Abstract
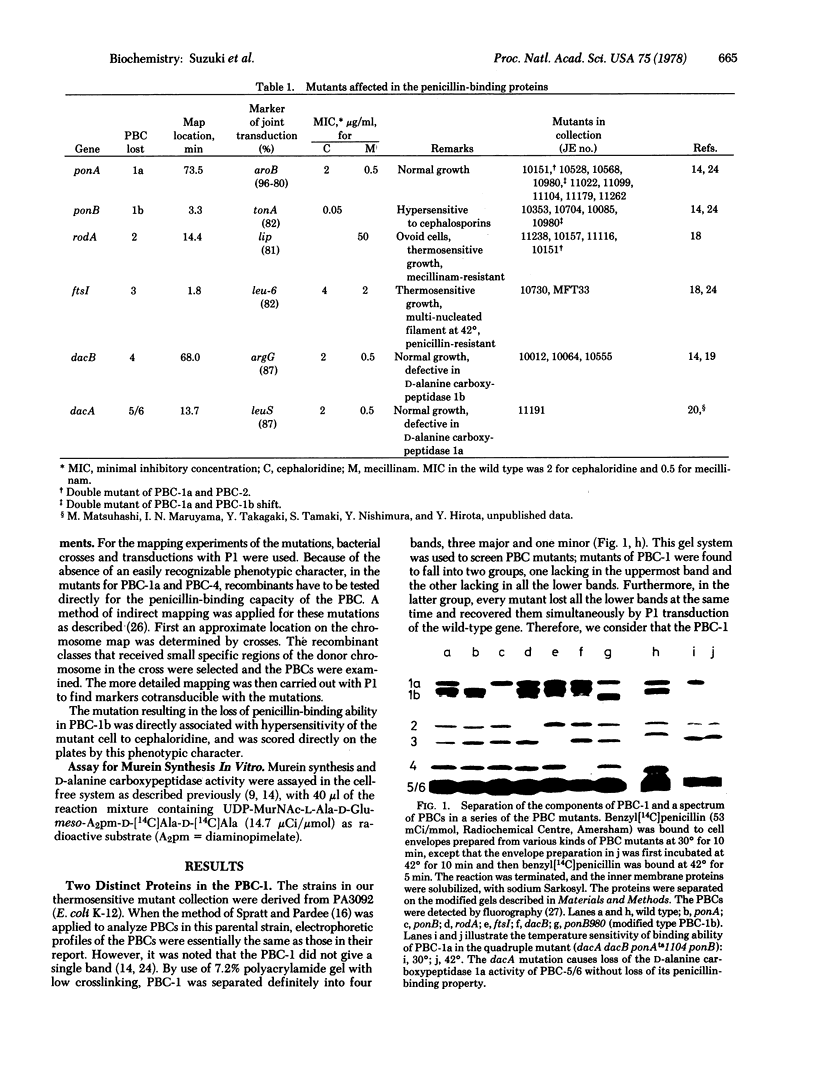
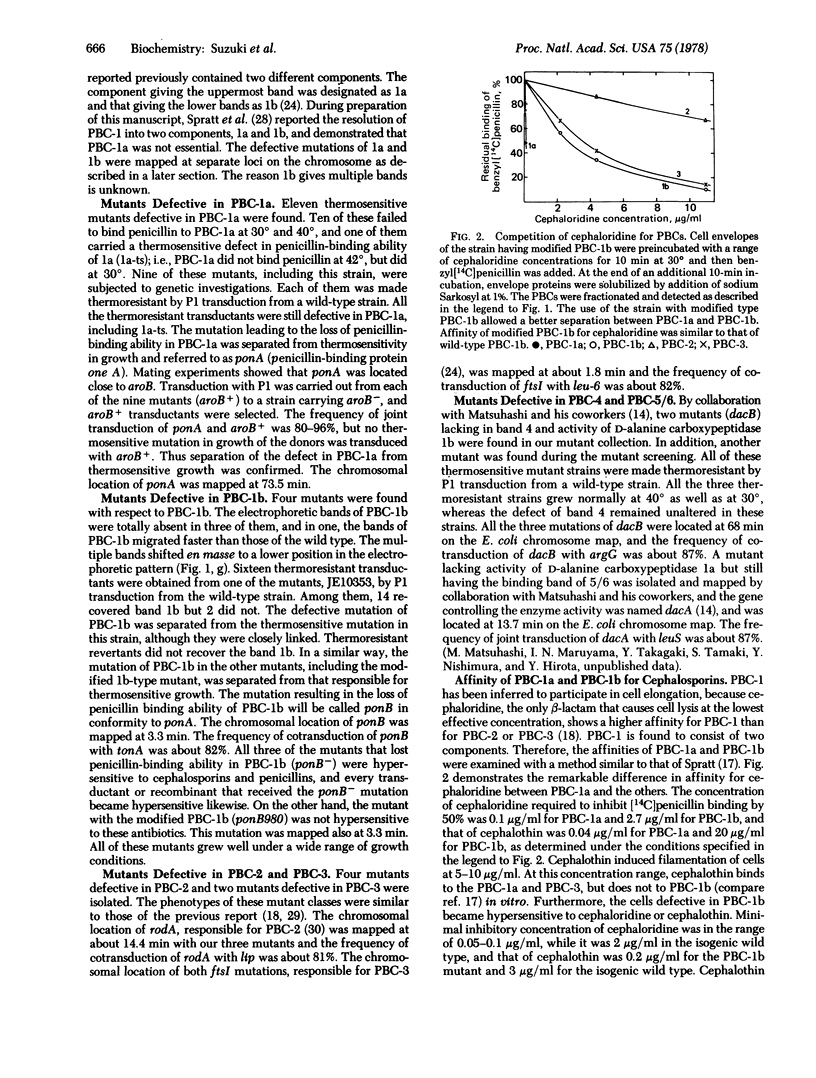
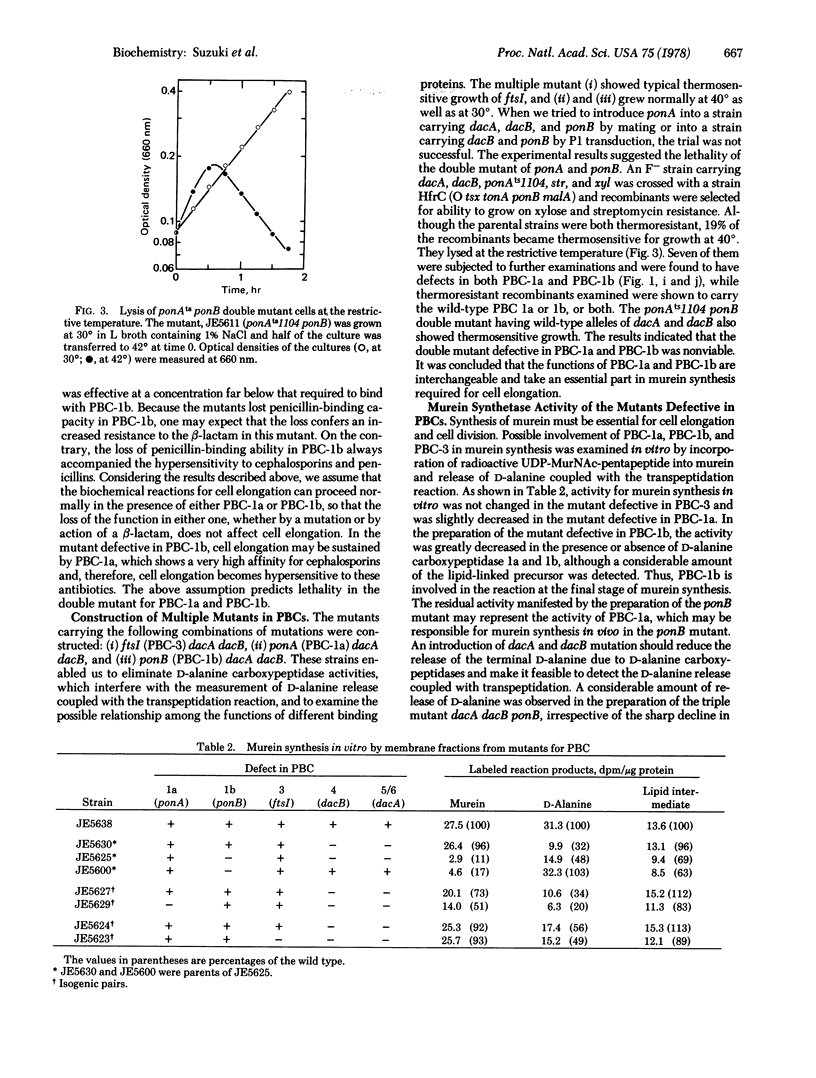
A series of mutants defective in penicillin-binding components (or protein) (PBCs) was isolated from a collection of thermosensitive mutants of Escherichia coli. The mutants included mutations for each PBC (1 through 4) and a mutation in the activity of D-alanine carboxypeptidase 1a (PBC-5/6). PBC-1 was resolved into two components, PBC-1a and PBC-1b, which were the products of different genes referred to as ponA and ponB, respectively. No mutation examined in ponA-- or ponB--, by itself, was associated with a thermosensitive defect in growth. The mutants having ponB-- were hypersensitive to cephalosporins, which showed unusually high affinity to PBC-1a. Murein synthetic activity decreased markedly in cell-free preparations from the ponB-- mutant. The double mutation ponA-- ponB-- was found to be lethal. A mutant having ponAts and ponB-- revealed thermosensitivity of growth and lysed at the restrictive temperature. It is concluded that PBC-1a and PBC-1b could share a biochemical reaction necessary for cell elongation so that the function of either one may bypass the loss of the function of the other. With the series of mutants, the chromosomal locus of the gene for each PBC was mapped: ponA (PBC-1a) 73.5 min, ponB (PBC-1b) 3.3 min, rodA (PBC-2) 14.4 min, ftsI (PBC-3) 1.8 min, dacB (PBC-4) 68 min, and dacA (PBC-5/6) 13.7 min.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Gefter M., Mindich L. A mutant of Escherichia coli defective in DNA polymerase II activity. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3238–3242. doi: 10.1073/pnas.69.11.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Strominger J. L. Simultaneous deletion of D-alanine carboxypeptidase IB-C and penicillin-binding component IV in a mutant of Escherichia coli K12. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2980–2984. doi: 10.1073/pnas.74.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIV. Purification and properties of two D-alanine carboxypeptidases from Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3193–3201. [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lazdunski C. J., Lazdunski A. M., Bourrillon M. J. Cell division in Escherichia coli: aspects of the defective gene product in a thermosensitive mutant Fts-A. Biochimie. 1973;55(10):1253–1259. doi: 10.1016/s0300-9084(74)80330-5. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Distèche M., Pollock J. J., Ghuysen J. M., Puig J., Reynolds P., Perkins H. R., Coyette J., Salton M. R. Sensitivity to ampicillin and cephalothin of enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):457–463. doi: 10.1111/j.1432-1033.1974.tb03287.x. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Takeda Y., Nishimura A., Suzuki H., Inouye M., Hirota Y. Synthetic ColE1 plasmids carrying genes for cell division in Escherichia coli. Plasmid. 1977 Nov;1(1):67–77. doi: 10.1016/0147-619x(77)90009-9. [DOI] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V. Mutants of Escherichia coli which lack a component of penicillin-binding protein 1 are viable. FEBS Lett. 1977 Jul 15;79(2):374–378. doi: 10.1016/0014-5793(77)80824-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Izaki K., Matsuhashi M., Tipper D. J. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Fed Proc. 1967 Jan-Feb;26(1):9–22. [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Wickus G. G., Strominger J. L. Penicillin-sensitive transpeptidation during peptidoglycan biosynthesis in cell-free preparations from Bacillus megaterium. I. Incorporation of free diaminopimelic acid into peptidoglycan. J Biol Chem. 1972 Sep 10;247(17):5297–5306. [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]




