Abstract
OBJECTIVE
Approximately one-third of the adult U.S. population has the metabolic syndrome. Its prevalence is the highest among Hispanic adults, but variation by Hispanic/Latino background is unknown. Our objective was to quantify the prevalence of the metabolic syndrome among men and women 18–74 years of age of diverse Hispanic/Latino background.
RESEARCH DESIGN AND METHODS
Two-stage area probability sample of households in four U.S. locales, yielding 16,319 adults (52% women) who self-identified as Cuban, Dominican, Mexican, Puerto Rican, Central American, or South American. The metabolic syndrome was defined according to the American Heart Association/National Heart, Lung, and Blood Institute 2009 Joint Scientific Statement. The main outcome measures were age-standardized prevalence of the metabolic syndrome per the harmonized American Heart Association/National Heart, Lung, and Blood Institute definition and its component abnormalities.
RESULTS
The metabolic syndrome was present in 36% of women and 34% of men. Differences in the age-standardized prevalence were seen by age, sex, and Hispanic/Latino background. The prevalence of the metabolic syndrome among those 18–44, 45–64, and 65–74 years of age was 23%, 50%, and 62%, respectively, among women; and 25%, 43%, and 55%, respectively, among men. Among women, the metabolic syndrome prevalence ranged from 27% in South Americans to 41% in Puerto Ricans. Among men, prevalences ranged from 27% in South Americans to 35% in Cubans. In those with the metabolic syndrome, abdominal obesity was present in 96% of the women compared with 73% of the men; more men (73%) than women (62%) had hyperglycemia.
CONCLUSIONS
The burden of cardiometabolic abnormalities is high in Hispanic/Latinos but varies by age, sex, and Hispanic/Latino background. Hispanics/Latinos are thus at increased, but modifiable, predicted lifetime risk of diabetes and its cardiovascular sequelae.
Introduction
Many areas of the world experience a high population burden of metabolic abnormalities collectively known as metabolic syndrome (1). Included in this syndrome are insulin resistance; adiposity; hyperglycemia; hyperlipidemia; elevated blood pressure; and a sustained, mild proinflammatory profile (1). Over the past decade, heavier U.S. adults have gained more adiposity, and ethnic disparities in BMI and waist circumference have grown (2). BMI in men and women, as well as waist circumference in men, increased linearly, but among women waist circumference increased at a steeper rate at higher percentile values (2).
In the context of a widespread obesity epidemic, the cardiometabolic abnormalities associated with excess weight and ectopic fat deposition are of considerable clinical and public health interest (3) since the metabolic syndrome confers an elevated risk of the development of type 2 diabetes and cardiovascular sequelae, and mortality (4–6). Based on a recently harmonized definition of the metabolic syndrome (7), the age-adjusted prevalence of metabolic syndrome in the U.S. is between 34 and 39%, depending on the thresholds used to define abdominal adiposity. The highest prevalence of the metabolic syndrome in the U.S. was observed among Mexican Americans in the National Health and Nutrition Examination Survey (NHANES), 1988–1994 (8). In this report, we provide estimates of the prevalence of the metabolic syndrome among U.S. Hispanic/Latino individuals who were 18–74 years of age, were from different Hispanic backgrounds, and were recruited from randomly selected households in 4 of the 10 largest Hispanic/Latino urban U.S. communities by the Hispanic Community Health Study/Study of Latinos (HCHS/SOL).
Research Design and Methods
The National Institutes of Health–supported HCHS/SOL was designed to examine the prevalence of risk factors and protective factors for chronic diseases, and their association with the incidence of newly developed disease among Hispanic/Latinos (http://www.cscc.unc.edu/hchs/). The HCHS/SOL design and sampling methods have been published (9). Between March 2008 and June 2011, 16,415 self-identified Hispanic/Latino persons 18–74 years of age were recruited from randomly selected households in the Bronx, New York; San Diego, California; Chicago, Illinois; and Miami, Florida. The study was designed to include participants from Cuban, Dominican, Mexican, Puerto Rican, Central American, and South American backgrounds in pre-established proportions. Households were chosen using a stratified two-stage area probability sample design. Census block groups were randomly selected in specified geographic areas of each study site, and households were randomly selected in each sample block group. Households were screened for eligibility, and self-identified Hispanic/Latino persons 18–74 years of age were selected in each household. Oversampling occurred at each stage (block groups in areas of high concentration of Hispanic/Latinos, households associated with an Hispanic/Latino surname, and persons 45–74 years of age at were sampled at rates higher than younger household members). Sampling weights that reflect the probabilities of selection at each stage were used in the statistical analyses. Approval by institutional review boards was obtained at each participating institution, and written informed consent was obtained from all study participants.
Study Measurements
All examinations and interviewer-administered questionnaires were conducted by centrally trained and certified study personnel following a standardized protocol, which included ongoing quality assurance procedures. All study protocol manuals are available at http://www.cscc.unc.edu/hchs/. Study participants were asked to fast and to abstain from smoking 12 h prior to the examination and also to avoid vigorous physical activity on the morning of the examination. Body weight was measured to the nearest 0.1 kg, and height was recorded to the nearest centimeter. Abdominal girth was measured in duplicate using standardized reference points. Three seated blood pressure measurements were obtained after a 5-min rest using an oscillometric automated sphygmomanometer. The average of the second and third measurements was used in these analyses.
Blood samples were obtained following a nontraumatic venipuncture protocol; fresh as well as frozen specimens were shipped to the HCHS/SOL Central Laboratory for assays and long-term storage. HDL cholesterol (HDL-C) was measured by a magnesium/dextran sulfate method, and plasma glucose was measured using a hexokinase enzymatic method (Roche Diagnostics, Indianapolis, IN). Triglycerides were measured in serum on a Roche Modular P chemistry analyzer, using a glycerol blanking enzymatic method (Roche Diagnostics). The assay methodologies and their performance are described in HCHS/SOL Manual 7 (Addendum; at http://www.cscc.unc.edu/hchs/public/docfilter.php?study=hchs&filter_type=public).
Participants were asked to bring all prescription and nonprescription medications and supplements taken during the preceding 4 weeks to the examination, where all preparations, and their concentrations and units were coded. Interviewer-administered questionnaires were used to obtain information on demographic factors, education and income, country of origin and generational status, length of residence in the U.S., and language preference.
The metabolic syndrome was defined according to the American Heart Association/ National Heart, Lung, and Blood Institute 2009 Joint Scientific Statement (7), namely, subjects had to have three or more of the following criteria: 1) waist circumference ≥102 cm in men and ≥88 cm in women; 2) triglyceride level ≥150 mg/dL; 3) HDL-C level <40 mg/dL in men and <50 mg/dL in women; 4) blood pressure ≥130 mmHg systolic and/or ≥85 mmHg diastolic, and/or the subject was receiving medication; and 5) fasting glucose level ≥100 mg/dL and/or the subject was receiving medication.
Statistical Analysis
Summary statistics and their variances were weighted to adjust for sampling probability and nonresponse (9). Means, medians, and prevalence estimates were computed by sex and Hispanic/Latino background, and were age-standardized to the year 2010 U.S. population. The frequency of the component abnormalities of the metabolic syndrome also was determined. All statistical tests were two-sided at a significance level of 0.05. Analyses were performed using SAS version 9.2 (SAS Institute) and SUDAAN release 10.0.0 (RTI).
Results
Of the 39,384 sampled individuals who met eligibility criteria and were selected, 41.7% enrolled in the study. After the exclusion of individuals with missing/underreported Hispanic background (n = 81) or missing covariates (n = 48), 9,789 women and 6,530 men were available for these analyses. As shown in Table 1, the mean baseline age was 41.1 years and was comparable for all Hispanic/Latino groups. Individuals of Mexican origin represented close to 40% of all sampled individuals, followed by those of Puerto Rican (17%), Cuban (14%), Central American (11%), Dominican (9%), and South American (7%) origin. Consistent with national surveys (10), 77% of the HCHS/SOL participants were overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥30 kg/m2).
Table 1.
Characteristics of the 16,319 HCHS/SOL study participants included in this report (HCHS/SOL baseline examination, 2008–2011)
| Characteristics | Values |
|---|---|
| Age, years (mean) (SE) | 41.1 (0.25) |
| Women (%) | 52.2 ± 0.6 |
| Hispanic/Latino background, N (%)* | |
| Dominican | 1,457 (8.9) |
| Central American | 1,725 (10.5) |
| Cuban | 2,343 (14.3) |
| Mexican | 6,451 (39.3) |
| Puerto Rican | 2,702 (16.5) |
| South American | 1,063 (6.5) |
| Mixed/other | 497 (3.0) |
| BMI (%)† | |
| Underweight/normal (<25 kg/m2) | 23.1 ± 0.5 |
| Overweight (25–29.9 kg/m2) | 37.1 ± 0.6 |
| Obese (≥30 kg/m2) | 39.6 ± 0.7 |
| Missing information | 0.2 ± 0.05 |
| Years in the U.S. >15 years (%) | 57.0 ± 1.1 |
| Not born in the 50 U.S. states (%) | 77.3 ± 0.8 |
| Preference for Spanish (%) | 74.9 ± 0.9 |
| Education (%) | |
| <High school | 31.9 ± 0.7 |
| High school | 27.9 ± 0.6 |
| >High school | 38.4 ± 0.8 |
| Missing information | 1.8 ± 0.1 |
Data are N (%) or % ± SE, unless otherwise indicated.
*Unweighted proportions; all other values displayed in this report are weighted for survey design and nonresponse.
†A total of 248 men (3.7%) and 732 women (7.4%) had BMI values >40 kg/m2. A total of 130 individuals had BMI values <18.5 kg/m2 (<1%).
Approximately 77% of the participants were not born in the U.S. Although 57% of the participants had lived in the U.S. >15 years, 75% indicated a preference for conducting the interviews in Spanish. About 32% of the participants had less than a high school education, 28% had completed high school, and 38% had more than a high school education. Educational achievement did not differ by Hispanic background.
The age-standardized prevalence of the metabolic syndrome was 33.7% (95% CI 32.2–35.2%) in men and 36.0% (95% CI 34.6–37.4%) in women (Table 2). The prevalence of the metabolic syndrome increased steadily with age overall and in both men and women, although a greater increase with age was seen in women (P value for interaction 0.004). Variability in the prevalence of the metabolic syndrome was seen by Hispanic/Latino background and by sex (Table 2). The overall prevalence of the metabolic syndrome was highest among Puerto Ricans (37%, not statistically significantly different from other groups among the men), and significantly (P < 0.05) lower among South Americans (27%) compared with other Hispanic/Latino backgrounds overall and in women. The prevalence of the metabolic syndrome was significantly (P < 0.005) higher in Puerto Rican women compared with Puerto Rican men.
Table 2.
Age-standardized prevalence of the metabolic syndrome by Hispanic/Latino background and sex, 2008–2011
| Characteristics | All participants (N = 16,319) | Men (N = 6,530) | Women (N = 9,789) |
|---|---|---|---|
| Overall | 35.0 (34.0–36.1) | 33.7 (32.2–35.2) | 36.0 (34.6–37.4) |
| Hispanic/Latino background | |||
| Dominican (n = 1,457) | 31.5 (29.0–34.0) | 30.6 (26.3–35.2) | 32.2 (28.9–35.8) |
| Central American (n = 1,725) | 35.8 (33.0–38.7) | 32.6 (28.5–36.9) | 37.7 (34.7–40.8) |
| Cuban (n = 2,343) | 34.8 (32.6–37.0) | 34.7 (31.9–37.6) | 34.9 (32.0–37.9) |
| Mexican (n = 6,451) | 35.0 (33.2–36.9) | 33.7 (31.3–36.2) | 36.0 (33.5–38.6) |
| Puerto Rican (n = 2,702) | 37.1 (34.4–39.9) | 32.6 (28.7–36.8) | 40.9 (37.4–44.6)* |
| South American (n = 1,063) | 27.3 (24.2–30.7)† | 27.0 (22.3–32.4) | 26.8 (23.1–30.9)‡ |
| Age-groups (years) | |||
| 18–29 (n = 2,644) | 12.7 (11.1–14.4) | 12.9 (10.8–15.3) | 12.4 (10.3–14.9) |
| 30–39 (n = 2,375) | 24.7 (22.5–27.1) | 27.1 (23.6–30.9) | 22.4 (19.5–25.7) |
| 40–49 (n = 4,194) | 36.7 (34.5–39.0) | 36.1 (32.9–39.4) | 37.3 (34.5–40.1) |
| 50–59 (n = 4,323) | 48.6 (45.9–51.4) | 44.8 (41.3–48.4) | 51.6 (48.2–55.1) |
| 60–69 (n = 2,283) | 56.8 (53.8–59.8) | 52.3 (47.7–56.9) | 60.6 (56.3–64.7) |
| 70–74 (n = 500) | 66.6 (60.3–72.3) | 58.0 (49.6–65.9) | 72.0 (63.5–79.3) |
Data are % (95% CI).
Values were weighted for survey design and nonresponse, and were age-standardized to the population described by the 2010 U.S. Census.
*Statistically significant differences (P < 0.05) were seen between sexes.
†Statistically significant differences (P < 0.05) were seen among Hispanic/Latino backgrounds overall.
‡Statistically significant differences (P < 0.05) were seen among women.
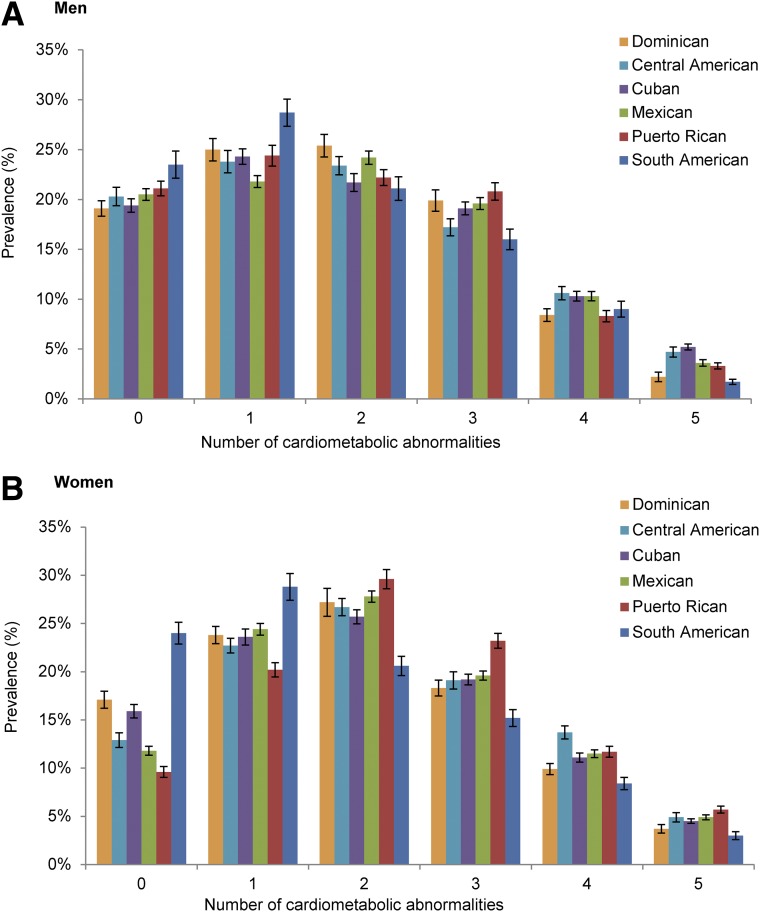
Figure 1 displays the prevalence of the number of individual cardiometabolic abnormalities of the metabolic syndrome by sex and Hispanic/Latino background. Overall, South Americans and Dominicans had the lowest prevalence of individual abnormalities (and thus of the metabolic syndrome). Puerto Rican women had a higher burden of metabolic abnormalities compared with Puerto Rican men and with women of other Hispanic/Latino background groups (Fig. 1). South American men had the lowest prevalence of metabolic syndrome abnormalities. Among women, the prevalence of zero, one, two, three, four, and five cardiometabolic abnormalities was 13.5%, 23.3%, 27.3%, 19.9%, 11.3%, and 4.6%, respectively. The corresponding prevalences among men were 20.7%, 22.9%, 22.9%, 19.6%, 10.0%, and 3.8%, respectively.
Figure 1.
Prevalence (%) of the number of individual cardiometabolic abnormalities in men (A) and women (B) in the HCHS/SOL cohort, by Hispanic/Latino background. Error bars represent the SE.
A characteristic common to both sexes is the strong association between the number of cardiometabolic abnormalities and age. The prevalence of (all) five cardiometabolic abnormalities was 0.2% in women 18–29 years of age, 10% in women 60–69 years of age, and 14% in women 70–74 years of age. Conversely, the prevalence of zero cardiometabolic abnormalities was 27% in women 18–29 years of age, 2% in women 60–69 years of age, and 0.5% in women 70–74 years of age. This pattern was comparable in men in that the prevalence of the five cardiometabolic abnormalities was 0.8% in men 18–29 years of age, 8% in men 60–69 years of age, and 10% in men 70–74 years of age. However, unlike women, the prevalence of zero cardiometabolic abnormalities was 43% in men 18–29 years of age, 6% in men 60–69 years of age, and 8% in men 70–74 years of age.
The profile of the cardiometabolic abnormalities among Hispanic/Latinos differed by sex. The most prevalent component of the metabolic syndrome in women was abdominal obesity irrespective of age group (Supplementary Table 1). Among women, the prevalence of low HDL-C level was higher in those 18–44 years of age, whereas the prevalences of elevated blood pressure and hyperglycemia were higher in those 75–74 years of age. Prevalences were higher for hypertriglyceridemia and low HDL-C level in men 18–44 years of age, whereas prevalences were higher for elevated blood pressure and hyperglycemia in men 45–64 and 75–74 years of age (Supplementary Table 2). The degree to which the median value of an individual risk factor was elevated relative to the threshold was greater with increasing age and with the number of risk factors, in men and in women (Supplementary Tables 1 and 2).
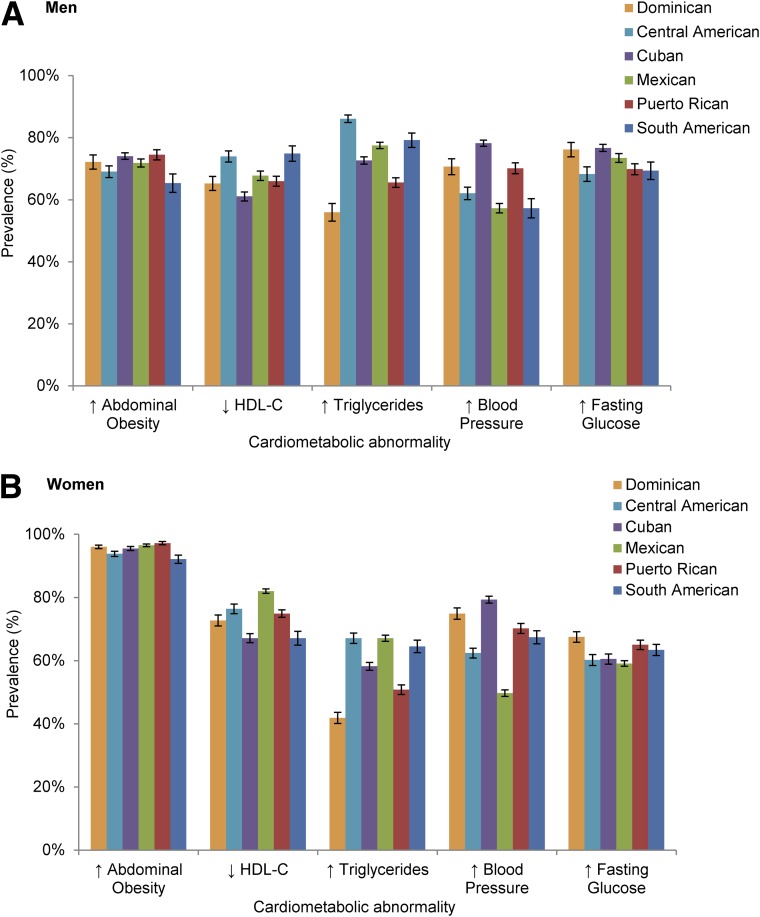
Among participants with the metabolic syndrome, 73% of the men and 96% of the women had abdominal obesity (exceeding waist circumference thresholds of 102 and 88 cm, respectively); 73% of men and 59% of women had hypertriglyceridemia; 67% of men and 75% of women had low HDL-C levels; 66% of men and 64% of women had elevated blood pressure or were receiving antihypertensive treatment; and 73% of men and 62% of women had hyperglycemia or were receiving hypoglycemic agents. Low HDL-C values were remarkably common in women 18–44 years of age with the metabolic syndrome, while both elevated triglyceride levels and low HDL-C levels were higher among the men with metabolic syndrome who were >65 years of age. Some variability in the prevalence of individual cardiometabolic abnormalities across Hispanic/Latino backgrounds was observed (Fig. 2). Among men with the metabolic syndrome, Dominicans had the lowest prevalence of low HDL-C compared with men of other Hispanic/Latino backgrounds, except Puerto Ricans. Among women with the metabolic syndrome, the prevalence of abdominal adiposity was high regardless of Hispanic/Latino background. Dominican women had the lowest prevalence of hypertriglyceridemia compared with other Hispanic/Latino backgrounds. Mexican women had the highest prevalence of hypertriglyceridemia compared with women of other Hispanic/Latino backgrounds, except for South Americans, and had the lowest prevalence of elevated blood pressure compared with women of other Hispanic/Latino backgrounds.
Figure 2.
Prevalence (%) of individual cardiometabolic abnormalities in men (A) and women (B) with the metabolic syndrome in the HCHS/SOL cohort, by Hispanic/Latino background. Error bars represent the SE.
Conclusions
Hispanics/Latinos, who are the largest U.S. minority group, experience a high burden of cardiovascular risk factors (11). Obesity, the metabolic syndrome, and diabetes have been found to be prevalent in Latinos/Hispanics at alarming rates, but limitations in the available data, combined with public health concerns led to recommendations for additional research in Hispanics/Latinos to understand the risk profile of this population (12). We add to this body of information by describing the prevalence of metabolic syndrome in its harmonized definition (7) among U.S. Hispanic/Latino adults of diverse backgrounds.
The prevalence of the metabolic syndrome in the population sampled by the HCHS/SOL was 34% in men and 36% in women, which is comparable to reports based on national probability samples indicating a higher frequency of occurrence in U.S. Hispanics than in whites (10,13). Among HCHS/SOL participants, 21% of men and 14% of women had no cardiometabolic abnormalities, 34% of men and 36% of women had three or more cardiometabolic abnormalities, and 3.8% of men and 4.6% of women had five or more abnormalities.
The remarkable features in these data are the high proportion of women who meet the metabolic syndrome criterion of three or more factors in each age stratum by virtue of exceeding the threshold value for abdominal girth, the high median values of waist circumference observed, and the progressively larger increments in median waist values across increasing numbers of risk factors present. This suggests that abdominal adiposity is the salient contributor to the metabolic syndrome among the women in the HCHS/SOL, to a greater degree than, for example, elevated blood pressure or the impairments in lipid or glucose metabolism that are often associated with the former. A limitation of these data is the low response at the level of the sampled households, which was 33.5%. All estimates are adjusted for nonresponse (9).
As is the case for much of the existing research on the health status of Hispanic/Latino groups in the U.S., most previous reports on the metabolic syndrome among Hispanics are based on Mexican Americans or a pooled heterogeneous group of Hispanics/Latinos. Although limited by small numbers, a prior report (14) identified heterogeneity in the frequency of the metabolic syndrome and its components in women by Hispanic/Latino background. As also observed among the women in the HCHS/SOL, the prevalence of metabolic syndrome in the New Jersey site of the Study of Women’s Health Across the Nation was greatest in Puerto Rican women (48%) and was lowest in Dominican women at this Study of Women’s Health Across the Nation site (13%), although the SEs for these estimates were rather large. Similar patterns were observed in the Multi-Ethnic Study of Atherosclerosis, in that Dominican men and women had a lower prevalence of the metabolic syndrome than Puerto Ricans (15).
The Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA) study (16) compared the prevalence of the metabolic syndrome in residents 25–64 years of age (average age 45 ± 11 years) in seven Latin American cities between 2003 and 2005 (∼1,600 examinees per city). The prevalence of the metabolic syndrome defined according to the Adult Treatment Panel III criteria, age- and sex-adjusted to the sample from each city, ranged from 14% in Quito, Ecuador, to 27% in Mexico City, Mexico. For comparison, use of the Adult Treatment Panel III criteria in the 2003–2006 NHANES showed a prevalence of 33% among Mexican American men and 41% among Mexican American women (13). Among the sites surveyed by the CARMELA study, Mexico City had the highest prevalence of obesity (31%), the metabolic syndrome (27%), and diabetes (9%). The prevalence of the metabolic syndrome observed in the HCHS/SOL (34% in men and 36% in women) is somewhat higher than that estimated by the CARMELA study for Mexico City, and was notoriously higher than those observed in Barquisimeto, Venezuela; Bogota, Colombia; Buenos Aires, Argentina; Lima, Peru; Quito, Ecuador; and Santiago, Chile.
Other noteworthy findings from the HCHS/SOL population are that 96% of women and 73% of men with the metabolic syndrome had abdominal obesity using the conventional 102/88 cm threshold. The International Diabetes Federation conceived of the metabolic syndrome based on waist circumference thresholds that differ for race and ethnic groups, and are considerably lower than those originally used in the National Cholesterol Education Program criteria. In its 2012 scientific statement on health disparities in endocrine disorders (17), the Endocrine Society called for the study and adoption of ethnic-specific cut points for central obesity, to avoid misclassification and for appropriate risk management. A number of reports (18–20) have raised concerns about the threshold values for waist circumference used by the current definitions of abdominal obesity, particularly as applied to Asian, African American, Polynesian, and Hispanic/Latino populations. The prevalence of the metabolic syndrome according to different waist circumference thresholds has been published based on NHANES 2003–2006 data (10). When less restrictive definitions of central obesity were used, prevalence estimates increased as expected, whereas associations with cardiometabolic correlates were not visibly affected.
Recognizing that the risk associated with a given waist measurement differs across populations, waist circumference thresholds recommended for ethnic Central and South American populations in the 2009 consensus Joint Scientific Statement (11) are ≥90 cm for men and ≥80 cm for women. Instead, the data presented in this report are based on the waist circumference thresholds familiar to clinical practitioners in the U.S. (≥102 cm in men and ≥88 cm in women), and are used as a common metric in reports from the NHANES (15). For comparability with studies based in other countries, we replicated our analyses using the ≥90 cm/≥80 cm thresholds for men and women, respectively, recommended by the consensus Joint Scientific Statement (11) for ethnic Central and South American populations (these data are presented in Supplementary Table 3).
There have been several attempts to establish waist circumference cutoff values for abdominal obesity suitable to women in Latin America, drawing on various criteria such as detection of diabetes (21), abnormal carotid artery intima media thickness (22), blood lipid profile and other risk factors (23), and hypertension (24). Based on an area of visceral adipose tissue ≥100 cm2 measured by computed tomography scan at the 5th lumbar vertebra, Aschner et al. (25) recommended 94 cm for men and 90 cm for women as the threshold of abdominal obesity. We examined the impact of waist circumference threshold values recommended by various authors for Hispanic/Latino populations on the prevalence of the metabolic syndrome in the HCHS/SOL populations. Overall, their impact on the prevalence of the metabolic syndrome in women in the HCHS/SOL was minor. For example, the use of the 90 cm threshold (instead of 88 cm), as recommended by Aschner et al. (25), reduced the prevalence of the metabolic syndrome in the women of the HCHS/SOL samples by only 1–2%.
Various names and definitions have been applied to the metabolic syndrome since its original description by Reaven (26) as a cluster of metabolic risk factors related to insulin resistance. There is consensus at this time that insulin resistance underlies the clustering of metabolic syndrome abnormalities, and that these are associated with an increased risk of type 2 diabetes and cardiovascular sequelae (7). Such associations are well-established for the individual components of this syndrome, regardless of whether they occur in isolation, in combination, or as a qualitatively defined syndrome based on any three cardiometabolic abnormalities. Although the metabolic syndrome is embedded in clinical management guidelines, there is no consensus about the value of the metabolic syndrome as a tool to screen for future risk of type 2 diabetes or cardiovascular diseases. Whether the cardiometabolic risk factors that constitute the metabolic syndrome occur alone or in clusters, evidence indicates that all such risk factors should be addressed individually, and managed effectively (27). To our knowledge, no evidence has been put forward to date for nonadditivity of these metabolic factors on the risk of type 2 diabetes, incident cardiovascular disease, or mortality (i.e., that the risk associated with the metabolic syndrome exceeds the risk conferred by the sum of the individual cardiometabolic risk factors that contributes to the syndrome) (27–29).
To aid in the interpretation of waist circumference, Lemieux et al. (30) proposed the concurrent measurement of fasting triglycerides as an inexpensive means to screen for the atherogenic metabolic dysregulation triad characterized by hyperinsulinemia, elevated apolipoprotein B level, and small, dense LDL-C. In the HCHS/SOL data, fasting triglyceride levels, HDL-C levels, systolic blood pressure, and fasting glucose levels each were associated with waist circumference in a monotonically increasing (graded) linear fashion, without indications of an inflection point or a threshold for waist circumference (data not shown).
As a qualitative approach to the characterization of cardiometabolic abnormalities associated with insulin resistance, the metabolic syndrome has been endorsed by major professional and scientific organizations (7). Although the Endocrine Society endorsed the use of the metabolic syndrome in clinical practice guidelines as a tool for primary prevention of type 2 diabetes and cardiovascular disease, its relevance still is subject to disagreement (31). The lack of management or therapies specific to this syndrome—as opposed to its individual component abnormalities—makes its use in clinical settings counterintuitive, and the need for sex-, race-, and ethnic group-specific thresholds for abdominal adiposity for a “universal” definition of the metabolic syndrome makes this construct susceptible to misclassification and remains a source of controversy (25).
The metabolic syndrome traits are known to have high heritability (30–70%), and candidate gene approaches, linkage studies, and genome-wide association studies (32) have identified susceptibility regions and loci for individual metabolic syndrome components. The heritability of the metabolic syndrome is reportedly 30%, although little of this heritability has been accounted for (33). Evidence for a common genetic underpinning of the broad spectrum of the metabolic syndrome has not been forthcoming, despite reports from genome-wide association studies of genetic variants associated with more than one of the metabolic abnormalities included in the metabolic syndrome (34). Although well-conducted studies failed to identify significant genetic susceptibility to multiple metabolic syndrome components (35), the modulation of metabolic syndrome expression by gene × environment interaction—such as gene × energy expenditure interaction (36)—warrants further study.
The clustering of metabolic impairments and the observed temporal trends in the prevalence of the metabolic syndrome are thought to result from excess food consumption and/or reduced levels of physical activity. Excess nutrient intake leads to adiposity and activation of stress signaling, which in turn results in chronic activation of proinflammatory kinase pathways that desensitize the metabolic response to insulin (37). Development of the metabolic syndrome in humans is also thought to be promoted by high levels of saturated fats, supported by animal models where high-fat diets induced metabolic disease (38). Recent work has highlighted the role of intestinal microbiota in promoting the cardiometabolic abnormalities associated with the metabolic syndrome, such as adiposity, by increasing the capacity of the host to extract energy from ingested food (39), or through interaction with the innate immune system in modulating inflammatory signaling (40). Murine models suggest that the loss of Toll-like receptor 5 function in the intestinal mucosa changes gut microbiota that induce low-grade inflammatory signaling, which may desensitize insulin receptor signaling, leading to excess food consumption and the associated cardiometabolic abnormalities of the metabolic syndrome (40). The excess caloric consumption thought to drive the current epidemic in metabolic syndrome may therefore be influenced in part by host-microbiota interactions.
In conclusion, the prevalence of the metabolic syndrome, and that of the cardiometabolic abnormalities that are considered to be components of the metabolic syndrome, is high in Hispanic/Latinos and varies by sex and across Hispanic/Latino backgrounds. Abdominal adiposity predominates in Hispanic/Latino women; in men, the characterization by Hispanic/Latino backgrounds shows heterogeneity in the profiles of the component cardiometabolic abnormalities. The prevention of metabolic abnormalities and their clinical management may benefit from awareness of the diversity in cardiometabolic dysregulation by sex and Hispanic/Latino background. This is reinforced by the lack of prevention policies or clinical management guidelines that are specific to the metabolic syndrome per se, as opposed to its component factors. Efforts to control the population burden of cardio-metabolic risk among Hispanics/Latinos will benefit from the observed differences by sex and Hispanic/Latino backgrounds.
Supplementary Material
Article Information
Acknowledgments. The authors thank the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) participants, who generously contributed their time and provided the study data. The authors also thank the HCHS/SOL staff members for their dedication and expertise. A complete list of staff and investigators was published in the Annals of Epidemiology 2010;20:642–649 and is also available on the study website (http://www.cscc.unc.edu/hchs).
Funding. The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), the University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes, centers, or offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Center on Minority Health and Health Disparities, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements.
The funding agency had a role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; and in the review and approval of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Authors Contributions. G.H. researched the literature, interpreted the data, and organized and wrote the manuscript. M.L.S., N.S., M.M.L., C.C., M.C., R.K., A.G., L.G., L.L., and L.A.-S. contributed to the interpretation of the data and to the writing of the manuscript, and were involved in editing during manuscript preparation. Y.T. conducted the statistical analyses, provided comments, and had oversight of the verification of results. G.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. A set of preliminary data from this study was presented at the Epidemiology and Prevention/Nutrition, Physical Activity, and Metabolism 2013 Scientific Sessions of the American Heart Association/American Stroke Association, New Orleans, LA, 19–22 March 2013.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-2505/-/DC1.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official position of the National Institutes of Health, the U.S. Department of Health and Human Services, or the federal government.
References
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med 2007;356:213–215 [DOI] [PubMed] [Google Scholar]
- 2.Beydoun MA, Wang Y. Gender-ethnic disparity in BMI and waist circumference distribution shifts in US adults. Obesity (Silver Spring) 2009;17:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 2005;352:1138–1145 [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 2008;31:1898–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballantyne CM, Hoogeveen RC, McNeill AM, et al. Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC study. Int J Obes (Lond) 2008;32(Suppl. 2):S21–S24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709–2716 [DOI] [PubMed] [Google Scholar]
- 7.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. National Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–359 [DOI] [PubMed] [Google Scholar]
- 9.Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes 2010;2:180–193 [DOI] [PubMed] [Google Scholar]
- 11.Daviglus ML, Talavera GA, Avilés-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA 2012;308:1775–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson JA, Kannel WB, Lopez-Candales A, et al. Avoiding the looming Latino/Hispanic cardiovascular health crisis: a call to action. Ethn Dis 2007;17:568–573 [PubMed] [Google Scholar]
- 13.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Rep 2009:1–7 [PubMed]
- 14.Derby CA, Wildman RP, McGinn AP, et al. Cardiovascular risk factor variation within a Hispanic cohort: SWAN, the Study of Women’s Health Across the Nation. Ethn Dis 2010;20:396–402 [PMC free article] [PubMed] [Google Scholar]
- 15.Allison MA, Budoff MJ, Wong ND, Blumenthal RS, Schreiner PJ, Criqui MH. Prevalence of and risk factors for subclinical cardiovascular disease in selected US Hispanic ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2008;167:962–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schargrodsky H, Hernández-Hernández R, Champagne BM, et al. CARMELA Study Investigators CARMELA: assessment of cardiovascular risk in seven Latin American cities. Am J Med 2008;121:58–65 [DOI] [PubMed] [Google Scholar]
- 17.Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab 2012;97:E1579–E1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katzmarzyk PT, Bray GA, Greenway FL, et al. Ethnic-specific BMI and waist circumference thresholds. Obesity (Silver Spring) 2011;19:1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palaniappan LP, Wong EC, Shin JJ, Fortmann SP, Lauderdale DS. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond) 2011;35:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumner AE, Sen S, Ricks M, Frempong BA, Sebring NG, Kushner H. Determining the waist circumference in African Americans which best predicts insulin resistance. Obesity (Silver Spring) 2008;16:841–846 [DOI] [PubMed] [Google Scholar]
- 21.Barbosa PJ, Lessa I, de Almeida Filho N, Magalhães LB, Araújo J. Criteria for central obesity in a Brazilian population: impact on metabolic syndrome. Arq Bras Cardiol 2006;87:407–414 [DOI] [PubMed] [Google Scholar]
- 22.Medina-Lezama J, Pastorius CA, Zea-Diaz H, et al. PREVENCION Investigators Optimal definitions for abdominal obesity and the metabolic syndrome in Andean Hispanics: the PREVENCION study. Diabetes Care 2010;33:1385–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez M, Casas JP, Cubillos-Garzon LA, et al. Using waist circumference as a screening tool to identify Colombian subjects at cardiovascular risk. Eur J Cardiovasc Prev Rehabil 2003;10:328–335 [DOI] [PubMed] [Google Scholar]
- 24.Sánchez-Castillo CP, Velázquez-Monroy O, Berber A, Lara-Esqueda A, Tapia-Conyer R, James WP, Encuesta Nacional de Salud (ENSA) 2000 Working Group Anthropometric cutoff points for predicting chronic diseases in the Mexican National Health Survey 2000. Obes Res 2003;11:442–451 [DOI] [PubMed] [Google Scholar]
- 25.Aschner P, Buendía R, Brajkovich I, et al. Determination of the cutoff point for waist circumference that establishes the presence of abdominal obesity in Latin American men and women. Diabetes Res Clin Pract 2011;93:243–247 [DOI] [PubMed] [Google Scholar]
- 26.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 27.Kahn R, Buse J, Ferrannini E, Stern M, American Diabetes Association. European Association for the Study of Diabetes The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005;28:2289–2304 [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 2008;28:629–636 [DOI] [PubMed] [Google Scholar]
- 29.Bruno G, Fornengo P, Segre O, et al. What is the clinical usefulness of the metabolic syndrome? The Casale Monferrato study. J Hypertens 2009;27:2403–2408 [DOI] [PubMed] [Google Scholar]
- 30.Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 2000;102:179–184 [DOI] [PubMed] [Google Scholar]
- 31.Rosenzweig JL, Ferrannini E, Grundy SM, et al. Endocrine Society Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3671–3689 [DOI] [PubMed] [Google Scholar]
- 32.Aguilera CM, Olza J, Gil A. Genetic susceptibility to obesity and metabolic syndrome in childhood. Nutr Hosp 2013;28(Suppl. 5):44–55 [DOI] [PubMed] [Google Scholar]
- 33.Benyamin B, Sørensen TI, Schousboe K, Fenger M, Visscher PM, Kyvik KO. Are there common genetic and environmental factors behind the endophenotypes associated with the metabolic syndrome? Diabetologia 2007;50:1880–1888 [DOI] [PubMed] [Google Scholar]
- 34.Dastani Z, Hivert MF, Timpson N, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet 2012;8:e1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristiansson K, Perola M, Tikkanen E, et al. Genome-wide screen for metabolic syndrome susceptibility loci reveals strong lipid gene contribution but no evidence for common genetic basis for clustering of metabolic syndrome traits. Circ Cardiovasc Genet 2012;5:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos DM, Katzmarzyk PT, Diego VP, et al. Genotype by energy expenditure interaction with metabolic syndrome traits: the Portuguese healthy family study. PLoS One 2013;8:e80417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 2008;8:923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57:1470–1481 [DOI] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031 [DOI] [PubMed] [Google Scholar]
- 40.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010;328:228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.