Abstract
Objective
The aim of this study was to compare the growth of infants born to women with antenatal major depressive disorder (MDD), either treated or untreated with SSRI antidepressants, to a non-depressed, non-medicated control group, across the first year of life.
Method
In this prospective observational study, pregnant women were evaluated at weeks 20, 30 and 36 of gestation, and mother and infant pairs were assessed at 2, 12, 26 and 52 weeks postpartum. Three non-overlapping groups of women were defined according to their pregnancy exposures: 1) No SSRI, no MDD (N=97), no exposure to any antidepressant or MDD; 2) SSRI (N=46), SSRI exposure; and 3) MDD, No SSRI (N=31), presence of MDD without antidepressant exposure. Maternal demographic and clinical characteristics and newborn outcomes were compared across exposure groups. Infant weight, length and head circumference were measured by a physician or physician’s assistant who was blind to MDD and SSRI exposure status at each postpartum time point.
Results
Both adjusted and unadjusted analyses revealed no significant association of either antenatal MDD or SSRI exposure with the infant growth parameters of weight, length or head circumference compared to no exposure. In addition, the interaction of group and pre-pregnancy BMI was also evaluated and no significant synergistic effect was identified. Similarly, no differential effect of group over time was observed for weight, length or head circumference.
Conclusions
In utero exposure to MDD or SSRI did not impact infant growth with respect to weight, length or head circumference through 12 months of age.
Introduction
The incidence and period prevalence of major depressive disorder (MDD) during pregnancy and postpartum are 7.5% and 12.7%, respectively (1). Pregnant women often discontinue both psychotherapy and pharmacotherapy and do not resume care after birth (2, 3). Pregnant women who stop their medication proximate to conception have a higher risk for relapse (68%) compared to those who maintain treatment (26%)(4). These low treatment and high relapse rates are juxtaposed against mounting evidence that MDD increases the risk for adverse pregnancy outcomes.
During pregnancy, MDD is associated with risk directly related to the physiological dysregulation of psychiatric disorder as well as to associated maternal behaviors, such as smoking, poor nutrition, substance abuse, inadequate obstetrical care, and interpersonal isolation and suicide (5). Women with antenatal MDD have higher rates of small for gestational age (6) infants and preterm delivery (7). In a metaanalysis, Grote et al (8) found that MDD or clinically significant depressive symptoms were associated with an increase in the relative risks for preterm birth by 39%, for low birth weight by 49%, and for intrauterine growth restriction by 45%. Several causal pathways have been suggested, such as dysregulation of the hypothalamic-pituitary-adrenocortical (HPA) axis, increased uterine artery resistance with placental hypoperfusion in response to stress (9) and the exaggerated inflammatory responses associated with MDD (10).
In contrast, studies of the relationship of postpartum depressive symptoms on young children’s growth have not shown a significant relationship (6, 11, 12). Ramsay et al (13) performed a prospective study of the impact of depressive symptoms on neonatal sucking and found no effect on feeding practices, infant feeding abilities, or growth.
Antidepressant treatment of pregnant women has also been associated with adverse outcomes. Meta-analyses have shown that SSRI-treated women have a 2 to 3-fold greater risk for preterm birth as well as a higher rate of delivering low birth weight infants compared to women not exposed to SSRI (14, 15). In our previously published prospective investigation (7), preterm birth rates were similar in women exposed continuously to MDD (no SSRI) or to SSRI (23% and 21%, respectively). These rates were significantly higher than women with neither exposure (6%). In that study(7), growth parameters measured at birth (weight, length and head circumference, corrected for gestational age) did not significantly differ(7). In a recent study by Marroun et al.(16), maternal depression was associated with slower rates of fetal body and head growth, while pregnant women treated with SSRI had fetuses with no impact on body growth but with delayed head growth and higher rates of preterm birth.
Few data exist on the later physical growth of infants following in utero exposure to MDD or SSRI, and available studies do not include a control group with MDD without antidepressant exposure. In SSRI or SNRI treated women compared to controls (17), antenatal drug exposure was associated with lower infant birth weight, shorter birth length and head circumference, and these differences persisted at 1 month of age (18). Infants born to women continuously treated with SSRI (n=21) or controls (N=20) were evaluated on HPA and insulin-like growth factor (IGF)-I axes for relationship to growth and hormonal profiles. The SSRI-exposed infants had significantly decreased length and smaller head circumference. SSRI-exposed infants also had significantly lower cord blood levels of cortisol and higher levels of thyroid-stimulating hormone. Placental IGF-I receptor expression was significantly higher in the SSRI group than in controls, and urine 5-hydroxyindoleacetic acid (the major metabolite of serotonin) level was negatively correlated with birth weight and with dehydroepiandrosterone level. The authors concluded that fetal exposure to SSRI resulted in impaired intrauterine growth accompanied by alterations in the IGF-I and HPA axes. However, no evidence that prenatal SSRI exposure reduced neonatal bone quality was observed (19), although head circumference (but not birth weight or length) was significantly decreased in the SSRI-exposed compared to the control group in the same investigation.
Another factor for consideration is post-birth exposure to SSRI through breastmilk, which could affect infant growth. However, the method of infant feeding (i.e., providing breastmilk compared to formula) also has a substantial impact on infant growth. Compared to formula fed infants, breastfed infants have faster growth in the first few months of life followed by slower growth in the last half of the first year. Breastfed infants have lower weight than formula fed infants at 12 months (20, 21).
In a retrospective cohort design, Chambers et al (22) reported that infants of women who took the SSRI fluoxetine during pregnancy and lactation had less optimal growth than women who took fluoxetine during pregnancy but not during breastfeeding. Infants who received fluoxetine through breast milk had poorer weight gain in the first 6 months of life than breastfed infants of unmedicated mothers. However, the amount of fluoxetine exposure to women during pregnancy was also greater in those who continued the drug during lactation. Ten percent of women in the group that was unmedicated during breastfeeding were treated with antidepressants in the third trimester compared to 100% in the group who took fluoxetine during breastfeeding.
Consideration of the impact of both antenatal SSRI and MDD exposures on fetal and infant growth is an understudied component of the risk-benefit decision process for developing treatment plans for depressed pregnant women (23). Epidemiologic studies have demonstrated that infants born either small or large for gestational age have higher rates of chronic illnesses such as diabetes and cardiovascular disease as adults (24, 25). Differentiating between the contribution of MDD or depressive symptoms and that of antidepressant treatment on growth presents a clinical conundrum because (6): 1) investigators usually study the effects of one exposure without controlling for the other; 2) antidepressant use occurs at different antenatal times and dosages; 3) recognition and treatment of MDD by the physician is associated with symptom severity and episode duration, and 4) MDD is associated with use of other prescription and over the counter drugs as well as multiple confounders, such as smoking, substance use, overweight/obesity and elevated life stress. Evidence from meta-analyses (8) and other sources (7) suggests that untreated antenatal MDD is as likely to be associated with poor birth outcomes as treatment with SSRIs. To our knowledge, no study of the impact of SSRI on infant growth has included a comparison group of unmedicated women with MDD.
The aim of this study was to compare the measurements of weight, length and head circumference among infants born to pregnant women treated with SSRI, MDD (no SSRI), or neither exposure through 1 year postpartum. We hypothesized that no significant difference in anthropometric measurements would be observed among the infants exposed to MDD, SSRI or neither MDD nor SSRI, after controlling for key variables.
Method
Details of the parent study, Antidepressant Use during Pregnancy (MH R01 060335; PI, KLW), from which the subjects in this analysis were derived, have been previously published (7). In this prospective observational study, women were evaluated at weeks 20, 30 and 36 of gestation. The mother and infant pairs were assessed at 2, 12, 26 and 52 weeks postpartum.
Subjects
Pregnant women aged 15–44 years of age were enrolled at or before week 20. Women were eligible to participate if they had DSM4-defined MDD with or without antidepressant treatment or if they did not have MDD or antidepressant exposure (controls). Consultation about MDD management during pregnancy was provided to each depressed subject and a summary letter was sent both to the woman and her physician(s) as a benefit of participation. Enrollment did not depend upon acceptance of consultation recommendations or choice of treatment during or after pregnancy in this observational study. Medications were managed by the women’s physicians and no psychiatric treatment was prescribed by the study team. Pregnant women with psychosis, bipolar disorder, active substance use, any antenatal exposure to benzodiazepines or an FDA-defined prescription drug in classes D or X were excluded. Women with multiple births or chronic medical illnesses such as insulin-dependent diabetes also were excluded. Subjects were recruited through physician referral, advertising, self-referral, and screening within the obstetrical ultrasound suite. Written informed consent was obtained from all subjects.
Descriptive data for the childbearing study sample included: demographics (age, race, education, employment, marital status) and clinical characteristics (pre-pregnancy body mass index, parity, smoking status, alcohol intake, presence of lifetime DSM4 defined anxiety disorder, depression symptom score on the Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS29) (26)). The delivery and infant data included: rate of preterm birth (<37 weeks gestation (27)), infant sex, growth parameters (weight, length and head circumference) at birth, and breastfeeding status, Duration of gestation and newborn clinical status data were collected from the maternity and pediatric records by independent evaluators who were blind to study hypotheses and design. The measure was the Perinatal Events Scale (28).
Exposures
Beginning with conception, antidepressant exposure was documented by charting the subjects’ drug doses across each week of gestation. The vast majority of the women were treated with SSRI antidepressant monotherapy; only two were treated with additional bupropion or tricyclic augmentation. The antidepressant-treated women had serum levels of the drug obtained during all prenatal interviews to confirm that SSRI exposure actually occurred (7). The diagnosis of MDD was made according to the Structured Clinical Interview for DSM4 (SCID). To track MDD, we adapted the timeline technique (29) to chart mood episodes across time. For interviews after intake, the Longitudinal Interval Follow-up Evaluation (LIFE) was used in conjunction with the SCID to assess for MDD diagnostic status change. Additional exposures to prescribed drugs, over the counter medications, environmental agents, alcohol or smoking also were recorded at each assessment. Urine drug screens were obtained for all subjects at study enrollment. To accomplish the aim of differentiating the impact of SSRI from MDD exposures, we evaluated three non-overlapping groups of subjects according to their pregnancy exposures:
No SSRI, no MDD (N=97)-- No exposure to any antidepressant or MDD.
SSRI (N=46)-- The majority of women with SSRI exposure were treated continuously (66%) throughout gestation. Exposures also included first and/or second trimester, but not the third (21.3%); and second and/or third trimester, but not the first (12.7%).
MDD, No SSRI (N=31)-- Presence of full syndromal MDD at any point in pregnancy and without any antidepressant exposure. Women with MDD had the following characteristics: 26.5% were continuously depressed throughout pregnancy; 42.9% were depressed in the first and/or second trimester, but not the third; and 30.6% were depressed in the second and/or third trimester, but not the first. This group was included to evaluate the effects of active antenatal MDD on pregnancy and fetal outcomes.
Growth Assessments
Infant weight, length and head circumference were measured by a physician or physician’s assistant who was blind to MDD and SSRI exposure status both during pregnancy and postpartum. Weight was measured on a standard digital scale accurate to 1 gram. Length (cm) was measured by stretching the infant from the crown of the head to the heel on a pediatric examination table with a built-in ruler at each time point. Head circumference (cm) was measured with a pull-through tape to 1 mm, which was firmly tightened around the maximum circumference of the head. Although the target times for study visits were 2, 12, 26 and 52 postpartum, some variability in time of measurement occurred as anticipated and the growth data were analyzed according to the exact week after birth that the infants were evaluated. Preterm infants were seen at the age-corrected visit; that is, the number of weeks to 40 weeks (full term) was added to the planned assessment target time and their data were plotted with this correction.
Statistical Methods
Descriptive statistics for the population are reported as means and standard deviations for continuous variables and percentages for discrete variables. The comparison of subject characteristics across the three exposures groups was conducted with a chi-square test for discrete variables and an analysis of variance for continuous variables. Mixed effect regression models were used to assess the impact of antenatal exposure group on infant weight, length and head circumference at 2 weeks, and 3, 6.5 and 12 months. All demographic and clinical characteristics that significantly differed between the three exposure groups were included in the modeling procedures. To include the potential impact of continuing maternal MDD on growth, the models also included the presence or absence of the diagnosis of MDD at each postpartum assessment time. Indeed, exposure to MDD at each follow up follow-up timepoint was found to be significantly associated (independent of the effect of time) with exposure; therefore, it was included as a covariate in analyses. Main fixed effects were included for antenatal exposure group, time, and baseline characteristics that differed between the three exposure groups. A two-way fixed effect interaction was included for exposure group by pre-pregnancy BMI and exposure group by time. Random effects were included for intercept and slope. A p-value of .05 was used to designate statistical significance and the Bonferroni correction was used for multiple comparisons (set at p<.0167).
Results
Of the 238 pregnant women enrolled in the parent study (7), 71 (29.8%) were classified as having SSRI exposure, 36 (15.1%) had MDD exposure (no SSRI) and 131 (55.0%) had neither MDD nor SSRI exposure (controls). Of these original study subjects, 46 (65%) of the women exposed to SSRI, 31 (86%) of the women with MDD and 97 (74%) of the controls had sufficient longitudinal data to be included in this analysis. The inclusion rate did not significantly differ across the three groups (p=.136).
The comparison of the characteristics determined at intake across the three exposure groups is presented in Table 1. However, many significant differences in the women’s demographic and clinical characteristics were observed even after Bonferroni correction. The only three variables that did not differ across groups in this subsample were age, smoking and alcohol intake during pregnancy. With respect to race, more minority women were present in the MDD group, and the post-hoc pairwise comparisons yielded a significant difference between the MDD vs. no exposure (p=.01) and the MDD vs. SSRI groups (p<.01). The SSRI and control groups had similar high educational status and both significantly differed from the less well-educated MDD group (p=.01 and p<.01, respectively). Women in the control group were significantly more likely to be employed compared to the SSRI group (p<.01) and the MDD group (p=.01). Although marital status differed (p=.05), with more women in the MDD group being single, the post-hoc comparisons were not significant after Bonferroni correction. There was a significant difference in the average (SD) pre-pregnancy BMI (Control= 25.0 ± 5.6; MDD= 29.3 ± 10.5; SSRI= 27.3 ± 6.0; p<.02). Women with in the SSRI and MDD groups had more children than controls (2.1 ± .95 and 2.2 ± 1.2 vs. 1.9 ± 0.95, respectively), without significant differences after adjustment for multiple comparisons.
Table 1.
Maternal demographic and clinical measures
| Exposure during pregnancy | P values | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure | All (N=174) |
None (N=97) |
SSRI (N=46) |
MDD (N=31) |
Overall | SSRI v None |
MDD v None |
MDD v SSRI |
| Age | 30.5 ± 5.7 | 30.6 ± 5.4 | 31.3 ± 5.1 | 28.7 ± 7.0 | 0.126 | |||
| Race | 0.004 | 0.353 | 0.012* | 0.002* | ||||
| White | 141 (81.0) | 81 (83.5) | 42 (91.3) | 18 (58.1) | ||||
| Black | 28 (16.1) | 14 (14.4) | 3 (6.5) | 11 (35.5) | ||||
| Other | 5 (2.9) | 2 (2.1) | 1 (2.2) | 2 (6.5) | ||||
| White race | 141 (81.0) | 81 (83.5) | 42 (91.3) | 18 (58.1) | <.001 | 0.209 | 0.003* | <.001* |
| Education | 0.002 | 0.842 | <.001* | 0.012* | ||||
| <High school | 10 (5.7) | 3 (3.1) | 2 (4.3) | 5 (16.1) | ||||
| High school | 15 (8.6) | 7 (7.2) | 3 (6.5) | 5 (16.1) | ||||
| Some college | 30 (17.2) | 11 (11.3) | 8 (17.4) | 11 (35.5) | ||||
| College | 72 (41.4) | 45 (46.4) | 20 (43.5) | 7 (22.6) | ||||
| Graduate school | 47 (27.0) | 31 (32.0) | 13 (28.3) | 3 (9.7) | ||||
| College degree | 119 (68.4) | 76 (78.4) | 33 (71.7) | 10 (32.3) | <.001 | 0.386 | <.001* | <.001* |
| Employed | 103 (59.5) | 68 (70.1) | 21 (46.7) | 14 (45.2) | 0.006 | 0.007* | 0.012* | 0.897 |
| Marital status | 0.053 | |||||||
| Single | 38 (21.8) | 17 (17.5) | 8 (17.4) | 13 (41.9) | ||||
| Married/cohabiting | 131 (75.3) | 77 (79.4) | 36 (78.3) | 18 (58.1) | ||||
| Divorced/separated | 5 (2.9) | 3 (3.1) | 2 (4.3) | 0 (0.0) | ||||
| Widowed | 5 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Married/cohabiting | 131 (75.3) | 77 (79.4) | 36 (78.3) | 18 (58.1) | 0.049 | 0.878 | 0.018 | 0.058 |
| Pre-pregnancy BMI | 26.4 ± 7.0 | 25.0 ± 5.6 | 27.3 ± 6.0 | 29.3 ± 10.5 | 0.016 | 0.008* | 0.062 | 0.979 |
| Parity | 1.9 ± 0.95 | 1.7 ± 0.81 | 2.1 ± 0.95 | 2.2 ± 1.2 | 0.016 | 0.017 | 0.025 | 0.823 |
| Parity | 0.087 | |||||||
| 1 | 74 (42.5) | 49 (50.5) | 15 (32.6) | 10 (32.3) | ||||
| 2 | 61 (35.1) | 33 (34.0) | 17 (37.0) | 11 (35.5) | ||||
| 3+ | 39 (22.4) | 15 (15.5) | 14 (30.4) | 10 (32.3) | ||||
| Smoked tobacco during pregnancy | 17 (9.8) | 8 (8.2) | 5 (10.9) | 4 (12.9) | 0.718 | |||
| Drank alcohol during pregnancy | 0.061 | |||||||
| No | 119 (68.4) | 67 (69.1) | 30 (65.2) | 22 (71.0) | ||||
| Once a week or less | 42 (24.1) | 27 (27.8) | 11 (23.9) | 4 (12.9) | ||||
| More than once a week | 13 (7.5) | 3 (3.1) | 5 (10.9) | 5 (16.1) | ||||
| Anxiety (lifetime) | 57 (32.8) | 23 (23.7) | 21 (45.7) | 13 (41.9) | 0.016 | 0.008* | 0.049 | 0.747 |
| Baseline SIGH-ADS29 | 11.8 ± 7.1 | 8.2 ± 4.8 | 15.5 ± 7.1 | 17.6 ± 7.0 | <.001 | <.001* | <.001* | 0.208 |
Abbreviations BMI body mass index; SIGH-ADS Structured interview guide for the Hamilton rating scale for depression with atypical depression supplement.
Significant after Bonferroni correction.
Table 2 displays the infant demographic and clinical measures. The preterm birth rate was significantly different across the groups (p=.03), with the pot-hoc comparison of the SSRI vs. control group having a higher rate of preterm birth (19.1% vs. 5.0%, p=.01) and no significant difference in rate between the MDD vs. the SSRI group (9.7% vs. 19.1%, p=.34) or MDD vs. controls (9.7% vs. 5.0%, p=.39). Infant sex also differed across groups (p <.01) with significantly fewer males in the SSRI group compared to controls (p <.01). Unlike the population from the parent study (7), this subsample of women had infants that significantly differed with respect to birth weight and length, but not head circumference, after correction for gestational age. The rate of breastfeeding did not differ across the three groups.
Table 2.
Infant demographic and clinical measures.
| Exposure during pregnancy | P values | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure | All (N=178) |
None (N=100) |
SSRI (N=47) |
MDD (N=31) |
Overall | SSRI v None |
MDD v None |
MDD v SSRI |
| Weeks gestation at delivery <37 | 17 (9.6) | 5 (5.0) | 9 (19.1) | 3 (9.7) | 0.025 | 0.013* | 0.393 | 0.344 |
| Sex | 0.006 | 0.002* | 0.551 | 0.057 | ||||
| Male | 99 (55.6) | 64 (64.0) | 17 (36.2) | 18 (58.1) | ||||
| Female | 79 (44.4) | 36 (36.0) | 30 (63.8) | 13 (41.9) | ||||
| Birth weight (g) | 3470 ± 577 | 3563 ± 531 | 3343 ± 626 | 3366 ± 605 | 0.052 | |||
| Birth length (cm) | 51.1 ± 2.8 | 51.5 ± 2.7 | 50.2 ± 3.0 | 51.1 ± 2.7 | 0.038 | 0.011* | 0.529 | 0.190 |
| Birth head circumference (cm) | 34.6 ± 1.7 | 34.7 ± 1.7 | 34.3 ± 1.8 | 34.6 ± 1.6 | 0.354 | |||
| Ever breastfed | 138 (77.5) | 83 (83.0) | 35 (74.5) | 20 (64.5) | 0.083 | |||
Significant after Bonferroni correction.
Maternal clinical measures included the presence of a comorbid lifetime anxiety disorder, which differed across groups (p <.02). More than 40% of women in both the MDD and SSRI groups had anxiety disorders compared to 24% of controls. However, the post-hoc comparison was significant only for SSRI vs. no exposure (p <.01). The SIGH-ADS29 score for depressive symptom level at intake differed across groups as expected (p <.01), with both the MDD (17.6 ± 7.0) and SSRI-treated (15.5 ± 7.1) groups significantly different from controls (8.2 ± 4.8) (p<.01).
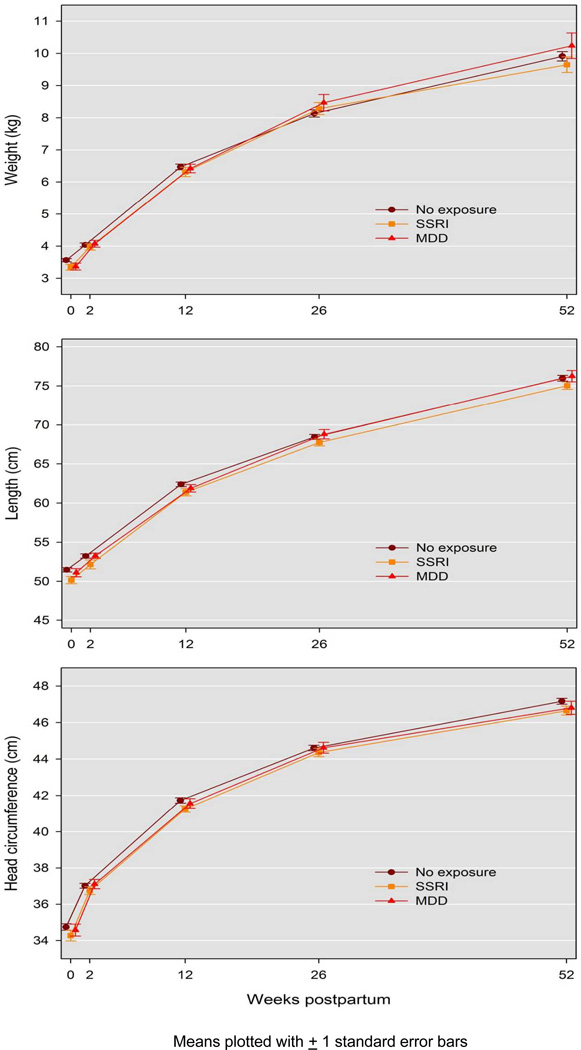
The longitudinal measurements of infant weight, length and head circumference by group are presented in Figure 1. No significant association between prenatal SSRI or MDD exposure and growth with respect to weight, length or head circumference was observed. The unadjusted analysis revealed no association of prenatal exposure to weight (p=.20), length (p=.29) or head circumference (p=.26). After controlling for the characteristics that differed between exposure groups (race, education, employment, marital status, parity, presence of lifetime anxiety disorder, infant sex and preterm birth), and including presence of MDD at each postpartum time point, no significant association of exposure with weight (p=.60), length (p=.93) or head circumference (p=.93) was observed. In addition, because maternal body weight impacts infant growth parameters, we evaluated the interaction of group and pre-pregnancy BMI, which was also non-significant and no synergistic effect was identified for weight (p=.87), length (p=.79), and head circumference p= (.97).
Figure 1.
Infant Weight, Length and Head Circumference by Post-birth Assessment Time
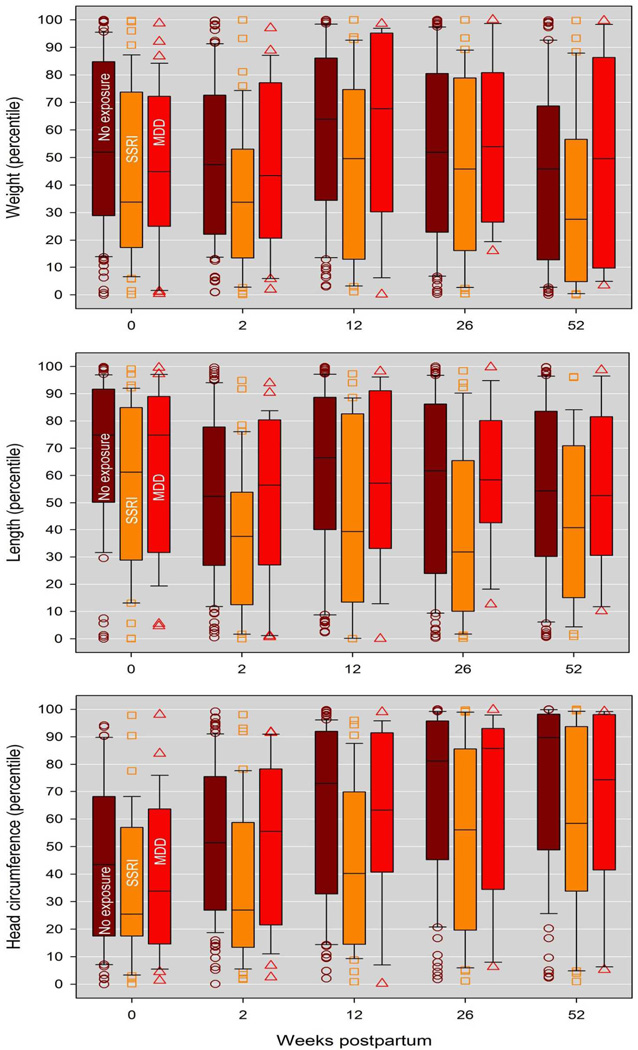
The values observed in our sample were compared to the population statistics from the Centers for Disease Control and Prevention (www.cdc.gov/growthcharts/clinical_charts.htm) (Figure 2). The mean length and weight measurements in all exposure groups were within the inter-quartile range (25th to 75th percentiles) of the general population of infants; therefore, the study sample is reasonably similar to the general population. The mean head circumferences of all exposure groups were within the inter-quartile range except infants prenatally exposed to MDD at 26 weeks of age and infants with no exposure at 26 and 52 weeks of age (all of which exceeded the 75th percentile.)
Figure 2.
Infant Weight, Length and Head Circumference Percentile Comparisons to CDC Data by Antenatal Exposure Group and Post-birth Assessment Time
Discussion
In this longitudinal observational study of in utero exposure to MDD or SSRI, we found no significant impact of either exposure on infant growth parameters (weight, length or head circumference) through 12 months of age compared to infants with no exposure. This investigation exemplifies the challenges of conducting observational reproductive outcome studies of pregnant women with diseases and the drugs used to treat them, a situation for which randomization into treatment groups creates ethical dilemmas (7). The demographic and clinical characteristics differed markedly across the depressed and non-depressed exposure groups and these variables also may differentially impact reproductive outcomes. Likewise, the distribution of women with MDD into SSRI-treated and non-medicated groups does not occur at random. Variables that differed across groups were included in the modeling procedures; however, other non-identified, unmeasured variables that differ across groups may also impact reproductive outcomes and always are a concern when interpreting data from observational studies.
Few studies of MDD during pregnancy have considered anxiety comorbidity. The presence of an anxiety disorder in over 40% of the group with MDD (with or without SSRI treatment) compared to only 24% in the control group without MDD is consistent with epidemiologic findings (30). Anxiety is another exposure (for which we adjusted our models) that impacts reproductive outcomes, such as higher risk for preterm birth, low birth weight and fetal and infant neurodevopment (31, 32).
The strengths of the study are: inclusion of not only an SSRI exposed but also an MDD exposed group of mothers; detailed exposure data collected prospectively; use of a urine drug screen to identify and exclude substance users; exclusion of women with antenatal exposure to FDA class D or X agents, which may be associated with increased risk for adverse pregnancy outcomes; blinded assessments of infant growth parameters; and collection of growth data for infants across the first postpartum year. The major weakness is a relatively small sample size, particularly for the mothers with MDD. From 65% to 86% of the original groups from the parent study contributed data. A larger sample of subjects would have been preferable, particularly with the inclusion of a substantial number of variables that differed between exposure groups in the modeling procedures across time.
Further research to expand the sample size, which likely would require multi-site studies, would allow subcategorization of the SSRI group based upon the degree to which the treated woman achieved symptom reduction (such as responders or remitters) to evaluate the question of whether the outcomes from effective SSRI treatment without the presence of MDD are more favorable than women with untreated MDD. This is the reason that pregnant women are treated with pharmacotherapy; that is, the anticipated overall benefit is greater than the risk (23). Prescribing a drug for a pregnant woman results from the physician’s judgment that her health is best served by treatment of the disorder, yet our literature is largely focused upon negative outcomes rather potential positive impacts on maternal disease and child outcomes (33, 34). For example, Hunter et al (35), demonstrated that infants born to mothers with anxiety disorders had impaired P50 auditory gating (a marker of infant attentional processing). Their novel finding was that maternal antidepressant treatment during pregnancy improved sensory gating in the offspring.
Women who have both SSRI exposure and continue to fulfill criteria for MDD may be at the highest risk for adverse reproductive outcomes. Much larger scale studies also would provide the number subjects required to evaluate reproductive outcomes related to individual SSRI. The impact of SSRI dose (3), or more directly maternal serum drug levels or other measures of biological impact on reproductive outcomes, are also needed to drive the process of risk-benefit decision-making to a new level of sophistication (23).
Acknowledgements
Dr. Sit reports donations of light boxes from Uplift Technologies for a K23study on light therapy for bipolar depression. Dr. Wisniewski reports advisory panel consulting for Cyberonic Inc. (2005–2009), ImaRx Therapeutics, Inc. (2006), Bristol-Myers Squibb Company (2007–08), Organon (2007), Case-Western University (2007), Singapore Clinical Research Institute (2009), Dey Pharmaceuticals (2010), Venebio (2010), Dey (2010).
We thank Ms. Emily Pinheiro for incorporating the references
Grant Support
National Institute of Mental Health; MH5R01 60335
Footnotes
Disclosures
The remaining authors report no competing interests.
References
- 1.Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evidence report/technology assessment (Summary) 2005;(119):1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett IM, Marcus SC, Palmer SC, Coyne JC. Pregnancy-related discontinuation of antidepressants and depression care visits among Medicaid recipients. Psychiatric services (Washington, DC) 2010;61(4):386–391. doi: 10.1176/ps.2010.61.4.386. [DOI] [PubMed] [Google Scholar]
- 3.Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. The American journal of psychiatry. 2007;164(8):1206–1213. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 4.Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 5.Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- 6.Grote V, Vik T, von Kries R, Luque V, Socha J, Verduci E, et al. Maternal postnatal depression and child growth: a European cohort study. BMC Pediatr. 2010;10:14. doi: 10.1186/1471-2431-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. The American journal of psychiatry. 2009;166(5):557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of general psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ (Clinical research ed) 1999;318(7177):153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian LM, Franco A, Iams JD, Sheridan J, Glaser R. Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain Behav Immun. 2010;24(1):49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ertel KA, Koenen KC, Rich-Edwards JW, Gillman MW. Maternal depressive symptoms not associated with reduced height in young children in a US prospective cohort study. PLoS One. 2010;5(10):e13656. doi: 10.1371/journal.pone.0013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos IS, Matijasevich A, Domingues MR, Barros AJ, Barros FC. Long-lasting maternal depression and child growth at 4 years of age: a cohort study. J Pediatr. 2010;157(3):401–406. doi: 10.1016/j.jpeds.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsay M, Gisel EG, McCusker J, Bellavance F, Platt R. Infant sucking ability, non-organic failure to thrive, maternal characteristics, and feeding practices: a prospective cohort study. Dev Med Child Neurol. 2002;44(6):405–414. doi: 10.1017/s0012162201002286. [DOI] [PubMed] [Google Scholar]
- 14.Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Archives of pediatrics & adolescent medicine. 2004;158(4):312–316. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 15.Lattimore KA, Donn SM, Kaciroti N, Kemper AR, Neal CR, Jr, Vazquez DM. Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol. 2005;25(9):595–604. doi: 10.1038/sj.jp.7211352. [DOI] [PubMed] [Google Scholar]
- 16.El Marroun H, Jaddoe VW, Hudziak JJ, Roza SJ, Steegers EA, Hofman A, et al. Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Archives of general psychiatry. 2012;69(7):706–714. doi: 10.1001/archgenpsychiatry.2011.2333. [DOI] [PubMed] [Google Scholar]
- 17.Lewis AJ, Galbally M, Opie G, Buist A. Neonatal growth outcomes at birth and one month postpartum following in utero exposure to antidepressant medication. Aust N Z J Psychiatry. 2010;44(5):482–487. doi: 10.3109/00048670903559593. [DOI] [PubMed] [Google Scholar]
- 18.Davidson S, Prokonov D, Taler M, Maayan R, Harell D, Gil-Ad I, et al. Effect of exposure to selective serotonin reuptake inhibitors in utero on fetal growth: potential role for the IGF-I and HPA axes. Pediatr Res. 2009;65(2):236–241. doi: 10.1203/PDR.0b013e318193594a. [DOI] [PubMed] [Google Scholar]
- 19.Dubnov-Raz G, Hemila H, Vurembrand Y, Kuint J, Maayan-Metzger A. Maternal use of selective serotonin reuptake inhibitors during pregnancy and neonatal bone density. Early Hum Dev. 2011 doi: 10.1016/j.earlhumdev.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Pediatric and Pregnancy Nutrition Surveillance System Health Indicators. [Accessed February 20, 2009]; [Google Scholar]
- 21.Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 1999–2005, CDC National Survey. [Date accessed February 20, 2009]; Vol http://www.cdc.gov/breastfeeding/data/nis_data/
- 22.Chambers CD, Anderson PO, Thomas RG, Dick LM, Felix RJ, Johnson KA, et al. Weight gain in infants breastfed by mothers who take fluoxetine. Pediatrics. 1999;104(5):e61. doi: 10.1542/peds.104.5.e61. [DOI] [PubMed] [Google Scholar]
- 23.Wisner KL, Zarin DA, Holmboe ES, Appelbaum PS, Gelenberg AJ, Leonard HL, et al. Risk-benefit decision making for treatment of depression during pregnancy. The American journal of psychiatry. 2000;157(12):1933–1940. doi: 10.1176/appi.ajp.157.12.1933. [DOI] [PubMed] [Google Scholar]
- 24.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ (Clinical research ed) 1989;298(6673):564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. American journal of epidemiology. 2007;165(8):849–857. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- 26.Williams J, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale With Atypical Depression Supplement (SIGH-ADS) New York: New York State Psychiatric Institute; 2003. [Google Scholar]
- 27.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118(3):1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 28.O'Hara MW, Varner MW, Johnson SR. Assessing stressful life events associated with childbearing: the Peripartum Events Scale. Journal of Reproductive and Infant Psychology. 1986;4(1–2):85–98. [Google Scholar]
- 29.Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. The American journal of psychiatry. 1988;145(7):844–848. doi: 10.1176/ajp.145.7.844. [DOI] [PubMed] [Google Scholar]
- 30.Regier DA, Rae DS, Narrow WE, Kaelber CT, Schatzberg AF. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. The British journal of psychiatry Supplement. 1998;(34):24–28. [PubMed] [Google Scholar]
- 31.Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Current opinion in psychiatry. 2012;25(2):141–148. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseini SM, Biglan MW, Larkby C, Brooks MM, Gorin MB, Day NL. Trait anxiety in pregnant women predicts offspring birth outcomes. Paediatric and perinatal epidemiology. 2009;23(6):557–566. doi: 10.1111/j.1365-3016.2009.01065.x. [DOI] [PubMed] [Google Scholar]
- 33.Wisner KL. The last therapeutic orphan: the pregnant woman. The American journal of psychiatry. 2012;169(6):554–556. doi: 10.1176/appi.ajp.2012.12030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyerly AD, Mitchell LM, Armstrong EM, Harris LH, Kukla R, Kuppermann M, et al. Risks, values, and decision making surrounding pregnancy. Obstetrics and gynecology. 2007;109(4):979–984. doi: 10.1097/01.AOG.0000258285.43499.4b. [DOI] [PubMed] [Google Scholar]
- 35.Hunter SK, Mendoza JH, D'Anna K, Zerbe GO, McCarthy L, Hoffman C, et al. Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. The American journal of psychiatry. 2012;169(6):616–624. doi: 10.1176/appi.ajp.2012.11091365. [DOI] [PMC free article] [PubMed] [Google Scholar]