Abstract
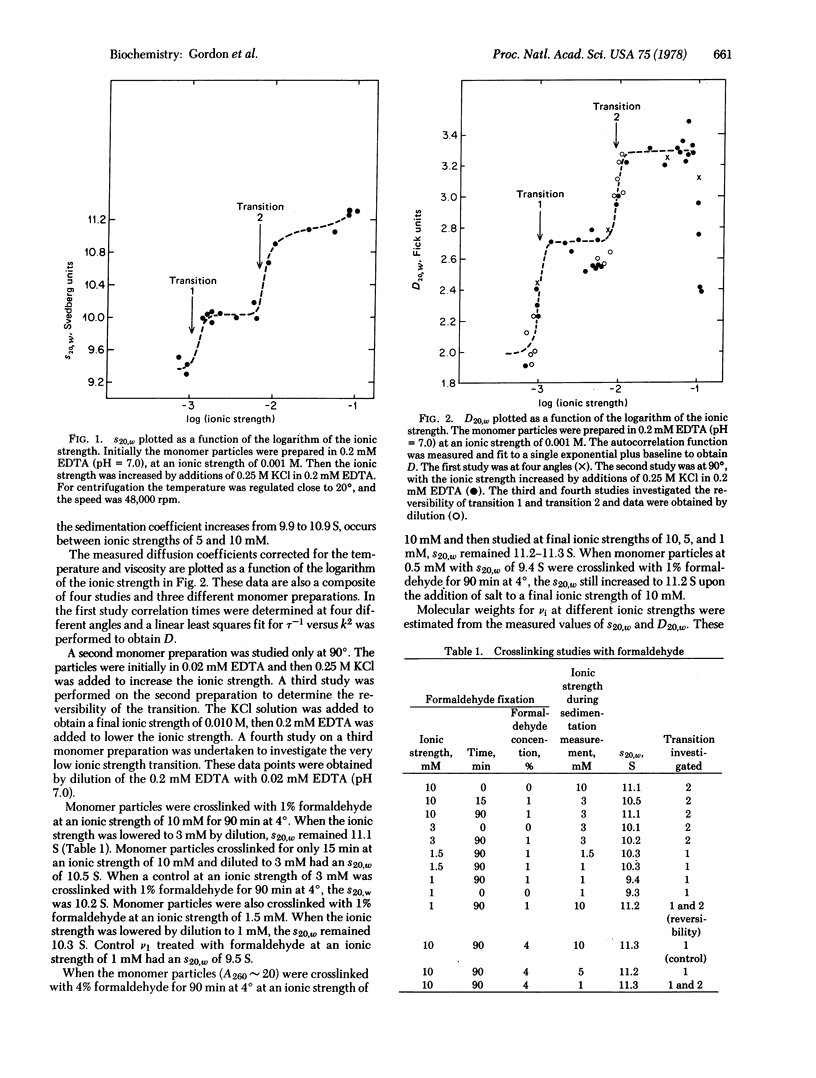
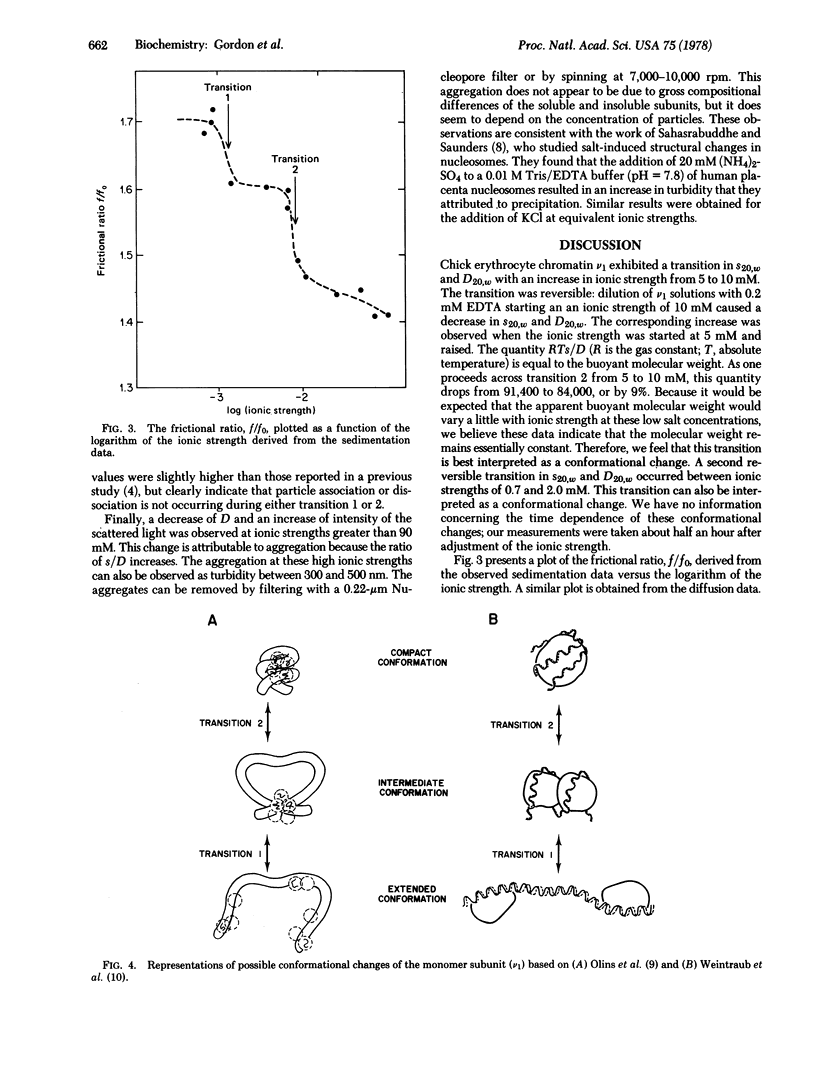
Hydrodynamic studies on monomer chromatin subunits (v1) as a function of ionic strength (0.7 mM to 100 mM KCl) indicate two salt-dependent conformational transitions. An abrupt transition occurs at about 7.5 mM ionic strength. Decreasing the ionic strength from 10 to 5 mM results in a decrease in s20,w of the v1 from 11.1 to 9.9 S. The diffusion coefficient D20,w decreases from 3.3 to 2.7 X 10(-7) cm2 sec--1. The v1 crosslinked with formaldehyde at 10 mM ionic strength do not undergo a similar salt-dependent change in s20,w. Another transition is observed at about 1 mM ionic strength; s20,w decreases to 9.4 S and D20,w decreases to 2.2 X 10(-7) cm2 sec--1. Throughout the entire salt range the molecular weight of the v1 remains reasonably constant, implying that salt-dependent changes in the frictional coefficient are being observed. Various hydrodynamic models are considered as possible interpretations of the observed changes in the frictional coefficient.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomfield V., Dalton W. O., Van Holde K. E. Frictional coefficients of multisubunit structures. I. Theory. Biopolymers. 1967 Feb;5(2):135–148. doi: 10.1002/bip.1967.360050202. [DOI] [PubMed] [Google Scholar]
- Chalkley R., Hunter C. Histone-histone propinquity by aldehyde fixation of chromatin. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1304–1308. doi: 10.1073/pnas.72.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Langmore J. P., Wooley J. C. Chromatin architecture: investigation of a subunit of chromatin by dark field electron microscopy. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2691–2695. doi: 10.1073/pnas.72.7.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M., Tatchell K. Chromatin core particle unfolding induced by tryptic cleavage of histones. Nucleic Acids Res. 1977 Jun;4(6):2039–2055. doi: 10.1093/nar/4.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Carlson R. D., Wright E. B., Olins D. E. Chromatin nu bodies: isolation, subfractionation and physical characterization. Nucleic Acids Res. 1976 Dec;3(12):3271–3291. doi: 10.1093/nar/3.12.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins D. E., Bryan P. N., Harrington R. E., Hill W. E., Olins A. L. Conformational states of chromatin nu bodies induced by urea. Nucleic Acids Res. 1977 Jun;4(6):1911–1931. doi: 10.1093/nar/4.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Saunders G. F. Salt-induced structural changes in nucleosomes. Nucleic Acids Res. 1977 Apr;4(4):853–866. doi: 10.1093/nar/4.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Worcel A., Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976 Nov;9(3):409–417. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]



