Summary
Histone lysine and arginine methylation involved in gene activation and silencing are dynamically regulated. However, partly limited to the research technologies previously available, the dynamics of global histone methylation on a site-specific basis have not been fully pursued. Heavy methyl-SILAC (Stable Isotope Labeling of Amino Acids in Cell Culture) labeling provides a remarkable signpost to distinguish the pre-existing and newly generated methyl marks on histones. Using this technology coupled with quantitative LC-MS analysis make it possible to monitor changes in the dynamics of histone site-specific methylation. In this chapter, we comprehensively describe the experimental strategy to determine the dynamics of multiple histone methylated residues including SILAC labeling, histone extraction/purification and mass spectrometry analysis.
Keywords: Histone, Methylation, Dynamics, Heavy methionine, SILAC, Quantitation, Mass spectrometry
1. Introduction
Eukaryotic DNA is packaged with histone proteins into the chromatin fiber. Histone proteins not only act as the structural proteins needed to maintain chromatin architecture, but also are subjected to numerous post-translation modifications (PTMs), including acetylation, methylation, ubiquitination, sumoylation and phosphorylation (1). Most of these modifications are located on the flexible N-terminal tails of histone proteins. These modifications serve to change histone steric properties and charges, so as to alter chromatin structure or influence the interactions among DNA, RNA and proteins. Furthermore, the PTMs are known to act as docking sites for the recruitment of specific proteins and complexes participating in diverse gene expression regulation mechanisms. Moreover, these modifications could be combinatorial and interdependent, forming a “histone code” of transcriptional activation or repression (2, 3). Methylation is unique among these PTMs due to its diverse forms and consequent variable influence over gene expression. Histone methylation can occur on lysine (mono-(me1), di- (me2) and trimethylation (me3)) and arginine (me1, symmetrical or asymmetrical me2). Multiple histone methyltransferases and demethylases are responsible for specific conversions between unmodified (me0), me1, me2 and me3 states of lysine and arginine residues on histone proteins. For example, G9a and Suv39h1 catalyze the production of H3K9me1/me2 and me3, respectively (4), whereas JHDM2A and HDM3A remove H3K9me2 (5) and me3 (6), respectively. Interestingly, various locations and degrees of methylation on specific histone residues result in different, even opposing functional outcomes. For example, methylation on H3K4 (7), H3K36 (8) and H3K79 (9)is correlated with gene activation, whereas that on H3K9 (10), H3K27 (11, 12)and H4K20 (12) is are generally associated with gene silencing. In addition, H3R2me1 is associated with gene activation while H3R2me2 is linked to gene repression (13).
For a long time lysine methylation was thought to be irreversible until very recently the first histone demethylase, Lysine Specific Demethylase 1 (LSD1) was discovered (14). The dynamic regulation of methylation by methyltransferases and demethylases is an important manner for regulation of gene expression. However, most previous studies regarded histone methylation as a static condition at a particular time, and partly due to technical limitations, only a limited number of investigations pursued the dynamic change of histone methylation. Some studies focused on methylation dynamics during cell cycle progression (15–17). For example, the levels of H3K9me1, me2 and me3 were found to dynamically regulated increase from interphase to metaphase and then decrease to the initial levels at the start of the next mitotic cycle (17). Radiolabeling of methionine was used to monitor the turnover of methylation of proteins like histone H3 (18), whereas this technique only could track methylation at the protein level, not on a site-specific basis. Immunoblot analysis could specifically study dynamic change of methylation sites (17). Nevertheless, this method still cannot distinguish between “old” and “new” synthesized methyl groups.
Recently we described a new strategy to investigate the site-specific dynamics of histone methylation (19) by combining heavy methyl-SILAC (stable isotope labeling by amino acids in cell culture) labeling technology and the quantitative mass spectrometry analysis previously developed in our lab (20). Methionine is an essential amino acid that mammal cells cannot synthesize de novo, and it is also the precursor of the S-adenosyl methionine (SAM), the sole donor of the methyl group. When heavy stable isotope form of methionine, L-Methionine-methyl-13C, D3 is used in the cell culture medium, heavy-methyl groups will be introduced by methyltransferases into lysine and arginine residues on histone proteins. Therefore, this labeling technology coupled with quantitative mass spectrometry technology will remarkably facilitate dynamics study of site-specific methylation of histone proteins.
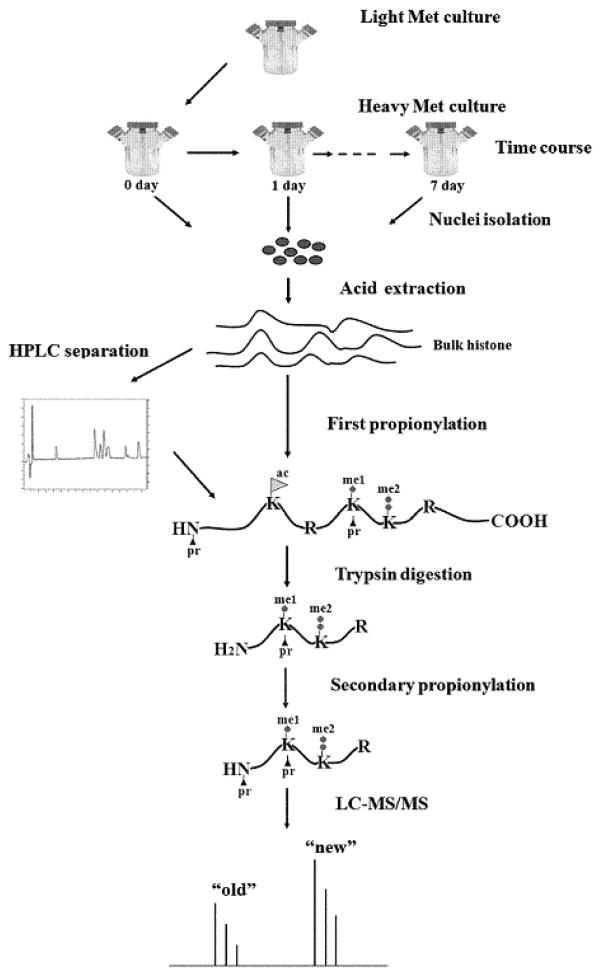
Our experimental scheme is shown in Figure 1. Briefly, HeLa cells are introduced into the heavy methionine-labeled medium and harvested at a series of time points. Histone proteins are obtained by nuclei isolation and acid extraction. Bulk histone proteins or individual histone variant separated by high performance liquid chromatography (HPLC) is sequentially subjected to propionylation, trypsin digestion and LC-MS/MS analysis. Finally the abundances of light, heavy and intermediate forms of the interesting methylation sites are extracted, and normalized to construct kinetic models towards investigating their dynamics.
Figure 1.
Strategy for using heavy methyl-SILAC labeling to study dynamics of histone methylation. HeLa cells are introduced into the heavy methionine-labeled medium to allow for incorporation of the heavy methyl group on histones. Cells are harvested at a series of time points and subsequently histones are extracted. Bulk histone proteins or individual histone variant separated by HPLC is sequentially subjected to propionylation, trypsin digestion and LC-MS/MS analysis.
2. Materials
2.1. Mammalian cell culture and heavy methionine labeling
-
1
Cell line: HeLa S3 cells, epithelial adenocarcinoma (ATCC).
-
2
Culture medium: Minimum Essential Medium Eagle, Joklik modification (SAFC Biosciences).
-
3
Components of homemade medium (all from Sigma-Aldrich): L-Arginine monohydrochloride; L-Cysteine dihydrochloride; L-Histidine dihydrochloride; L-Isoleucine; L-Leucine; L-Lysine monohydrochloride; L-Phenylalanine; L-Threonine; L-Tryptophan; L-Tyrosine; L-Valine; Choline chloride; Folic acid; myo-Inositol; Niacinamide; D-Pantothenic acid hemicalcium salt; Pyridoxal hydrochloride; Riboflavin; Thiamine hydrochloride; MgCl2; KCl; NaCl; Na2HPO4; Glucose; Phenol Red sodium salt; NaHCO3.
-
3
Heavy amino acids: L-Methionine-methyl-13C, D3 (Sigma-Aldrich); L-Lysine-13C6, 15N2 dihydrochloride (Cambridge Isotope Laboratories Inc).
-
4
Supplements: newborn calf serum (Thermo Scientific); dialyzed fetal bovine serum (Gemini Bioproducts); GlutaMAX (Invitrogen); penicillin/streptomycin (Invitrogen).
-
5
MilliQ water.
-
6
Other: sterile Dulbecco’s phosphate-buffered saline (DPBS, Invitrogen); filter units (Millipore); syringe filter (Pall); spinner flasks (Corning).
2.2. Nuclei isolation and histone Extraction
Nuclear Isolation Buffer (NIB-250): 15 mM Tris-HCl (pH 7.5), 60 mM KCl, 15 mM NaCl, 5 mM MgCl2, 1 mM CaCl2, 250 mM sucrose. Store at 4°C.
1 M DTT: store at −20°C. Add to NIB-250 to 1 mM when use.
10% NP-40.
Inhibitors: 5 μM Microcystin-LR (1000×, Sigma-Aldrich), 200 mM AEBSF (400×, Sigma-Aldrich) and 5 M sodium butyrate (500×, Sigma-Aldrich).
0.4 N (0.2 M) sulfuric acid (H2SO4), stored at 4°C.
Trichloroacetic Acid (TCA, Fisher Scientific), 100% stock solution stored at 4°C.
Acetone (Sigma-Aldrich), stored at −20°C.
deionized water.
Bradford quantification solution (Bio-Rad).
2.3 Reverse-phase HPLC separation of histone proteins
C18 column, 4.6 mm internal diameter × 250 mm (Vydac).
Manual injector valve (Rheodyne).
System Gold HPLC system (Beckman Coulter).
0.1% trifluoroacetic acid (TFA, Sigma-Aldrich).
-
LC gradient:
Buffer A: 0.5% acetonitrile/ 0.2% TFA;
Buffer B: 90% acetonitrile/0.188% TFA.
2.3. Propionic anhydride derivatization
100 mM ammonium bicarbonate (Sigma-Aldrich).
Ammonium hydroxide (Sigma -Aldrich).
Propionic anhydride (Sigma-Aldrich).
Isopropanol (anhydrous, Sigma-Aldrich).
Acetic acid (HPLC grade, Sigma-Aldrich).
Argon.
pH indicator strips (EMD Chemicals).
2.4. Trypsin digestion
Sequencing grade-modified porcine trypsin (Promega).
100 mM ammonium bicarbonate (Sigma-Aldrich).
Acetic acid (HPLC grade, Sigma-Aldrich).
2.5. StageTip purification
-
1
Empore C18 Disk (3M).
-
2
Conditioning solution: methanol (HPLC grade, Fisher Scientific).
-
2
Equilibrating/loading/washing solution : 0.1% acetic acid.
-
3
Eluting solution: 75% acetonitrile/1% acetic acid.
-
4
Gel loader tips.
-
5
Compressed air can.
2.6. LC-MS analysis
Prepare an analytical column by packing a 10-cm fused silica capillary (75 μm inner diameter, 360 μm outer diameter with a 1 μm homemade laser-pulled electrospray tip) with a 50% acetonitrile/50% isopropanol slurry of reverse-phase C18 resin (Magic C18, 5 μm particles, 200 Å pore size, Michrom BioResources) at a helium pressure (70 bar) using a bomb-loader device (Proxeon Biosystems).
-
LC gradient:
Buffer A: 0.1 M acetic acid;
Buffer B: 98% acetonitrile/ 0.1 M acetic acid.
AS2 autosampler (Eksigent)
1200 binary HPLC system (Agilent)
LTQ-Orbitrap XL (Thermo Scientific)
3. Methods
3.1. Heavy labeled medium preparation
Since there is no commercially available Joklik modified medium deficient in methionine, we need to make it according to its standard formulation.
Prepare 1000× heavy methionine (15.4 mg/mL) stock solution by dissolving it in DPBS. Filter it with a 0.22 μm syringe filter (see Note 1). Store at −20°C.
Weigh the required amounts of all of the cell culture medium components except methionine and add them to 800 mL MilliQ water (See Note 2). Mix until completely dissolved.
Adjust pH to 7.2 (see Note 3) and supplement MilliQ water to 900 mL.
Filter the solution with a 0.22 μm membrane filter. Store at 4°C.
Add dialyzed FBS, heavy methionine stock solution and supplements to the medium to their working concentration when use.
3.2. HeLa S3 cells time -course labeling and harvesting
HeLa S3 cells are maintained at 37°C in 0.2 LPM CO2. Medium is replenished every day to maintain a cell density of 2–6 ×105 cells/ml throughout the experiment.
-
1
Suspension culture of HeLa S3 cells is initially maintained in normal Joklik modified medium supplemented with 10% newborn calf serum, penicillin/streptomycin, and 1% GlutaMAX.
-
2
Centrifuge cells at 600 × g for 5 min, decant the medium and wash cell pellet in sterile DPBS twice to remove the residual normal medium.
-
3
Resuspend cells in heavy methionine -labeled Joklik modified medium and culture at 37°C.
-
4
Daily aliquots are taken for continuous seven days or as desired (see Note 4). Centrifuge cells at 600 ×g for 5 min, decant the medium and wash cell pellet in DPBS twice.
-
6
Flash-freeze cell pellet in liquid nitrogen for 3 min and store at −80°C prior to further analysis.
3.3. Nuclei isolation and histone extraction
Put frozen cell pellet on ice to thaw.
Add NIB-250 with 0.3% NP-40 (final concentration) to cell pellet to a final ratio of 10:1 (10 mL buffer for 1 mL cell pellet).
Completely resuspend cells by pipetting gently and incubate on ice for 5 min.
Centrifuge at 600 × g for 5 min to pellet nuclei at 4°C. Transfer the supernatant (cytoplasmic extract) to a different tube or simply discard.
Resuspend nuclei pellet gently in 10:1 NIB -250 without NP-40.
Centrifuge at 600 ×g for 5 min at 4 °C and decant the supernatant.
Repeat steps 5–6.
Slowly add 0.4 N H2SO4 (See Note 5) to nuclei pellet at a 5:1 final ratio (5 mL solution for a 1 mL pellet)and vortex.
Place the tube on ice for at least 1 h or as long as overnight by mixing intermittently or by rotation on a roller at 4°C (See Note 6).
Centrifuge at 3400 × g (see Note 7) for 10 min at 4°C and transfer the supernatant containing histone proteins to a clean tube.
Add 100% TCA to the supernatant to give a final concentration of 20% TCA.
Place the tube on ice for at least 1 h or as long as overnight (See Note 8).
Centrifuge at 3400 ×g for at 10 min to pellet proteins and discard supernatant.
Wash protein pellet in pre-chilled acetone (see Note 9).
Centrifuge at 3400 ×g for 10 min and discard supernatant.
Repeat steps 14–15.
Air-dryprotein pellet at room temperature (see Note 10).
Resuspend dried protein pellet in deionized water (see Note 11), in 100 mM ammonium bicarbonate or in 0.1% TFA (use for HPLC separation).
Centrifuge at 3400 ×g for 10 min. Slowly and carefully transfer the supernatant to a clean tube.
Determine the yield of the extracted histone proteins by the Bradford assay (See Note 12).
Aliquot histone proteins and store at −80°C prior to further analysis.
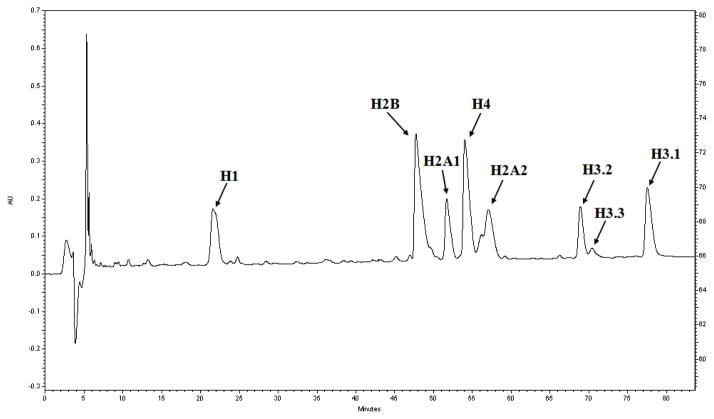
3.4. Reversed-phase HPLC separation of histone variants (See Figure 2)
Figure 2.
Separation of histone variants with reversed-phase HPLC.
Load about 150 μg histone proteins dissolved in 0.1% TFA onto a C18 preparative column by a manual sample injector valve.
Run a 100 min gradient from 30% to 60% Buffer B to separate histone variants at a flow rate of 0.8 mL/min, followed by 20 min 100% Buffer B.
Automatically collect fractions every 2 min throughout the gradient and pool fractions spanning a single variant.
Lyophilize and store at −80°C prior to further analysis.
3.5. Propionic anhydride derivatization (see Note 13)
Dilute histone sample with 100 mM ammonium bicarbonate to the concentration about 0.5–1 μg/μL.
Take 10–20 μL histone sample. Add 0.5 μL of ammonium hydroxide to sample to make sure pH is between 7–9.
Prepare propionylation reagent by adding 25 μL of propionic anhydride to 75 μL of isopropanol (or in a 1:3 ratio of a suitable volume) (See Note 14).
Add about 5–10 μL propionic anhydride reagent (half relative to sample volume) to histone sample, vortex and spin down quickly.
Immediately add 5–7 μL ammonium hydroxide, check pH to make sure pH ~ 8.0. If pH < 8, continue to add ammoniumhydroxide until pH is approximately 8.
Incubate at 37°C for 15 min.
Dry sample down to < 5 μL.
Repeat steps 2–7 (see Note 15).
Store in −80°C or use for trypsin digestion.
3.6. Trypsin digestion
Dilute sample with 50–100 μL of 100 mM ammonium bicarbonate.
Add trypsin to sample to a 1:20 (wt/wt) ratio of trypsin to sample. Incubate at 37°C for 6 –10 h.
Quench trypsin digestion by adding 4 –8 μL glacial acetic acid.
Dry sample down to approximately 5 μL.
Repeat steps 3.5 2–8 to perform propionic anhydride derivatization to N-termini of histone peptides.
Lyophilize histone peptides.
3.7. StageTip purification
Pouch out a small (~1 mm diameter) piece of Empore C18 disk using a pipette tip with the end cut off and transfer it into a gel loader tip. Carefully use a fused silica capillary with a large outer diameter to push the disk out and jam it into the tapered part of the gel loader tip.
Repeat step 1(See Note 16).
Reconstitute histone peptides with 20 μL 0.1% acetic acid. Check pH to make sure it acidic.
Condition the StageTip by loading 20 μL methanol and slowly pushing through using a compressed air can (~ 30 sec) (See Note 17).
Equilibrate the StageTip by loading 20 μL 0.1% acetic acid (~ 30 sec).
Load histone sample to the StageTip (~ 1 min).
Re-load the sample if desired.
Wash the StageTip by loading 20 μL 0.1% acetic acid (~ 30 sec).
Elute the StageTip by loading 20 μL 75% acetonitrile/0.1% acetic acid (~ 30 sec).
Dry down to < 5 μL on a SpeedVac concentrator (See Note 18) and store in −80°C prior to LC-MS/MS analysis.
3.8. LC-MS/MS analysis
The sample is loaded onto the homemade C18 analytical column with an emission tip using an Eksigent AS2 autosampler. A 110-min LC gradient from 5 to 35% Buffer B at a flow rate of 200 nL/min (post-split) on a Agilent 1200 binary HPLC system is used to separate histone peptides.
LTQ-Orbitrap XL mass spectrometer is basically operated in data-dependent mode: a resolution of 30,000 for a full scan and 7 subsequent MS/MS scan in ion trap by collision-induced dissociation (CID). The selected ion monitoring (SIM) mode and target MS/MS are performed to some peptide ions with low-abundance modification states to increase their identification (such as K4 methylation). Lock mass calibration in MS mode is implemented using polysiloxane ions, 371. 1012 and 445.1200.
3.9. Data analysis
The RAW files are converted into maximal files by ReAdW program (21). Histone peptide sequence and modifications are confirmed by manual inspection of MS/MS spectra (see Note 19). The precursor ion tolerance is set to 10 ppm to distinguish acetylation and trimethylation, and the fragment ion tolerance is set to 0.5 Da. Propionylation of N-termini of peptides is considered as a static modification. The following variable modifications are considered: propionylation of unmodified and monomethylated lysines; dimethylation of lysine; trimethylation of lysine; acetylation of lysine; oxidation of methionine; monomethylation of H4R3.
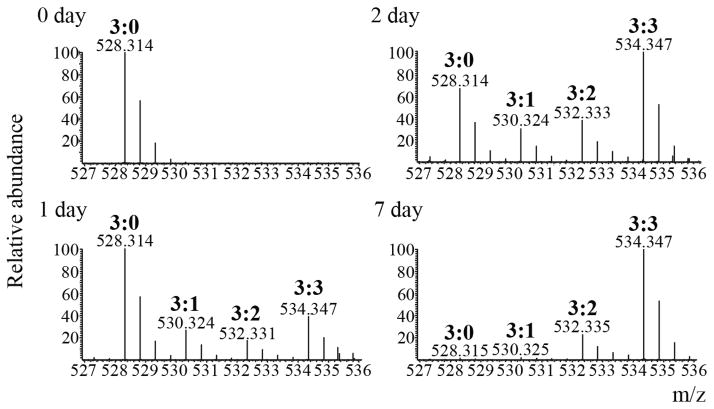
To describe unambiguously a specific methylated form, we append each residue with two numbers, the first referring to the total number of methyls and the second to the number of heavy labeled methyls. For example, H3K9me3:0 refers to unlabeled trimethylated H3K9, and H3K9me3:3 refers to fully labeled trimethylated H3K9, and H3K9me3:1 and H3K9me3:2 refer to intermediately labeled trimethylated H3K9 forms containing one and two unlabeled methyls, respectively (See Figure 3).
Peptide abundance is calculated by chromatographic peak integration using an in-house program or manually. The abundances of all labeled forms (light methyls, heavy methyls and possible intermediate methyls) for a specific methylation state (me1, me2 or me3) of a specified residue are summed and normalized to 1, hereby to obtain the relative abundance of each labeled form. From the relative abundances, one can apply various differential equations to obtain different kinetic parameters of methylation. For instance, by optimizing the relative abundances of the fully labeled methylated histone peptide over time to a single logarithmic function, one can extrapolate the time when the methylation labeling reached half of its maximum labeling efficiency, or essentially the inverse of a half-life measurement for each methylation state of a histone peptide (See Figure 4 and Figure 5A). While relatively direct to implement and interpret, such an approach also neglects a significant fraction of the MS data by assuming that each methylation state for a given histone peptide is formed and turned over independently of another methylation state for the same peptide. In order to take advantage of as much information about the histone peptide, one can alternatively fit all the fully labeled, partially labeled, and fully unlabeled peptides to a set of first order differential equations each representing the addition or removal of a methyl group (See Figure 5B). By establishing a stepwise relation between all the modified peptides, one can then determine specific rate constants between specific methylation states.
Figure 3.
Monitoring rate of H3.1K9me3. Full scan mass spectra of the [M+2H]2+ ions from the histone H3.1 peptide Kme3STGGKAPR extracted from the different time points (days 0, 1, 2 and 7) after pulse heavy methionine labeling. Intermediately labeled trimethylated forms (me3:1, 530.324 m/z and me3:2, 532.333 m/z) are present between the unlabeled trimethylated form (me3:0, 528.314 m/z) and the fully labeled trimethylated form (me3:3, 534.347 m/z) after day 0. Turnover of the old H3K9me3:0 can be seen in day 7, while the new H3K9me3:3 form is highest in abundance at that same time point.
Figure 4.
Plot of the incorporation of new labels between methylation state on H3.1K9 (from Figure 3)and general H 3.1 total protein turnover.
Figure 5.

Kinetic modeling of histone methylation dynamics. (A). Logarithmic function that fits a generic fully labeled monomethylated peptide. A = the final methyl labeling efficiency of the peptide; B = time constant. (B). Set of first order differential equations that fits a generic unmodified histone peptide with the respective unlabeled and labeled monomethylated peptides. α = rate of synthesis; k0→1 = monomethylation with light SAM; k′0→1 = monomethylation with heavy SAM; k1→0 = demethylation from the monomethylation state; t0 = degradation of unmodified state; t1 = degradation of monomethylated state.
Acknowledgments
B.A G. gratefully acknowledges funding from a National Science Foundation grant (CBET -0941143), an Agilent Thought Leader award, an NSF Early Faculty CAREER award, and an NIH Innovator award (DP2OD007447) from the Office Of The Director, NIH.
Footnotes
The concentration of normal methionine in Joklik medium is 15 mg/L, so the corresponding concentration of heavy methionine should be 15.4 mg/L considering the molar equivalent of light and heavy methionine. To minimize loss during filtering, the stock solutions of heavy amino acids can be directly added to the medium to its working concentration, and filtered together when making the medium.
We can make stock solutions of almost all the required components, store them at −20°C and then add MilliQ water to make their working concentration stocks when make the medium. For histone H3 or H4, the general protein turnover rates can be estimated from the peptides containing a methionine residue, while for H1 variants without any methionine residue, other heavy essential amino acids like L-Lysine-13C6, 15N2 would be necessary to be added into the medium to estimate the turnover rates.
The pH of the medium usually rises 0.1 –0.2 units during filtration.
The normal doubling time of HeLaS3 cell is about 24 h.
0.2 N HCl can be used as an alternative. Histone proteins are soluble in dilute H2SO4 or HCl buffer, while most of other nuclear proteins and nucleic acids are not and precipitate out (22, 23).
During the acid extraction procedure, technically you can use the maximal centrifuge force that your rotor and the associated tubes can endure.
The extraction time is recommended to extend to overnight when the number of cells is low (<100000 cells).
Extending incubation time can increase the yield of histone proteins.
Acetone can remove residual TCA which would interfere with the subsequent propionic derivatization, and meanwhile can also remove TCA precipitated NP-40 which would interfere with mass spectrometry analysis.
Cover the tube with parafilm, poke small holes in the film and lay it at room temperature. If lyophilizing the protein pellet in a SpeedVac concentrator, usually 2–3 min is enough to completely remove the acetone.
Not all precipitates are dissolved in water, but histone proteins being highly basic will be dissolved.
At least 100–200 μg bulk histone proteins can be extracted from 1 × 107 HeLa cells according to our experience.
The propionic anhydride derivatization is performed twice, at the protein level and at the peptide level, respectively (24). The purpose of the chemical derivatization to histone proteins is to block unmodified and monomethylated lysine residues, and therefore subsequent trypsin digestion results in proteolysis only C-terminal to arginine residues. In addition, the derivatization step neutralizes the charges at the N-terminal and unmodified and monomethylated lysine residues and introduces the bulky propionyl group. Propionylated histone peptides are also more hydrophobic, so they are more easily retained and resolved by standard reverse phase liquid chromatography.
When not in use, propionic anhydride and isopropanol should be stored under argon or nitrogen and sealed with parafilm to reduce moisture in the bottles.
Usually the propionylation reaction should be repeatedly performed to ensure maximum derivatization efficiency.
The loading capacity of a piece of Empore C18 disk (1 mm diameter, 0.5 mm length) is about 25 μg of total peptide (25). Therefore two pieces of the disk usually should be enough for LC-MS/MS analysis for several times.
Try to always keep the disk wet during this step of the procedure.
Be careful to not over day the sample to reduce losses before reconstitution, as you only have to remove the acetonitrile (20 × 75% = 15 μL).
The labeling experiment here involves more than ten kinds of variable modifications. Therefore it is not practical to identify peptides by making a database searching (false positive rate very high). Since high pure histone proteins can be obtained by acid extraction described here and the histone peptides are subjected to high resolution mass spectrometry analysis, most peptides can be determined by their masses and their MS/MS spectra after manual inspection to determine unambiguously the localization of the modifications.
The light, heavy and intermediate forms of methionine and methyl groups should be considered when analyzing peptides. Methionine containing peptides may also be oxidized.
References
- 1.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Rice JC, Briggs SD, Ueberheide B, Barber CM, Shabanowitz J, Hunt DF, Shinkai Y, Allis CD. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell. 2003;12:1591–1598. doi: 10.1016/s1097-2765(03)00479-9. [DOI] [PubMed] [Google Scholar]
- 5.Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Klose RJ, Yamane K, Bae Y, Zhang D, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006;442:312–316. doi: 10.1038/nature04853. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein BE, Humphrey EL, Erlich RL, Schneider R, Bouman P, Liu JS, Kouzarides T, Schreiber SL. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc Natl Acad Sci U S A. 2002;99:8695–8700. doi: 10.1073/pnas.082249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kizer KO, Phatnani HP, Shibata Y, Hall H, Greenleaf AL, Strahl BD. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol Cell Biol. 2005;25:3305–3316. doi: 10.1128/MCB.25.8.3305-3316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steger DJ, Lefterova MI, Ying L, Stonestrom AJ, Schupp M, Zhuo D, Vakoc AL, Kim JE, Chen J, Lazar MA, Blobel GA, Vakoc CR. DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol Cell Biol. 2008;28:2825–2839. doi: 10.1128/MCB.02076-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tschiersch B, Hofmann A, Krauss V, Dorn R, Korge G, Reuter G. The protein encoded by the Drosophila position-effect variegation suppressor gene Su (var)3–9 combines domains of antagonistic regulators of homeotic gene complexes. EMBO J. 1994;13:3822–3831. doi: 10.1002/j.1460-2075.1994.tb06693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 12.Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, Reinberg D, Jenuwein T. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18:1251–1262. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirmizis A, Santos-Rosa H, Penkett CJ, Singer MA, Green RD, Kouzarides T. Distinct transcriptional outputs associated with mono- and dimethylated histone H3 arginine 2. Nat Struct Mol Biol. 2009;16:449–451. doi: 10.1038/nsmb.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Thomas G, Lange HW, Hempel K. Kinetics of histone methylation in vivo and its relation to the cell cycle in Ehrlich ascites tumor cells. Eur J Biochem. 1975;51:609–615. doi: 10.1111/j.1432-1033.1975.tb03963.x. [DOI] [PubMed] [Google Scholar]
- 16.Pesavento JJ, Yang H, Kelleher NL, Mizzen CA. Certain and progressive methylation of histone H4 at lysine 20 during the cell cycle. Mol Cell Biol. 2008;28:468–486. doi: 10.1128/MCB.01517-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McManus KJ, Biron VL, Heit R, Underhill DA, Hendzel MJ. Dynamic changes in histone H3 lysine 9 methylations: identification of a mitosis-specific function for dynamic methylation in chromosome congression and segregation. J Biol Chem. 2006;281:8888–8897. doi: 10.1074/jbc.M505323200. [DOI] [PubMed] [Google Scholar]
- 18.Waterborg JH. Dynamic methylation of alfalfa histone H3. J Biol Chem. 1993;268:4918–4921. [PubMed] [Google Scholar]
- 19.Zee BM, Levin RS, Xu B, LeRoy G, Wingreen NS, Garcia BA. In vivo residue-specific histone methylation dynamics. J Biol Chem. 285:3341–3350. doi: 10.1074/jbc.M109.063784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plazas-Mayorca MD, Zee BM, Young NL, Fingerman IM, LeRoy G, Briggs SD, Garcia BA. One-pot shotgun quantitative mass spectrometry characterization of histones. J Proteome Res. 2009;8:5367–5374. doi: 10.1021/pr900777e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedrioli PG, Eng JK, Hubley R, Vogelzang M, Deutsch EW, Raught B, Pratt B, Nilsson E, Angeletti RH, Apweiler R, Cheung K, Costello CE, Hermjakob H, Huang S, Julian RK, Kapp E, McComb ME, Oliver SG, Omenn G, Paton NW, Simpson R, Smith R, Taylor CF, Zhu W, Aebersold R. A common open representation of mass spectrometry data and its application to proteomics research. Nat Biotechnol. 2004;22:1459–1466. doi: 10.1038/nbt1031. [DOI] [PubMed] [Google Scholar]
- 22.Murray K. The acid extraction of histones from calf thymus deoxyribonucleoprotein. J Mol Biol. 1966;15:409–419. doi: 10.1016/s0022-2836(66)80116-x. [DOI] [PubMed] [Google Scholar]
- 23.Shechter D, Dormann HL, Allis CD, Hake SB. Extraction, purification and analysis of histones. Nat Protoc. 2007;2:1445–1457. doi: 10.1038/nprot.2007.202. [DOI] [PubMed] [Google Scholar]
- 24.Garcia BA, Mollah S, Ueberheide BM, Busby SA, Muratore TL, Shabanowitz J, Hunt DF. Chemical derivatization of histones for facilitated analysis by mass spectrometry. Nat Protoc. 2007;2:933–938. doi: 10.1038/nprot.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rappsilber J, Ishihama Y, Mann M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem. 2003;75:663–670. doi: 10.1021/ac026117i. [DOI] [PubMed] [Google Scholar]