Abstract
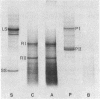
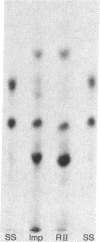
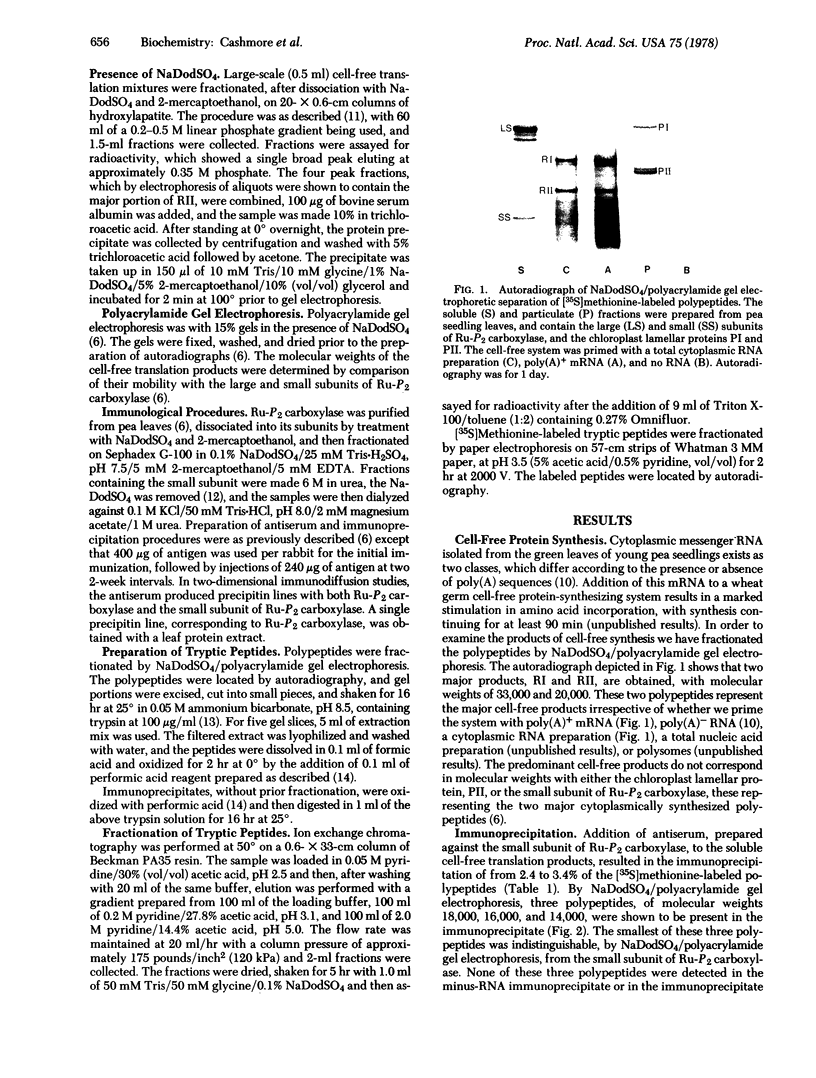
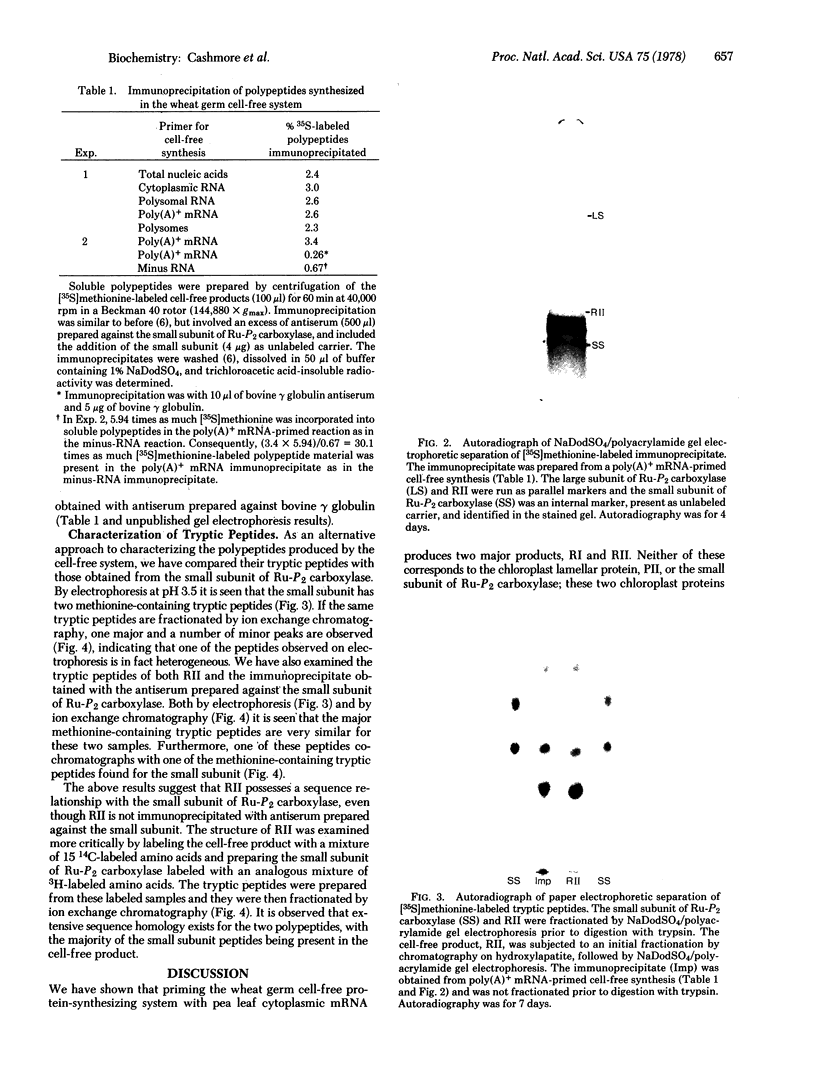
Cytoplasmic mRNA has been isolated from the leaves of pea seedlings. Translation of this RNA in the wheat germ cell-free system produces two major products, RI and RII, with molecular weights of 33,000 and 20,000, respectively. Both of these products are considerably larger than the small subunit of ribulose-1,5-bisphosphate carboxylase [3-phospho-D-glycerate carboxy-lyase (dimerizing), EC 4.1.1.39], which is the major product of cytoplasmic protein synthesis in vivo and has a molecular weight of 14,000. Antiserum prepared against the small subunit of ribulose-1,5-bisphosphate carboxylase precipitates from the cell-free products, in 2-3% yield, three polypeptides of molecular weights 18,000, 16,000 and 14,000. The smallest of these polypeptides is indistinguishable, by sodium dodecyl sulfate/polyacrylamide gel electrophoresis, from the small subunit of ribulose-1,5-bisphosphate carboxylase. Although the cell-free product RII is not precipitated with antiserum prepared against the small subunit of ribulose-1,5-bisphosphate carboxylase, the two polypeptides do show extensive sequence homology, as indicated by ion exchange chromatography of their tryptic peptides. The production of RII can also be achieved in a polysome-primed cell-free system, where protein synthesis is restricted to the completion of polypeptide chains that have already been initiated in vivo. These results indicate that RII is apparently a precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase. We suggest that the selective transport of cytoplasmically synthesized organelle proteins, like animal secretory proteins, may be achieved via the production of precursor polypeptides.
Keywords: immunoprecipitation, tryptic peptides, organelle protein biosynthesis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. N., Blobel G. The role of organelles in the chemical modification of the primary translation products of secretory proteins. FEBS Lett. 1976 Dec 31;72(2):215–226. doi: 10.1016/0014-5793(76)80973-8. [DOI] [PubMed] [Google Scholar]
- Cashmore A. R. Protein synthesis in plant leaf tissue. The sites of synthesis of the major proteins. J Biol Chem. 1976 May 10;251(9):2848–2853. [PubMed] [Google Scholar]
- Davies J. W., Samuel C. E. Translation of virus mRNA: comparison of reovirus and brome mosaic virus single-stranded RNAs in a wheat germ cell-free system. Biochem Biophys Res Commun. 1975 Jul 22;65(2):788–796. doi: 10.1016/s0006-291x(75)80214-2. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Steiner D. F., Chick W. L. Partial purification and characterization of the mRNA for rat preproinsulin. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3539–3543. doi: 10.1073/pnas.73.10.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Kekwick R. G. The synthesis of the small subunit of ribulose 1,5-bisphosphate carboxylase in the french bean Phaseolus vulgaris. Eur J Biochem. 1974 May 15;44(2):491–500. doi: 10.1111/j.1432-1033.1974.tb03507.x. [DOI] [PubMed] [Google Scholar]
- Gray R. E., Cashmore A. R. RNA synthesis in plant leaf tissue: the characterization of messenger RNA species lacking and containing polyadenylic acid. J Mol Biol. 1976 Dec 15;108(3):595–608. doi: 10.1016/s0022-2836(76)80139-8. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone. Evidence for an early biosynthetic precursor of proparathyroid hormone. J Biol Chem. 1976 Jul 10;251(13):3893–3899. [PubMed] [Google Scholar]
- Haslett B. G., Yarwood A., Evans I. M., Boulter D. Studies on the small subunit of fraction I protein from Pisum sativum L. and Vicia faba L. Biochim Biophys Acta. 1976 Jan 20;420(1):122–132. doi: 10.1016/0005-2795(76)90351-2. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Hydroxylapatite chromatography of protein-sodium dodecyl sulfate complexes. A new method for the separation of polypeptide subunits. J Biol Chem. 1972 Aug 25;247(16):5194–5198. [PubMed] [Google Scholar]
- Roy H., Patterson R., Jagendorf A. T. Identification of the small subunit of ribulose 1,5-bisphosphate carboxylase as a product of wheat leaf cytoplasmic ribosomes. Arch Biochem Biophys. 1976 Jan;172(1):64–73. doi: 10.1016/0003-9861(76)90048-5. [DOI] [PubMed] [Google Scholar]
- Shepard J. F., Shalla T. A. An antigenic analysis of potato virus X and of its degraded protein. I. Evidence for and degree of antigenic disparity. Virology. 1970 Dec;42(4):825–834. doi: 10.1016/0042-6822(70)90332-6. [DOI] [PubMed] [Google Scholar]
- Tse T. P., Taylor J. M. Translation of albumin messenger RNA in a cell-free protein-synthesizing system derived from wheat germ. J Biol Chem. 1977 Feb 25;252(4):1272–1278. [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]