failing G/MMC treatment as well as 1 additional intravesical treatment and refusal of any further therapy.
Table 1.
Patient characteristics (n = 47)
| Characteristic | |
|---|---|
| Age | |
| Median (range) | 70 (32–86) |
| Sex (M:F) | 36:11 |
| Location | |
| Iowa | 30 |
| San Francisco | 14 |
| Minnesota | 3 |
| American Society of Anesthesiologists (ASA) score | |
| Median (range) | 3 (1–4) |
| Stage and grade | |
| CIS | |
| CIS | 12 |
| Ta and CIS | 7 |
| T1 and CIS | 7 |
| Ta Only | |
| Ta Low grade | 6 |
| Ta High grade | 4 |
| T1 Only | |
| T1 Low grade | 0 |
| T1 High grade | 6 |
| Positive cytology | |
| High grade | 5 |
| Time from initial diagnosis to administration of G/MMC (mo) | |
| Median (range) | 33.5 (0–192) |
| No. of prior treatments | |
| Median (range) | 2.0 (0–6) |
| Type of prior treatment | |
| No prior treatments | 7 |
| BCG naive | 10 |
| 1 prior BCG failure | 11 |
| ≥2 or more prior BCG failures | 26 |
| Time to failure after BCG (mo) | |
| Median (range) | 5 (3–60) |
3.2. RFS
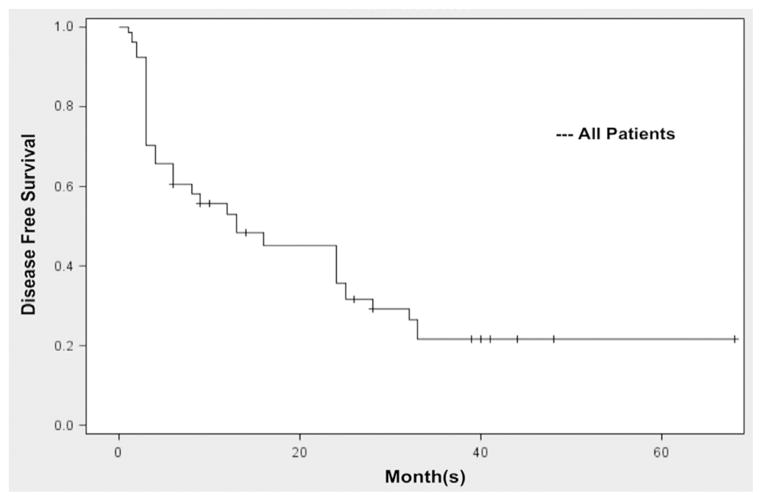
For all patients treated with G/MMC, the CR, 1-year RFS (1-RFS), and 2-year RFS (2-RFS) was 68%, 48%, and 38%, respectively (Fig. 1). In all, 14 of 47 patients (30%) remained free of recurrence with a median time to follow-up of 26 months (range 6–80 mo). The median time to recurrence for all patients who recurred was 4 months (range 1–33 mo).
Fig. 1.
Recurrence-free survival for all patients. (A) RFS based on stage. 68% CR, 48% 1-RFS, and 38% 2-RFS.
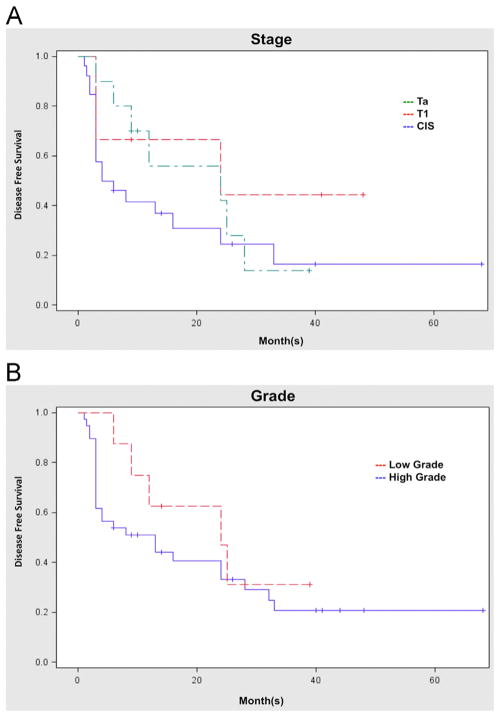
Stage of disease did not affect RFS (P = 0.30; Fig. 2A). Likewise, there was no significant difference in RFS when comparing high-grade and low-grade disease (P = 0.39; Fig. 2B).
Fig. 2.
Recurrence-free survival based on pathology. (A) RFS based on stage. CIS—58% CR, 38% 1-RFS, and 23% 2-RFS. Ta—90% CR, 50% 1-RFS, and 50% 2-RFS. T1—67% CR, 60% 1-RFS, and 60% 2-RFS. (B) RFS based on grade. High grade—62% CR, 44% 1-RFS, and 33% 2-RFS. Low grade—100% CR, 63% 1-RFS, and 57% 2-RFS. (Color version of figure is available online.)
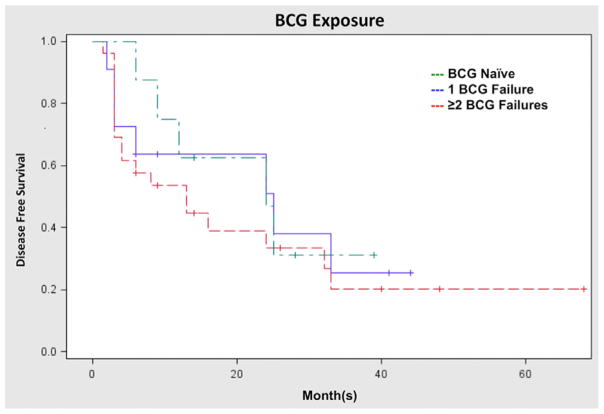
RFS of patients naive to BCG, patients with 1 prior BCG failure, and patients with 2 or more BCG failures were also compared (Fig. 3). Interestingly, the CR rate was nearly identical among the 3 groups, as was the 1-RFS. There was no significant difference in RFS when comparing prior BCG exposure (P = 0.69).
Fig. 3.
Recurrence-free survival based on prior BCG treatments. RFS based on prior BCG exposure. BCG naive—80% CR, 50% 1-RFS, and 44% 2-RFS. One prior BCG failure—73% CR, 56% 1-RFS, and 56% 2-RFS. Two or more prior BCG Failures—69% CR, 50% 1-RFS, and 32% 2-RFS. (Color version of figure is available online.)
3.3. Complications
Overall, the combination of G/MMC was well tolerated. Ten patients reported frequency, urgency, and/or dysuria, 3 reported a significant rash, and 1 developed pericarditis shortly after last treatment that was attributed to MMC exposure (all toxicities were considered grade I or II) [12]. Four of these patients, 1 with rash and 3 with frequency, urgency, and/or dysuria, discontinued MMC prior to completion of the 6-week induction (1 after 2 treatments, 1 after 4, and 2 after 5 treatments respectively); however, gemcitabine as a single agent was continued in such cases.
4. Discussion
Patients with BCG refractory NMIBC disease remain a very difficult population to treat. Cystectomy remains the gold standard for such patients but is associated with high morbidity. Many patients are deemed unfit or are simply unwilling to undergo cystectomy. It is this group of patients that many urologists struggle with. We reviewed the administration of sequential G/MMC in such patients. The overall CR rate for all patients was 68%. RFS at 1 and 2 years remained strong at 48% and 38%, respectively. The median follow-up was 26 months. Our study is limited by its retrospective nature as well as by the heterogenous population. Regardless, this study emphasizes the importance of continuing to find successful treatment options in this difficult to treat population.
Following recurrence of NMIBC after BCG therapy, second-line treatment options are limited and include repeat BCG treatment, an alternate immunotherapy regimen, chemotherapy, or device-assisted therapy. MMC is one agent that has been tried unsuccessfully as a second-line therapy for patients with prior BCG failure. In a study by Malmstrom et al., only 4 of 21 (19%) BCG-failure patients treated with MMC were disease free at 3 years [13]. Recent advances in the administration of MMC have brought into question the efficacy of MMC in the treatment of BCG refractory NMIBC. Electromotive intravesical administration of MMC has been used to improve drug delivery across biological membranes with increased accumulation in the bladder [14]. Perhaps an even more promising treatment option for patients failing BCG therapy involves microwave hyperthermia and administration of MMC. The addition of thermal energy to the administration of MMC allows for increased cellular permeability of the chemotherapeutic agent, increased DNA cross-linking, and inhibition of DNA repair [15,16].
Currently, gemcitabine is being evaluated as an intra-vesical treatment option in patients who have previously failed BCG therapy. Three initial studies evaluating the use of intravesical gemcitabine in the treatment of BCG refractory NMIBC have had widely variable results [17–19]. RFS rates these studies were 10% at 12 months [18], 60% at 18 months [17], and 75% at 12 months [19]. As the number of patients with high-grade NMIBC and/or carcinoma in situ (CIS) or both increased, the RFS decreased, whether they had received prior treatment or not. When evaluating our patients treated for CIS only (n = 26) or those treated for high-grade disease (n = 41) our study compares favorably to Dalbagni et al. [18]. Of the 3 previously mentioned studies Dalbagni et al. treated the highest proportion of patients with CIS or high-grade NMIBC. In their study, 30 patients with high-grade disease (23 with CIS, 4 with Ta high grade [TaHG], and 3 with T1 NMIBC) were treated with intravesical gemcitabine. They found a 1-RFS of 10% compared with 1-RFS of 44% in our high-grade population and 38% in our CIS population.
All patients in both the studies by Dalbagni et al. [18] and Mohanty et al. [17] had previously failed at least 1 prior BCG treatment, unlike the study by Bartoletti et al. [19] where 64 of 116 (55%) patients were undergoing treatment for their first occurrence of NMIBC and 70 of 116 (60%) had no prior intravesical treatment. In our study, both the 1-RFS and 2-RFS were 56% for patients who failed 1 prior BCG treatment, and 50% and 32% for those who failed 2 or more BCG treatments, respectively. The outcomes from our study are favorable compared with those presented by Dalbagni but not by Mohanty. The discrepancy between the results of our study and Mohanty’s may again be secondary to the grade of disease being treated. Of the 37 patients in our study with at least 1 prior BCG failure, 33 had grade III or high-grade disease prior to treatment; in contrast, of the 35 patients in Mohanty’s study, 13 had grade III disease, 14 had grade II disease, and 8 had grade I disease. Thus, a higher portion of patients with lower grade disease were found in Mohanty’s study.
The addition of MMC to gemcitabine would appear to compare favorably with gemcitabine alone in the treatment of patients with high-grade disease as well as patients with prior BCG failure; however, definitive comparisons or recommendations cannot be made from a retrospective study. The benefit of the other studies mentioned is that they are prospective in nature, further suggesting the importance of prospectively studying G/MMC in the treatment of NMIBC.
Our study is further complicated by a heterogeneous population in that there is variability in pathology as well as prior exposure to BCG therapy. However, our results are strengthened by the fact that this was a multi-institutional study, and the long-term follow-up (median 26 mo).
Results from even the most optimistic studies involving single-agent intravesical therapy in the treatment of patients with prior BCG failure have been mixed. Data from our medical oncology colleagues suggest that metastatic bladder cancer is best treated with multiple chemotherapeutic agents. Recently, this practice has been more widely accepted and attempted in the treatment of NMIBC [20–23]. Our data suggest that G/MMC may be a reasonable intra-vesical therapy for patients with high-grade disease as well as those with prior BCG failure who are not surgical candidates or refuse therapy, but further study is warranted.
Future directions for the administration of G/MMC would involve prospective evaluation of patients with high-grade and high-risk bladder cancer with prior BCG failure.
5. Conclusion
The use of G/MMC in the treatment of NMIBC specifically in patients with high-grade disease as well as those with prior BCG failure is promising but preliminary. Further prospective studies should be undertaken to evaluate the use of G/MMC in the treatment of NMIBC, specifically after BCG failure and in patients with high-grade disease. Currently, cystectomy remains the standard of care for high-risk patients who have failed BCG therapy.
References
- 1.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 2.Hussain MH, Wood DP, Bajorin DF, et al. Bladder cancer: narrowing the gap between evidence and practice. J Clin Oncol. 2009;27:5680–4. doi: 10.1200/JCO.2009.23.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Meijden AP, Sylvester R, Oosterlinck W, et al. EAU guidelines on the diagnosis and treatment of urothelial carcinoma in situ. Eur Urol. 2005;48:363–71. doi: 10.1016/j.eururo.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303–14. doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 5.Hall MC, Chang SS, Dalbagni G. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314–30. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Lightfoot AJ, Rosevear HM, O’Donnell MA. Recognition and treatment of BCG failure in bladder cancer. Sci World J. 2011;11:602–13. doi: 10.1100/tsw.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukui I, Sekine H, Kihara K. Intravesical combination chemotherapy with mitomycin C and doxorubicin for carcinoma in situ of the bladder. J Urol. 1989;141:531–4. doi: 10.1016/s0022-5347(17)40882-2. [DOI] [PubMed] [Google Scholar]
- 8.Maymí JL, Saltsgaver N, O’Donnell MA. Intravesical sequential gemcitabine-mitomycin chemotherapy as salvage treatment for patients with refractory superficial bladder cancer. J Urol. 2006;175(Suppl 4):271. [abstract no. 840] [Google Scholar]
- 9.Breyer BN, Whitson JM, Carroll PR, Konety BR. Sequential intra-vesical gemcitabine and mitomycin C chemotherapy regimen in patients with non-muscle invasive bladder cancer. Urol Oncol. 2010;28:510–4. doi: 10.1016/j.urolonc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wientjes MG, Badalament RA, Au JL. Use of pharmacologic data and computer simulations to design an efficacy trial of intravesical mitomycin C therapy for superficial bladder cancer. Cancer Chemother Pharmacol. 1993;32:255–62. doi: 10.1007/BF00686169. [DOI] [PubMed] [Google Scholar]
- 11.Grossman HB, O’Donnell MA, Cookson MS, Greenberg RE, Keane TE. Bacillus calmetteguerin failures and beyond: contemporary management of non-muscle-invasive bladder cancer. Rev Urol. 2008;10:281–9. [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute (U.S.) Common terminology criteria for adverse events (CTCAE) Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. Rev. ed. [Google Scholar]
- 13.Malmstrom PU, Wijkstrom H, Lundholm C, Wester K, Busch C, Norlen BJ. 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study Group. J Urol. 1999;161:1124–7. [PubMed] [Google Scholar]
- 14.Di Stasi SM, Giannantoni A, Stephen RL, et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: a prospective randomized study. J Urol. 2003;170:777–82. doi: 10.1097/01.ju.0000080568.91703.18. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijden AG, Kiemeney LA, Gofrit ON, et al. Preliminary European results of local microwave hyperthermia and chemotherapy treatment in intermediate or high risk superficial transitional cell carcinoma of the bladder. Eur Urol. 2004;46:65–72. doi: 10.1016/j.eururo.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Gofrit ON, Shapiro A, Pode D, et al. Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology. 2004;63:466–71. doi: 10.1016/j.urology.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Mohanty NK, Nayak RL, Vasudeva P, Arora RP. Intravesicle gemcitabine in management of BCG refractory superficial TCC of urinary bladder-our experience. Urol Oncol. 2008;26:616–9. doi: 10.1016/j.urolonc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Dalbagni G, Russo P, Bochner B, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guerin-refractory transitional cell carcinoma of the bladder. J Clin Oncol. 2006;24:2729–34. doi: 10.1200/JCO.2005.05.2720. [DOI] [PubMed] [Google Scholar]
- 19.Bartoletti R, Cai T, Gacci M, et al. Intravesical gemcitabine therapy for superficial transitional cell carcinoma: results of a Phase II prospective multicenter study. Urology. 2005;66:726–31. doi: 10.1016/j.urology.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 20.Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57:766–73. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oosterlinck W, Kirkali Z, Sylvester R, et al. Sequential intravesical chemoimmunotherapy with mitomycin C and bacillus Calmette-Guerin and with bacillus Calmette-Guerin alone in patients with carcinoma in situ of the urinary bladder: results of an EORTC genito-urinary group randomized phase 2 trial (30993) Eur Urol. 2011;59:438–46. doi: 10.1016/j.eururo.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 22.Moibi JA, Mak AL, Sun B, Moore RB. Urothelial cancer cell response to combination therapy of gemcitabine and TRAIL. Int J Oncol. 2011;39:61–71. doi: 10.3892/ijo.2011.1023. [DOI] [PubMed] [Google Scholar]
- 23.Cho DY, Bae JH, Moon DG, et al. The effects of intravesical chemoimmunotherapy with gemcitabine and Bacillus Calmette-Guerin in superficial bladder cancer: a preliminary study. J Int Med Res. 2009;37:1823–30. doi: 10.1177/147323000903700618. [DOI] [PubMed] [Google Scholar]