Abstract
Diagnostic Lumbar Puncture is one of the most commonly performed invasive tests in clinical medicine. Evaluation of an acute headache and investigation of inflammatory or infectious disease of the nervous system are the most common indications. Serious complications are rare, and correct technique will minimise diagnostic error and maximise patient comfort. We review the technique of diagnostic Lumbar Puncture including anatomy, needle selection, needle insertion, measurement of opening pressure, Cerebrospinal Fluid (CSF) specimen handling and after care. We also make some quality improvement suggestions for those designing services incorporating diagnostic Lumbar Puncture.
INTRODUCTION
HISTORY
The first reported Lumbar Punctures were performed in the late 19th Century by Heinrich Iraneus Quincke1-whose patient with meningitis survived 3 procedures, and around the same time Walter Essex Wynter reported four patients undergoing Lumbar Puncture all of whom died.1 No imaging techniques were available and these procedures were associated with high mortality, which may have led to an unjustified poor reputation of the procedure.
The advent of computed tomographic imaging allowed identification of brain lesions that would place a patient at risk of tentorial herniation. Prospective2 and retrospective3 studies have identified clinical features that are associated with low risks of complication. These include absence of focal neurological deficits, no history of seizure in the previous week, and immunocompetence.2 In trained hands, Lumbar Puncture is a straightforward procedure with few complications. According to The Health and Social Care Information Centre for England, there were 55,427 episodes of hospital care that included a diagnostic Lumbar Puncture in 2011-12, 0.53% of all hospital consultant episodes, which in Northern Ireland's health system, with 600,000 admissions annually, would equate to about 8 diagnostic Lumbar Punctures per day.4
INDICATIONS
There are many indications for Lumbar Puncture (Table 1), but obtaining CSF may be the only way of confirming or refuting subarachnoid haemorrhage, meningitis and neuro-inflammatory diseases. Lumbar Puncture is still required to obtain indirect measurements of intracranial pressure, although non-invasive methods of intracranial pressure estimation are undergoing validation.5
Table 1.
Indications for Lumbar Puncture
| To exclude subarachnoid haemorrhage in acute severe headache |
|---|
To investigate or exclude meningitis
|
To investigate neurological disorders
|
To demonstrate and manage disorders of Intracranial Pressure
|
To administer therapeutic or diagnostic agents*
|
The National Patient Safety Agency recommends formal training for those who undertake intrathecal injection6.
CONSENT AND DOCUMENTATION
Consent should include the risk of Post-Lumbar Puncture Headache (PLPH), which has a published incidence of 32%.7 Other risks to discuss include failure to obtain CSF, localised bruising, bleeding and local discomfort at the injection site. Iatrogenic meningitis and nerve root injury are exceptionally rare.8
The procedure should be documented in the patient's notes. The position and vertebral space selected, local anaesthesic (type, strength and volume), needle type, opening pressure, CSF appearance (clear, cloudy, blood-stained or pigmented) and number of samples collected should be documented, allowing another physician to retrospectively interpret the investigative findings accurately. A proforma (Figure 1) can serve as both aide memoire and audit tool. It is also advisable to document that post Lumbar Puncture advice has been given, and a patient information leaflet is one way to provide this information.
Fig 1.
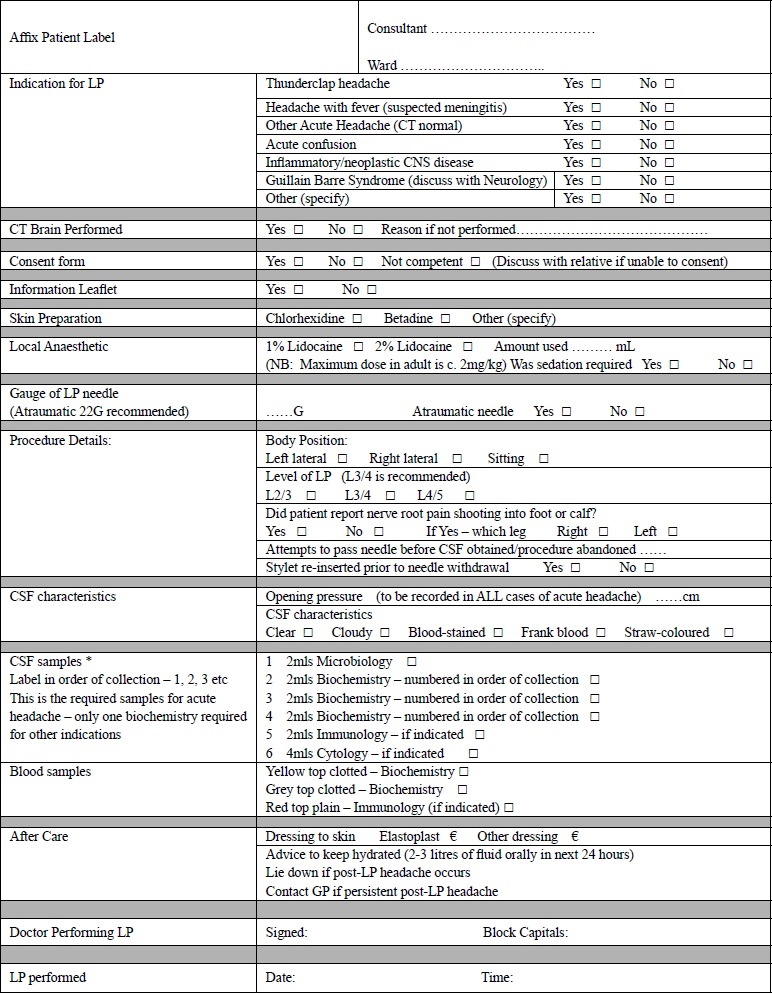
The lumbar puncture proforma currently in use in Craigavon Area Hospital
ANATOMY
Knowledge of the anatomy of the lumbar spine9 is essential for anyone performing Lumbar Puncture. The Lumbar Puncture needle pierces in order: skin, subcutaneous tissue, supraspinous ligament, interspinous ligament, ligamentum flavum, epidural space containing the internal vertebral venous plexus, dura, arachnoid, and finally the subarachnoid space. (Figure 2).
Fig 2.
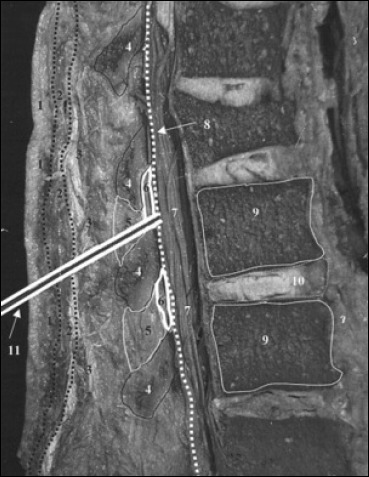
Cadaveric sagittal section through lumbar spine showing proper needle trajectory (from Boon et al.9)
Sagittal section of lumbar vertebrae illustrating the course of the lumbar puncture needle through skin (1), subcutaneous tissue (2), supraspinous ligament (3), interspinous ligament (5) between the spinous processes (4), ligamentum flavum (6), dura mater (8), into the subarachnoid space and between the nerve roots of the cauda equina (7). Lumbar vertebral bodies (9), intervertebral disc (10), and lumbar puncture needle (11).
If you are able to visualise the anatomy it will allow re-positioning of the needle, should the initial pass be unsuccessful-Figure 3 demonstrates the relationship between bony structures and the centre of the spinal canal.
Fig 3.
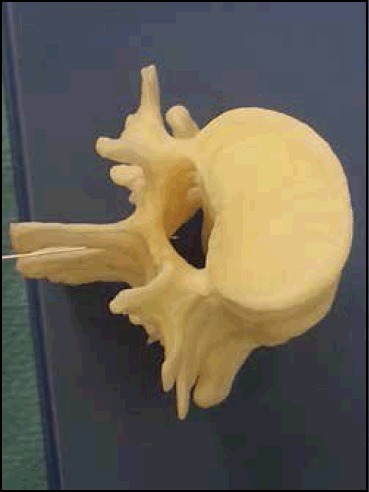
Correct position of tip of Lumbar Puncture needle in centre of Lumbar Spinal Canal at L3/4 level.
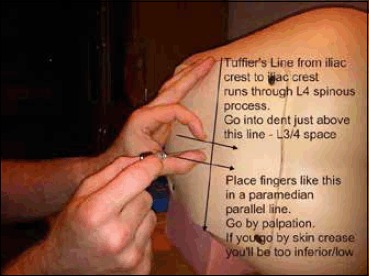
The most important bony landmark is the L4 spinous process, which is located at the intersection of the ‘intercristal’ or ‘Tuffier's’ line (the line between the top of the iliac crests) and the lumbar spine midline (Figure 4). Radiological studies have shown that this clinical landmark is accurate in over 95% of the population,10 although in females or obese people Tuffier's Line tends to be found at a higher level than L4.11
Fig 4.
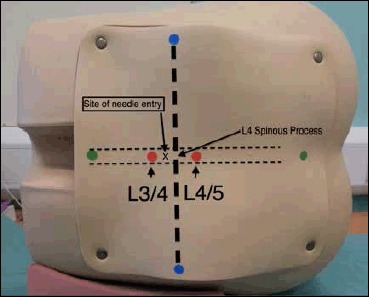
Surface Markings for Lumbar Puncture from Training Mannequin
Blue dots-Iliac crests, and the line connecting them is the Intercristal Line (eponymously Tuffier's Line). Red Dots are either side of the palpated L4 spinous process, the right hand one is in the L4/5 interspinal space and the left hand one in the L3/4 interspinal space. A diagnostic Lumbar Puncture should be performed at the L3/4 interspinal space, marked ‘x’.
The approximate distance from the skin to the epidural space is 45-55mm and the dura mater may be up to 7mm beyond that depth.12 Typically, a standard 90mm Whitacre needle (Vygon UK) will need to be inserted to two thirds of its length before it reaches the ligamentum flavum, with CSF obtained at about 10mm beyond that (Figure 5).
Fig 5.

The distance from surface to Ligamentum Flavum is approximately 55mm.
CSF SPECIMEN HANDLING
Prior to performing the Lumbar Puncture the practitioner should know which tests are required so that appropriate CSF samples are collected and where necessary paired serum (Table 2). Some samples require larger volumes (e.g. Cytology), and others require rapid transport to the laboratory (Cytospin testing for lymphoproliferative cells). Testing for CSF Xanthochromia (to detect bilirubin from blood breakdown) requires rapid transit of a light protected CSF sample to the laboratory. To protect from light the CSF bottle should be wrapped in foil or placed in an envelope. Samples should be correctly labelled with patient identifiers, time and date. Immediate transport by hand to the laboratory by an emergency porter is preferred to the use of vacuum tube delivery systems, as excessive movement of blood specimens has caused increased rates of haemolysis,32 which could lead to in vitro CSF oxyhaemoglobin synthesis which would affect interpretation of spectrophotometry analysis.33
Table 2.
Tests frequently performed on CSF
| Test | Utility | Volume | Form | Additional |
|---|---|---|---|---|
| Microbiology | Cell count, culture and sensitivity | 20 drops | Microbiology | |
| Biochemistry | Protein and glucose | 20 drops | Biochemistry | Paired serum protein and glucose samples |
| Xanthochromia | Spetrophotometry | 20 drops in each of 3 serially numbered bottles | Biochemistry | Transport in opaque envelope or wrap sample container in foil. |
| Oligoclonal bands | Investigation of CNS inflammation | 20 drops | Immunology | Paired serology serum sample |
| Cytology | Investigation of malignant meningitis | 50 drops | Neuropathology | Swift transport to laboratory |
| Cytospin | Investigation of CNS lymphoma | 20 drops | Haematology | Prior arrangement and swift transport to laboratory |
| Viral PCR | PCR for viral DNA | 20 drops | Virology | |
| ACE | Investigation of neurosarcoidosis | 20 drops | Biochemistry | Paired serum ACE sample |
| Lactate | Investigation of neurodegenerative disorders | 20 drops | Biochemistry | Paired serum lactate sample |
Isotope dilution studies show that an average adult makes about 500 ml of CSF every 24 hours, and that CSF is replaced about 4 times daily i.e. there is approximately 20 ml of CSF manufactured each hour.8 About 30 ml of CSF resides in the Lumbar Cistern, so taking about 10 ml of CSF to facilitate diagnostic testing is not likely to endanger the patient. The patient is more likely to come to harm from inadequate CSF samples.
ASEPTIC TECHNIQUE
Diagnostic Lumbar Puncture is an aseptic procedure, but as there is no direct injection into the spinal canal, the procedure can be done in the ward setting and does not need to be done in an operating theatre. It should be noted that The Association of Anaesthetists of Great Britain and Ireland recommends that injections into the spinal canal, such as epidural blood patching, should only be performed using aseptic techniques in a theatre environment.22 In a diagnostic Lumbar Puncture, standard bedside aseptic procedures apply with no-touch technique, sterile drapes and use of chlorhexidine or an equivalent antiseptic.
There has been wide variation in what clinicians, particularly anaesthetists, feel constitute aseptic technique for spinal procedures. Clinicians' views regarding hand-washing technique, donning of gowns or masks, and wearing of jewelry when placing an epidural catheter have been shown to vary.23 Wearing of masks may be associated with reduced bacterial transfer. A cluster of four cases of streptococcal meningitis was caused by a physician who had chronic tonsillitis.24
Bacteria in the orifices of sebaceous glands and hair follicles are protected by the stratum corneum from disinfectants.25 The overlying skin should therefore be cleaned with a solution that breaches this layer such as povidone-iodine or 0.5% chlorhexidine and 70% alcohol. Traditionally, chlorhexidine had not been recommended for procedures with meningeal exposure due to a possible association with arachnoiditis, but chlorhexidine does not seem to be associated with an increased incidence of neurological complications in spinal anaesthesia,26 and has been recommended for anaesthetic practice.24 We routinely use chlorhexidine to prepare the skin for the less hazardous procedure of diagnostic Lumbar Puncture. The Anaesthetic Literature supports use of 0.5% chlorhexidine with 70% alcohol as a suitable antiseptic.26
NEEDLE SELECTION
We suggest that the evidence for reduced frequency of PLPH from use of atraumatic needles mandates a change in practice, and physicians in training should no longer be taught to use classic needles routinely. A classic ‘Quincke’ needle has a cutting, bevelled tip, but an atraumatic needle has a pencil point tip with a side aperture (Figure 6). The recommended needle is a 22 gauge atraumatic needle. In our own practice we use a 22 gauge Whitacre needle, which is now the standard stock Lumbar Puncture needle in our institution. The biggest adjustment for people changing from traumatic to atraumatic needle is that the skin must be punctured first prior to insertion of the Lumbar Puncture needle, as an atraumatic needle will not pierce the epidermis. Options include using the introducer needle provided to pierce the skin, or using the puncture site made by the green 19G local anaesthetic needle as the needle entry point.
Fig 6.
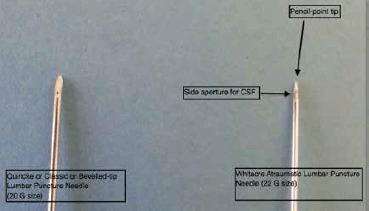
Classic (Quincke, or Bevelled-tip) and Whitacre Atraumatic Lumbar Puncture Needle
Passage of atraumatic needle is a completely different sensation to a classic needle, as the practitioner experiences greater resistance when traversing the tissue planes to the CSF. A good analogy is the difference in sensation felt when cutting through a banana (analogous to the standard needle) and a potato (analogous to an atraumatic needle).13 In 2005, The American Academy of Neurology recommended atraumatic needles as means of reducing the risk of PLPH7 but this practice has to date been poorly adopted. However, with appropriate training and support, medical staff will adopt use of atraumatic needles and reproduce the benefits demonstrated in clinical trials in clinical practice.14-16
Atraumatic needles have even been shown to be more cost-effective than classic cutting needles.15 Patients with PLPH can require in-patient care, which makes atraumatic needles both effective and cost-effective options for diagnostic Lumbar Puncture.17
Needles of smaller diameter have been demonstrated to be associated with a reduction of risk of PLPH from 24.4% to 12.2%.7 The smallest diameter needle with which the practitioner can confidently perform the procedure avoiding an increased number of attempts should be chosen,18 and it is known that larger bore atraumatic needles allow adequate pressure measurement and collection of sufficient CSF.19
PATIENT POSITIONING
A right-handed practitioner should position the patient in the left lateral decubitus position, with the vertebrae in line in the horizontal plane and the head in a neutral position and the knees flexed. Opening Pressure at Lumbar Puncture is a surrogate measurement of Intracranial Pressure. An accurate Opening Pressure requires the needle entry point to be on the same level as the midline of the spine (Figure 4), which should also be at the same level as the patient's head. A few centimetres of ‘head up’ bed tilt or more than one pillow could artificially increase the Opening Pressure measurement.
Always ensure that the patient is comfortable, and that the bed height is appropriate for the operator, as the practitioner risks compromising aseptic technique if the patient has to be re-positioned mid-procedure.
Lumbar Puncture can also be performed in the seated position, providing pressure measurement is not required. There are occasions when pressure measurement is sacrificed in order to obtain a CSF sample, for example in elective Lumbar Puncture for neuro-inflammatory disease. If the procedure is to be performed in the upright position, seated with the chin down and the feet supported, a table and pillow will improve comfort and optimise positioning. This position widens the interspinous distance.20 Remember that an elevated Opening Pressure may be the only sign of Cerebral Venous Sinus Thrombosis,21 or Idiopathic Intracranial Hypertension, so in acute headache a Lumbar Puncture should be performed in the lateral decubitus position, whenever possible, to allow a pressure measurement.
HOW TO GET CSF AND MEASURE OPENING PRESSURE
The best location to perform a Lumbar Puncture will depend on local facilities, but a treatment room, or somewhere calm and quiet is preferred to a ward bay. The attitude of operator and assistant should be one of calm confidence and reassurance. An experienced operator will have the patient consented, positioned and comfortable, and from the moment of washing hands until the first CSF sample appears should be about 8 to 10 minutes (personal observation).
Aseptic technique is required, and a pre-prepared epidural pack contains all the equipment needed other than your needle, lidocaine and manometer (Figure 7). The manometer (Figure 8) should be prepared prior to commencing the spinal injection, by connecting the two tubes and loosening the tap (which is always slightly stiff and is difficult to open if you do not loosen it prior to connecting it to the manometer).
Fig 7.
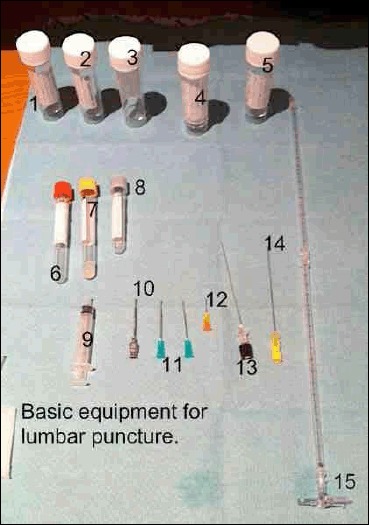
Equipment needed for Lumbar Puncture
1-5 CSF Specimen Bottles
6-7 Serum Specimen Bottles, 8 Serum glucose bottle (fluoride oxalate)
9 Syringe for local anaesthetic
10 Introducer for spinal needle (not always required)
11-12 19G and 25G hypodermic needles to draw up and inject anaesthetic
13 Whitacre 22G spinal needle (atraumatic needle)
14 Quincke 20G spinal needle (no longer recommended)
15 Manometer with 3-way tap
Fig 8.
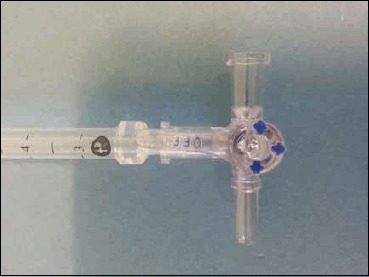
Three way tap attached to end of Manometer
Once skin is sterile, local anaesthetic can be administered. Our practice is to raise a small intradermal bleb of lidocaine, which produces almost immediate cutaneous anaesthesia (Figure 9). A small amount of lidocaine can be infiltrated into deeper tissues, but care must be taken not to distort local anatomy by administering too much local anaesthetic. If you anaesthetise the skin and have a correct trajectory, there is little benefit to infiltrating large volumes of anaesthetic.
Fig 9.
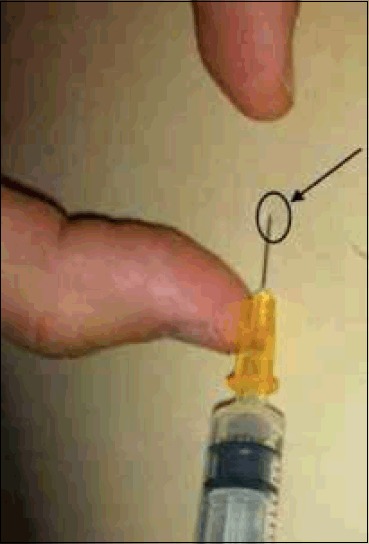
An intradermal bleb of 0.5ml of 1% lidocaine will produce almost immediate cutaneous anaesthesia
The aperture through which the needle must pass to reach the lumbar cistern is diamond shaped and surrounded by bony structures. The Lumbar Puncture needle should be inserted at an angle that will allow it to pass between the spinous processes (Figure 10). The most common problem encountered by operators is their needle impacting on a bony structure-either the superior surface of the L4 spinous process, or the inferior surface of the L3 spinous process. If the needle tip is advanced beyond 50mm and the needle hits bone, then you have probably impacted on bone around the intervertebral space. Consider the anatomy and needle angle, and thus which bone is likely to have been reached. Withdraw the needle and adjust the trajectory, gradually proceeding until a “give” is felt on passing through the ligamentum flavum (Figure 2), remembering that the distance to ligamentum flavum is approximately 2/3rds of the length of a standard needle in a non-obese patient. Whichever needle type has been selected, the stylet should be in place when the needle insertion commences, as there have been case reports of implantation of epidermal plugs resulting in the growth of spinal epidermoid tumours.27
Fig 10.
Insertion of needle at an angle to allow passage between spinous processes of L3 and L4-note anatomical landmarks.
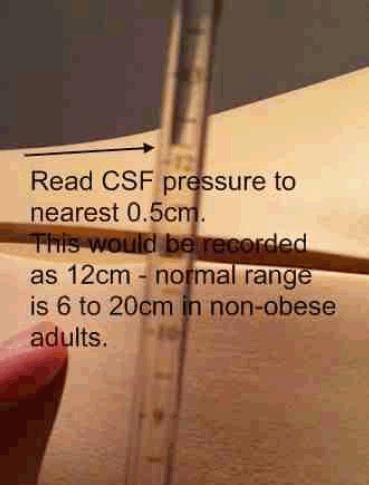
Once the needle is sufficiently advanced, withdraw the stylet slowly and wait about 5 seconds to see if CSF emerges. If it does not, replace the stylet and advance the needle another 2 or 3 mm and check again for CSF. Once CSF is obtained, connect the manometer and measure Opening Pressure (unless the Lumbar Puncture is performed in a seated position). It will take approximately one minute for CSF pressure to be measured and it is normal to observe the meniscus of CSF at the top of the manometer oscillate with respiration. Like all fluid measurements the pressure is the height of the lowest part of the meniscus at the top of the fluid column (Figure 11). Distracting the patient with conversation or other relaxation techniques may be used to ameliorate anxiety and allow a falsely elevated opening pressure to fall. CSF pressure measurements, from large series of adults indicate that CSF pressures between 60mm and 200mm are regarded as normal,8 although in overweight individuals pressures as high as 250mm can be asymptomatic.28 Patient anxiety, incorrect head position and excessive flexion of the patient's legs against the abdomen can all artificially elevate CSF pressure.
Fig 11.
Reading CSF pressure from the top of the CSF fluid column
Samples should ideally be taken either using sterile bottles by the individual performing the procedure, or by an assistant holding an open specimen container underneath the flow of CSF from the end of the spinal needle. Twenty drops per bottle is sufficient for most commonly performed tests (which provides about 2ml of CSF). A minimum volume of 5ml of CSF is required for cytopathological examination for example in suspected malignant meningitis. Replacement of the stylet is associated with a reduced incidence of post Lumbar Puncture headache,29 and failure to replace the stylet has been associated with nerve root herniation. 30 After the needle has been removed, a sterile dressing should be placed over the puncture site.
IF YOU ARE UNABLE TO GET CSF…
If you are unable to obtain CSF, having optimised position and needle trajectory, consider whether there is another suitably qualified physician available to attempt the procedure. Low CSF pressure, or viscous CSF in cases of malignant or tuberculous meningitis may mean that CSF will not appear despite perfect positioning and technique. Aspiration of CSF is not recommended, as it may cause spinal cord injury. Image guided procedures are often required in overweight people suspected of Idiopathic Intracranial Hypertension. Ultrasound identification of the interspinous space should become routine practice, now that evidence shows a reduced risk of complications and a higher success rate in obtaining CSF with ultrasonic identification of the inter-spinal space.31
AFTER CARE
A small sterile dressing is placed on the site: a pressure dressing is not required. The patient can mobilise as soon as it is comfortable to do so. Written information should be provided on what to do if a PLPH occurs, and recommended doses of regular analgesics such as paracetamol or NSAIDs are reasonable. Bed rest is more comfortable than being ambulant, but prolonged bed rest does not reduce the incidence of PLPH.39
COMPLICATIONS
The most common complication is Post Lumbar Puncture Headache (PLPH). In one meta-analysis, the frequency of PLPH was 32%.7 An important feature in the diagnosis of PLPH is the postural component; the patient will report a background headache which worsens within a few minutes of adopting upright posture and improves within a few minutes of lying flat.34 Risk factors include: younger age, female gender, and headache before or at the time of the procedure. The volume of fluid removed is not a risk factor, and the pathophysiology of PLPH is more plausibly explained as a loss of compliance of the spinal compartment, rather than due to loss of CSF volume from a continous CSF leak.35 Relief of PLPH by adopting a supine posture means that the brain and its supporting dura are not mechanically stretched by the loss of spinal compliance.
The symptoms of PLPH usually develop within 24 hours of Lumbar Puncture, and the natural history is for symptoms to resolve by about 10 days. The pain is usually diffuse, global or bitemporal headache, which can be accompanied by nausea, altered hearing, tinnitus, photophobia or neck stiffness. Low pressure may produce diplopia due to traction on the fourth or sixth cranial nerve.36
There is robust evidence that the most important factor in reducing the risk of PLPH is needle selection, and a 22G atraumatic needle is now recommended for diagnostic Lumbar Puncture.7 In our own practice we reduced the risk of PLPH from 50% to 10% with the introduction of a guideline that required initial attempts with an atraumatic needle.37 This observation is consistent with the experience from other Neurology Centres.14
There is anecdotal evidence that a straightforward procedure may increase the risk of PLPH due to the low levels of trauma and therefore clotting factors that might promote closure of the defect thus preventing CSF leakage.38 Directing the needle with the bevel parallel to the dural fibres (which run craniocaudally) has been shown to be associated with a reduction in PLPH.7 This advice only applies to classic ‘traumatic’ needles so is irrelevant when practitioners transition to using atraumatic needles due to the pencil point shape of the tip (Figure 5). Replacing the stylet has been shown to reduce the risk of PLPH, theoretically because a strand of arachnoid may splint the dural defect open if the needle is withdrawn without the stylet.29 A Cochrane Review has suggested that there is no benefit to routine bed rest or intravenous fluids in prevention of PLPH after Lumbar Puncture.39
Maintaining a supine posture, oral or intravenous fluids and symptomatic management with analgesia and antiemetics are logical first steps to conservative management of PLPH. There is some evidence for the use of intravenous caffeine or intravenous theophylline,40 but the definitive treatment if conservative management fails is epidural blood patching. The optimum timing of blood patching remains one of clinical judgement. The natural history is for resolution of PLPH by 10-14 days, and unless symptoms are completely disabling, it may be prudent to delay epidural blood patching for this length of time. However, epidural blood patching has been demonstrated to be effective41 and the prospect of near-immediate relief may be difficult to deny a patient in distress. An epidural blood patch is commonly viewed as causing a tamponade which seals the CSF leak; although it may be more correct to state that epidural blood injection increases spinal compartment compliance.35 Epidural Blood Patch should be performed under strict asepsis in an operating theatre environment.22 Prophylactic epidural blood patching is not currently recommended,41 as it is not proven to be effective and would require all diagnostic Lumbar Punctures to take place in operating theatre conditions.
Cortical vein thrombosis,42 and reversible cerebral vasoconstriction syndrome43 have been reported as very rare complications of low CSF pressure states. These may present with worsening headache following Lumbar Puncture, and require additional neuro-imaging to confirm their presence.
Imaging studies cannot completely exclude raised intracranial pressure, but they will exclude mass lesions which pose a risk of Tentorial Herniation. Tentorial Herniation is preceded by lateral brainstem shift,44 so a unilateral mass lesion poses most risk prior to Lumbar Puncture. Observational studies of patients with suspected meningitis indicate that Lumbar Puncture without prior brain imaging is safe in people with normal conscious level, no focal neurological signs and no prior history of immunosuppression.2
Severe thrombocytopenia, bleeding diathesis or anticoagulant therapy are contraindications to Lumbar Puncture, although it can be performed safely at platelet counts of 50 x 109/l or above.45 A survey showed that 45% of physicians and paediatricians perform coagulation studies and platelet counts prior to Lumbar Puncture.46 Aspirin therapy is not associated with a high risk of bleeding in spinal anaesthetic interventions47, but data on clopidogrel and other GP IIa/IIIb receptor antagonists are lacking. Guidance on spinal anaesthetic procedures suggest that procedures should be avoided until platelet function has recovered.48 Warfarin should be stopped 5-7 days in advance of procedures and the INR should be less than 1.2. Low molecular weight heparin can be used in the interim but treatment dose heparin should be stopped for twenty-four hours prior to spinal procedures. Prophylactic doses should be avoided within twelve hours.48
FUTURE DEVELOPMENTS
Diagnostic Lumbar Puncture is an essential skill for emergency medicine and neurology services. Historically, specialists used to be critical of the unthinking use of Lumbar Puncture,49 but more recently specialists have been critical of underuse, especially in suspected meningitis.3 It is important that Lumbar Puncture is performed by practitioners who do them frequently enough to maintain their skills. Lumbar Puncture mannequins (as seen in Figure 4) have been shown to useful for skill development in trainee doctors,50 and could be used for maintaining or revalidating skills.
Fluoroscopically-guided Lumbar Puncture is an option if a standard Lumbar Puncture has been unsuccessful, but due to x-ray exposure they are not suitable for pregnant women or for repeated procedures. A recent systematic review of the use of ultrasound guidance for both Lumbar Puncture and epidural anaesthesia concluded that compared with standard palpation methods, ultrasound imaging reduced the number of insertion attempts, of needle redirections, and failed or traumatic procedures.31 Pre-procedural scanning is used to identify vertebral levels, the midline, and the depth to the spinal canal. Dynamic or real time scanning can be used to visualise the progression of the needle. In modern medical practice there is a growing trend toward ultrasound guidance for invasive procedures such as chest drain insertion50 and vascular procedures.52
It is likely that ultrasound guidance will become a routine part of Lumbar Puncture practice in future, particularly in the context of increasing rates of obesity. However, this will probably require the procedure to be concentrated into the hands of people who are both competent in ultrasound localisation and spinal puncture.
Acknowledgments
The authors have no conflict of interest.
The Lumbar Puncture Mannequin was donated to the Neurology Department as a result of the fundraising efforts of the late Helen Annesley.
The Lumbar Puncture Proforma was written by Elaine Johnston who co-ordinated our local audits of practice.
BIBLIOGRAPHY
- 1.Pearce JM. Walter Essex Wynter, Quincke, and lumbar puncture. J Neurol Neurosurg Psychiatry. 1994;57(2):179. doi: 10.1136/jnnp.57.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345(24):1727–33. doi: 10.1056/NEJMoa010399. [DOI] [PubMed] [Google Scholar]
- 3.Kneen R, Solomon T, Appleton R. The role of lumbar puncture in suspected CNS infection--a disappearing skill? ArchDis Child. 2002;87(3):181–3. doi: 10.1136/adc.87.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leeds: HSCIC. Health and Social Care Information Centre; 2012. Hospital Episode Statistics, Admitted Patient Care-England, 2011-12. [Google Scholar]
- 5.Ragauskas A, Matijosaitis V, Zakelis R, Petrikonis K, Rastenyte D, Piper I, et al. Clinical assessment of noninvasive intracranial pressure absolute value measurement method. Neurology. 2012;78(21):1684–91. doi: 10.1212/WNL.0b013e3182574f50. [DOI] [PubMed] [Google Scholar]
- 6.Safer practice with epidural injections and infusions. London: National Patient Safety Agency; 2007. Department of Health, Social Services and Public Safety, Northern Ireland. NPSA Alert 21. [Google Scholar]
- 7.Armon C, Evans RW. Addendum to assessment: Prevention of postlumbar puncture headaches: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65(4):510–2. doi: 10.1212/01.wnl.0000173034.96211.1b. [DOI] [PubMed] [Google Scholar]
- 8.Fishman RA. 2nd ed. Philadephia: W.B. Saunders Co.; 1992. Cerebrospinal fluid in diseases of the nervous system. [Google Scholar]
- 9.Boon JM, Abrahams PH, Meiring JH, Welch T. Lumbar puncture: anatomical review of a clinical skill. Clin Anat. 2004;17(7):544–53. doi: 10.1002/ca.10250. [DOI] [PubMed] [Google Scholar]
- 10.Ievins FA. Accuracy of placement of extradural needles in the L3-4 interspace: comparison of two methods of identifying L4. Br J Anaesth. 1991;66(3):381–2. doi: 10.1093/bja/66.3.381. [DOI] [PubMed] [Google Scholar]
- 11.Chakraverty R, Pynsent P, Isaacs K. Which spinal levels are identified by palpation of the iliac crests and the posterior superior iliac spines? JAnat. 2007;210(2):232–6. doi: 10.1111/j.1469-7580.2006.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan QH. Epidural anatomy: new observations. Can J Anaesth. 1998;45:R40–8. doi: 10.1007/BF03019206. (5 Pt 2) [DOI] [PubMed] [Google Scholar]
- 13.Hatfalvi BI. The dynamics of post-spinal headache. Headache. 1977;17(2):64–6. doi: 10.1111/j.1526-4610.1977.hed1702064.x. [DOI] [PubMed] [Google Scholar]
- 14.Vakharia VN, Lote H. Introduction of Sprotte needles to a single-centre acute neurology service: before and after study. JRSM Short Reports. 2012;3(12):82. doi: 10.1258/shorts.2012.012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung CE. Education research: changing practice. Residents' adoption of the atraumatic lumbar puncture needle. Neurology. 2013;80(17):e180–2. doi: 10.1212/WNL.0b013e31828f1866. [DOI] [PubMed] [Google Scholar]
- 16.Thomas SR, Jamieson DR, Muir KW. Randomised controlled trial of atraumatic versus standard needles for diagnostic lumbar puncture. BMJ. 2000;321(7267):986–90. doi: 10.1136/bmj.321.7267.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dakka Y, Warra N, Albadareen RJ, Jankowski M, Silver B. Headache rate and cost of care following lumbar puncture at a single tertiary care hospital. Neurology. 2011;77(1):71–4. doi: 10.1212/WNL.0b013e318220abc0. [DOI] [PubMed] [Google Scholar]
- 18.Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006;296(16):2012–22. doi: 10.1001/jama.296.16.2012. [DOI] [PubMed] [Google Scholar]
- 19.Carson D, Serpell M. Choosing the best needle for diagnostic lumbar puncture. Neurology. 1996;47(1):33–7. doi: 10.1212/wnl.47.1.33. [DOI] [PubMed] [Google Scholar]
- 20.Sandoval M, Shestak W, Sturmann K, Hsu C. Optimal patient position for lumbar puncture, measured by ultrasonography. Emerg Radiol. 2004;10(4):179–81. doi: 10.1007/s10140-003-0286-3. [DOI] [PubMed] [Google Scholar]
- 21.Cumurciuc R, Crassard I, Sarov M, Valade D, Bousser MG. Headache as the only neurological sign of cerebral venous thrombosis: a series of 17 cases. J Neurol Neurosurg Psychiatry. 2005;76(8):1084–7. doi: 10.1136/jnnp.2004.056275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Infection control in anaesthesia. Association of Anaesthetists of Great Britain and Ireland. Anaesthesia. 2008;63(9):1027–36. doi: 10.1111/j.1365-2044.2008.05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sellors JE, Cyna AM, Simmons SW. Aseptic precautions for inserting an epidural catheter: a survey of obstetric anaesthetists. Anaesthesia. 2002;57(6):593–6. doi: 10.1046/j.1365-2044.2002.02509_3.x. [DOI] [PubMed] [Google Scholar]
- 24.Hebl JR. The importance and implications of aseptic techniques during regional anesthesia. Reg Anesth Pain Med. 2006;31(4):311–23. doi: 10.1016/j.rapm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Sukeyuki S, Dan K. Human skin flora as a potential source of epidural abscess. Anestheiology. 1996;85(6):1276–82. doi: 10.1097/00000542-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Scott M, Stones J, Payne N. Antiseptic solutions for central neuraxial blockade: which concentration of chlorhexidine in alcohol should we use? Br J Anaesth. 2009;103(3):456. doi: 10.1093/bja/aep214. [DOI] [PubMed] [Google Scholar]
- 27.McDonald JV, Klump TE. Intraspinal epidermoid tumors caused by lumbar puncture. Arch Neurol. 1986;43(9):936–9. doi: 10.1001/archneur.1986.00520090064019. [DOI] [PubMed] [Google Scholar]
- 28.Corbett JJ, Mehta MP. Cerebrospinal fluid pressure in normal obese subjects and patients with pseudotumor cerebri. Neurology. 1983;33(10):1386–8. doi: 10.1212/wnl.33.10.1386. [DOI] [PubMed] [Google Scholar]
- 29.Strupp M, Brandt T, Muller A. Incidence of post-lumbar puncture syndrome reduced by reinserting the stylet: a randomized prospective study of 600 patients. J Neurol. 1998;245(9):589–92. doi: 10.1007/s004150050250. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqi TS, Buchheit WA. Herniated nerve root as a complication of spinal tap. Case report. J Neurosurg. 1982;56(4):565–6. doi: 10.3171/jns.1982.56.4.0565. [DOI] [PubMed] [Google Scholar]
- 31.Shaikh F, Brzezinski J, Alexander S, Arzola C, Carvalho JC, Beyene J, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346 doi: 10.1136/bmj.f1720. f1720. [DOI] [PubMed] [Google Scholar]
- 32.Ellis G. An episode of increased hemolysis due to a defective pneumatic air tube delivery system. Clin Biochem. 2009;42(12):1265–9. doi: 10.1016/j.clinbiochem.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Cruickshank A, Auld P, Beetham R, Burrows G, Egner W, Holbrook I, et al. Revised national guidelines for analysis of cerebrospinal fluid for bilirubin in suspected subarachnoid haemorrhage. Ann Clin Biochem. 2008;45(Pt 3):238–44. doi: 10.1258/acb.2008.007257. [DOI] [PubMed] [Google Scholar]
- 34.The International Classification of Headache Disorders. Cephalalgia. (2nd edition) 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 35.Levine DN, Rapalino O. The pathophysiology of lumbar puncture headache. J Neurol Sci. 2001;192(1-2):1–8. doi: 10.1016/s0022-510x(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 36.Thomke F, Mika-Gruttner A, Visbeck A, Bruhl K. The risk of abducens palsy after diagnostic lumbar puncture. Neurology. 2000;54(3):768–9. doi: 10.1212/wnl.54.3.768. [DOI] [PubMed] [Google Scholar]
- 37.Peukert T, Gray O, Forbes R. Lumbar puncture audit: do atraumatic needles matter? Abstracts from the ABN Annual Meeting Gateshead. J Neurol Neurosurg Psychiatry. 2012;83(e1) [Google Scholar]
- 38.Rizzoli P. Taking the sting out of lumbar puncture. BMJ. 2013;346 doi: 10.1136/bmj.f1734. f1734. [DOI] [PubMed] [Google Scholar]
- 39.Arevalo-Rodriguez I, Ciapponi A, Munoz L, Roque i, Figuls M, Bonfill Cosp X. Posture and fluids for preventing post-dural puncture headache. Cochrane Database Syst Rev. 2013;(7) doi: 10.1002/14651858.CD009199.pub2. Cd009199. [DOI] [PubMed] [Google Scholar]
- 40.Bezov D, Ashina S, Lipton R. Post-dural puncture headache: Part II--prevention, management, and prognosis. Headache. 2010;50(9):1482–98. doi: 10.1111/j.1526-4610.2010.01758.x. [DOI] [PubMed] [Google Scholar]
- 41.Boonmak P, Boonmak S. Epidural blood patching for preventing and treating post-dural puncture headache. Cochrane Database Syst Rev. 2010;(1) doi: 10.1002/14651858.CD001791.pub2. Cd001791. [DOI] [PubMed] [Google Scholar]
- 42.Laverse E, Cader S, de Silva R, Chawda S, Kapoor S, Thompson O. Peripartum isolated cortical vein thrombosis in a mother with postdural puncture headache treated with an epidural blood patch. Case Rep Med. 2013;2013 doi: 10.1155/2013/701264. 701264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schievink WI, Maya MM, Chow W, Louy C. Reversible cerebral vasoconstriction in spontaneous intracranial hypotension. Headache. 2007;47(2):284–7. doi: 10.1111/j.1526-4610.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 44.Fisher CM. Brain herniation: a revision of classical concepts. Can J Neurol Sci. 1995;22(2):83–91. doi: 10.1017/s0317167100040142. [DOI] [PubMed] [Google Scholar]
- 45.British Committee for Standards in Haematology. Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br Haematol. 2003;122(1):10–23. [Google Scholar]
- 46.Waldman W, Laureno R. Precautions for lumbar puncture: a survey of neurologic educators. Neurology. 1999;52(6):1296. doi: 10.1212/wnl.52.6.1296. [DOI] [PubMed] [Google Scholar]
- 47.Horlocker TT, Wedel DJ, Schroeder DR, Rose SH, Elliott BA, McGregor DG, et al. Preoperative antiplatelet therapy does not increase the risk of spinal hematoma associated with regional anesthesia. Anesth Analg. 1995;80(2):303–9. doi: 10.1097/00000539-199502000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Layton KF, Kallmes DF, Horlocker TT. Recommendations for anticoagulated patients undergoing image-guided spinal procedures. AJNR Am J Neuroradiol. 2006;27(3):468–70. [PMC free article] [PubMed] [Google Scholar]
- 49.Dripps RD, Vandam LD. Hazards of lumbar puncture. J Am Med Assoc. 1951;147(12):1118–21. doi: 10.1001/jama.1951.03670290026007. [DOI] [PubMed] [Google Scholar]
- 50.Uppal V, Kearns RJ, McGrady EM. Evaluation of M43B Lumbar puncture simulator-II as a training tool for identification of the epidural space and lumbar puncture*. Anaesthesia. 2011;66(6):493–6. doi: 10.1111/j.1365-2044.2011.06710.x. [DOI] [PubMed] [Google Scholar]
- 51.Anonymous. London: British Thoracic Society; 2008. Guidance for the implementation of local Trust policies for the safe insertion of chest drains for pleural effusions in Adults, following the NPSA Rapid Response Report —NPSA/2008/RR003. [Google Scholar]
- 52.Hind D, Calvert N, McWilliams R, Davidson A, Paisley S, Beverley C, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. doi: 10.1136/bmj.327.7411.361. [DOI] [PMC free article] [PubMed] [Google Scholar]


