Abstract
OBJECTIVE
To compare the efficacy and safety of two doses of once-weekly dulaglutide, a glucagon-like peptide 1 receptor agonist, to sitagliptin in uncontrolled, metformin-treated patients with type 2 diabetes. The primary objective was to compare (for noninferiority and then superiority) dulaglutide 1.5 mg versus sitagliptin in change from baseline in glycosylated hemoglobin A1c (HbA1c) at 52 weeks.
RESEARCH DESIGN AND METHODS
This multicenter, adaptive, double-blind, parallel-arm study randomized patients (N = 1,098; mean baseline age 54 years; HbA1c 8.1% [65 mmol/mol]; weight 86.4 kg; diabetes duration 7 years) to dulaglutide 1.5 mg, dulaglutide 0.75 mg, sitagliptin 100 mg, or placebo (placebo-controlled period up to 26 weeks). The treatment period lasted 104 weeks, with 52-week primary end point data presented.
RESULTS
The mean HbA1c changes to 52 weeks were (least squares mean ± SE): −1.10 ± 0.06% (−12.0 ± 0.7 mmol/mol), −0.87 ± 0.06% (9.5 ± 0.7 mmol/mol), and −0.39 ± 0.06% (4.3 ± 0.7 mmol/mol) for dulaglutide 1.5 mg, dulaglutide 0.75 mg, and sitagliptin, respectively. Both dulaglutide doses were superior to sitagliptin (P < 0.001, both comparisons). No events of severe hypoglycemia were reported. Mean weight changes to 52 weeks were greater with dulaglutide 1.5 mg (−3.03 ± 0.22 kg) and dulaglutide 0.75 mg (−2.60 ± 0.23 kg) compared with sitagliptin (−1.53 ± 0.22 kg) (P < 0.001, both comparisons). The most common gastrointestinal treatment-emergent adverse events in dulaglutide 1.5- and 0.75-mg arms were nausea, diarrhea, and vomiting.
CONCLUSIONS
Both dulaglutide doses demonstrated superior glycemic control versus sitagliptin at 52 weeks with an acceptable tolerability and safety profile.
Introduction
The American Diabetes Association and European Association for the Study of Diabetes recommend metformin for initial drug treatment of type 2 diabetes (1). If alternative or combination therapy is necessary, other agents such as sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, or insulin may be used (1). DPP-4 inhibitors and GLP-1 receptor agonists are being prescribed with increasing frequency in these patients (1).
Both GLP-1 receptor agonists and DPP-4 inhibitors act via the GLP-1 pathway by increasing the interaction of native GLP-1, or a respective receptor agonist, with the GLP-1 receptor. Treatment with GLP-1 receptor agonists is based on subcutaneous administration of exogenous peptides with different degrees of structural homology with native GLP-1. Oral DPP-4 inhibitors increase concentrations of endogenous GLP-1 by inhibiting the rapid inactivation by the DPP-4 protease (1,2). Increased availability of GLP-1 or a receptor agonist stimulates insulin secretion in a glucose-dependent manner and inhibits glucagon secretion (3,4). GLP-1 receptor agonists also slow gastric emptying and suppress appetite, actions that can contribute to overall glucose-lowering potency (1). In recently reported trials, GLP-1 receptor agonists and the DPP-4 inhibitor sitagliptin have been compared over a 26-week treatment period (5,6). The results of these studies indicate greater glucose- and body weight–lowering effects of GLP-1 receptor agonists. Additional data from longer duration trials are important to confirm these observations and further characterize safety profiles of these agents.
Dulaglutide is a long-acting human GLP-1 receptor agonist in development as a once-weekly subcutaneous injection for the treatment of type 2 diabetes (7,8). The molecule consists of two identical, disulfide-linked chains, each containing an N-terminal GLP-1 analog sequence covalently linked to a modified human immunoglobulin G4 heavy chain by a small peptide linker (7). In contrast to native GLP-1, dulaglutide is resistant to degradation by DPP-4 and has a large size that slows absorption and reduces renal clearance. These molecular features result in a soluble formulation and a prolonged half-life of ∼5 days, making it suitable for once-weekly subcutaneous administration (7). Dulaglutide exhibits GLP-1–mediated effects, including glucose-dependent potentiation of insulin secretion, inhibition of glucagon secretion, delay of gastric emptying, and weight loss (7–10).
Initial clinical trials have shown that dulaglutide treatment results in dose-dependent reductions in fasting plasma glucose (FPG), postprandial plasma glucose, and glycosylated hemoglobin A1c (HbA1c) (7,11,12). Nausea and diarrhea were the most commonly reported adverse events (7,11,12). This trial, Assessment of Weekly AdministRation of LY2189265 [dulaglutide] in Diabetes-5 (AWARD-5), evaluated multiple sets of objectives, including selection of one or two dulaglutide doses (from a range of seven doses) for further assessment in this and other phase 3 trials (13–15) and safety and efficacy of selected dulaglutide doses in comparison with sitagliptin over a period of 104 weeks and placebo up to 26 weeks in metformin-treated patients with type 2 diabetes. In this study, we present results for the selected doses and comparators up to the primary end point at 52 weeks.
Research Design AND Methods
Eligible patients were those 18–75 years old, had type 2 diabetes (≥6 months) with an HbA1c value of >8% (64 mmol/mol) and ≤9.5% (80 mmol/mol) on diet and exercise alone or ≥7% (53 mmol/mol) and ≤9.5% (80 mmol/mol) on oral antihyperglycemic medication (OAM) monotherapy or combination therapy (metformin plus another OAM), a BMI between 25 and 40 kg/m2, and a stable weight during the 3-month period before entering the study. Patients were excluded if they were taking GLP-1 receptor agonists during the 6 months prior to screening or were on chronic insulin therapy. The protocol was approved by local ethics review boards, and all patients provided written informed consent. The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization guideline on good clinical practices (16).
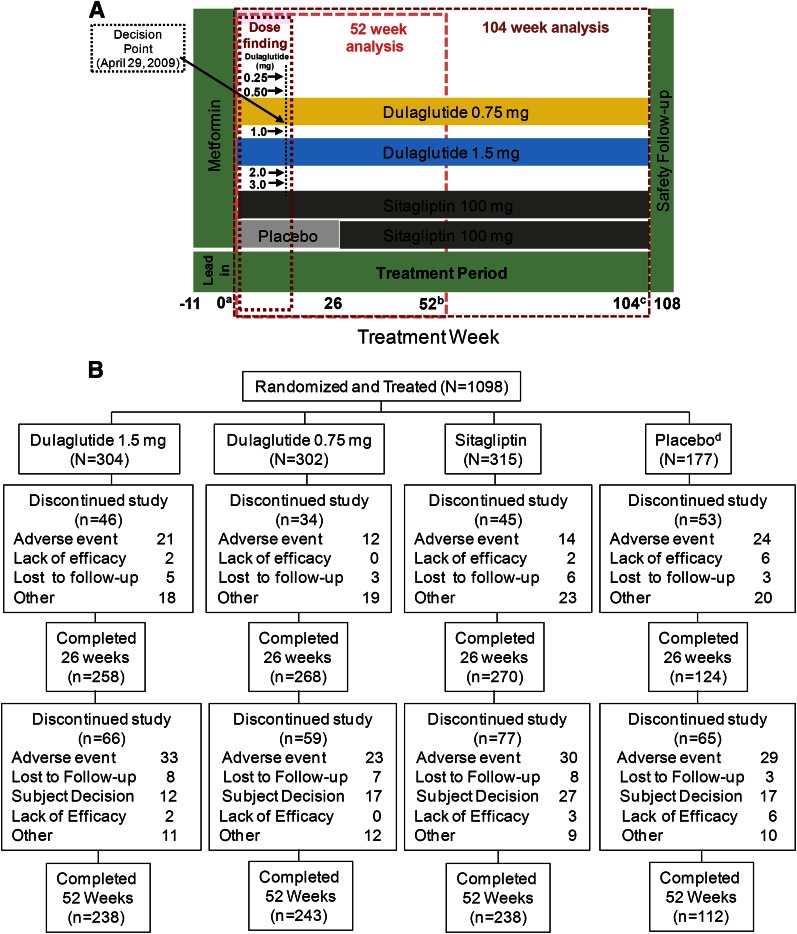
The study used an adaptive, seamless, parallel-arm design (Fig. 1A) to address two groups of objectives: 1) selection of one or two doses for further longer-term assessment (dose-finding) (17); and 2) comparison of efficacy and safety of selected dulaglutide doses versus sitagliptin (100 mg once daily) at 52 and 104 weeks and versus placebo at 26 weeks (confirmatory objectives). Eligible patients entered a lead-in period that lasted up to 11 weeks. Patients were required to be treated with metformin monotherapy (minimum dose ≥1,500 mg/day) for ≥6 weeks prior to randomization, to then be continued during the treatment period; all other OAMs were discontinued. This lead-in period allowed patients to achieve a stable dose of metformin and washout of other OAMs. Following the lead-in period, patients were assigned to treatment by one of two sequential randomization schemes: adaptive randomization during the dose-finding portion, followed by fixed randomization after dose selection (13–15). A total of 230 patients were adaptively randomized during the dose-finding portion. Dulaglutide 1.5- and 0.75-mg doses were selected for further assessment at the completion of the dose-finding portion (18). After dose selection occurred, patients from nonselected arms were discontinued. Additional patients were assigned in a fixed randomization manner to the remaining arms: dulaglutide 1.5 mg, dulaglutide 0.75 mg, sitagliptin 100 mg, or placebo (replaced with sitagliptin after 26 weeks to keep blinding) in a 2:2:2:1 ratio (17). The treatment period lasted 104 weeks. Patients who developed persistent or worsening hyperglycemia based on prespecified thresholds (Supplementary Table 1) were discontinued from the study, and an adverse event of hyperglycemia was reported in the database.
Figure 1.
Study design (A) and patient disposition (B). All patients underwent a metformin run-in period that lasted up to 11 weeks, to be continued for the duration of the study; other OAMs were discontinued. Seven doses of dulaglutide were evaluated in the dose-finding portion along with sitagliptin and placebo. At dose selection, dulaglutide 1.5 and 0.75 mg were selected for further evaluation. Only patients assigned to selected dulaglutide doses and comparators continued forward in the study. Placebo-treated patients continued until week 26 and were then switched to sitagliptin for blinding purposes. aRandomization. bPrimary end point. cFinal end point. dThe placebo period lasted for 26 weeks followed by a switch to sitagliptin to keep the arm blinded.
The primary outcome measure was mean change in HbA1c from baseline to 52 weeks. Secondary efficacy measures included change from baseline in HbA1c at other time points; percentage of patients with HbA1c <7.0% (53 mmol/mol) or ≤6.5% (48 mmol/mol); body weight, FPG (central laboratory measure), and fasting insulin; β-cell function and insulin sensitivity indices (updated homeostasis model assessment [HOMA2]) (19); and lipids.
Safety assessments included hypoglycemic episodes, vital signs, electrocardiograms, laboratory parameters, adverse events, and dulaglutide antidrug antibodies (ADAs). Adjudication of pancreatic events was performed by an independent clinical end point committee. The following events were adjudicated to assess for development of pancreatitis: investigator-reported pancreatitis, adverse events of serious or severe abdominal pain without known cause, and cases of confirmed pancreatic enzyme elevations (≥3 times the upper limit of normal). Laboratory analyses were performed at a central laboratory (Quintiles Laboratories). Immunogenicity testing was performed by BioAgilytix (Durham, NC) and Millipore (St. Charles, MO). Hypoglycemia was defined as plasma glucose ≤70 mg/dL (≤3.9 mmol/L) and/or symptoms and/or signs attributable to hypoglycemia (20). Severe hypoglycemia was defined as an episode requiring the assistance of another person to actively administer therapy (20).
Statistical Analysis
A sample size of 263 patients was chosen per arm (131 placebo) based on a predictive power calculation at dose selection of at least 85% to show superiority relative to sitagliptin at 52 weeks (14).
Primary and secondary analyses were based on the intent-to-treat (ITT) population, defined as all randomized patients. All results presented are based upon patients who had at least one outcome measure after initiation of treatment. All patients randomized in this study received at least one dose of study drug. The data from the placebo arm were excluded from any analysis after 26 weeks.
The analyses for the primary (noninferiority of dulaglutide 1.5 mg to sitagliptin at 52 weeks) and key secondary efficacy objectives (HbA1c change from baseline at 26 weeks vs. placebo and at 52 weeks vs. sitagliptin) used a tree-gatekeeping strategy to control the family-wise type 1 error rate with adjusted P values (21). Superiority or noninferiority (noninferiority margin of 0.25%) of a dulaglutide dose to a comparator treatment was concluded if the (one-sided) adjusted P value was <0.02.
The change from baseline in HbA1c and weight at 26 and 52 weeks was analyzed using ANCOVA with factors for treatment, country, and baseline value as a covariate. The last observation was carried forward (LOCF) in the case of missing data. All continuous measures, including sensitivity analyses of HbA1c and weight over time, were also analyzed using a mixed-effects, repeated-measures (MMRM) analysis with additional factors for visit and treatment-by-visit interaction. Least squares (LS) means and SEs are reported. The percentage of patients achieving HbA1c targets (LOCF) was analyzed using a logistic regression model. Total hypoglycemia included events that were documented symptomatic, documented asymptomatic, probable, and/or severe (20). Adverse events were analyzed using a χ2 test, unless there were not sufficient data to meet the assumptions of the analysis, in which case a Fisher exact test was conducted. The two-sided significance level was 5% for secondary end points and 10% for interactions.
Results
The ITT population comprised 1,098 patients randomized to dulaglutide (1.5 or 0.75 mg), sitagliptin, or placebo arms (Fig. 1A). Baseline characteristics were balanced across arms (Table 1). The metformin dose was similar across arms at baseline, and changes in dose were rare. The most common reasons for early study discontinuation were adverse events and subject decision (Fig. 1B).
Table 1.
Baseline characteristics and demographics of randomized patients
| Variable | DU 1.5 mg (N = 304) | DU 0.75 mg (N = 302) | SITA (N = 315) | PL (N = 177) |
|---|---|---|---|---|
| Sex | ||||
| Men | 146 (48) | 134 (44) | 151 (48) | 90 (51) |
| Women | 158 (52) | 168 (56) | 164 (52) | 87 (49) |
| Age (years) | 54 ± 10 | 54 ± 10 | 54 ± 10 | 55 ± 9 |
| Race | ||||
| Aboriginal | 0 (0) | 0 (0) | 1 (<1) | 0 (0) |
| Black | 16 (5) | 12 (4) | 7 (2) | 9 (5) |
| White | 157 (52) | 162 (54) | 158 (50) | 91 (51) |
| East Asian | 50 (16) | 47 (16) | 52 (17) | 28 (16) |
| Hispanic | 54 (18) | 51 (17) | 67 (21) | 38 (22) |
| Native American | 0 (0) | 0 (0) | 1 (<1) | 0 (0) |
| West Asian | 27 (9) | 30 (10) | 28 (9) | 11 (6) |
| BMI (kg/m2) | 31 ± 5 | 31 ± 4 | 31 ± 4 | 31 ± 4 |
| Weight (kg) | 87 ± 17 | 86 ± 18 | 86 ± 17 | 87 ± 17 |
| Diabetes duration (years) | 7 ± 6 | 7 ± 5 | 7 ± 5 | 7 ± 5 |
| HbA1c [% (mmol/mol)] | 8.1 ± 1.1 (65 ± 12) | 8.2 ± 1.1 (66 ± 12) | 8.1 ± 1.1 (65 ± 12) | 8.1 ± 1.1 (65 ± 12) |
| Antihyperglycemic medicationa | ||||
| OAM | 290 (95) | 284 (94) | 294 (93) | 167 (94) |
| One medication class | 203 (67) | 193 (64) | 218 (69) | 114 (64) |
| Two medication classes | 83 (27) | 89 (30) | 76 (24) | 49 (28) |
| More than two medication classes | 4 (1) | 2 (1) | 0 (0) | 4 (2) |
| SBP (mmHg) | 129 ± 13 | 128 ± 14 | 127 ± 13 | 128 ± 13 |
| DBP (mmHg) | 78 ± 8 | 78 ± 9 | 77 ± 9 | 78 ± 8 |
Data are means ± SD or n (%) unless otherwise indicated.
DU, dulaglutide; PL, placebo; SITA, sitagliptin.
aAt screening.
Efficacy
Of 1,098 patients included in the ITT population, 13 did not contribute to the primary analysis due to missing baseline or postbaseline HbA1c measurements. The mean HbA1c changes from baseline to the primary end point at 52 weeks were (LS mean ± SEs): −1.10 ± 0.06% (−12.0 ± 0.7 mmol/mol) for dulaglutide 1.5 mg, −0.87 ± 0.06% (−9.5 ± 0.7 mmol/mol) for dulaglutide 0.75 mg, and −0.39 ± 0.06% (−4.3 ± 0.7 mmol/mol) for sitagliptin (Fig. 2A). Compared with sitagliptin, the LS mean changes from baseline were significantly greater (adjusted P < 0.001, both comparisons) with dulaglutide 1.5 mg (LS mean difference −0.71% [−7.8 mmol/mol]; nominal 95% CI: −0.87 to −0.55% [−9.5 to −6.0 mmol/mol]); and also with dulaglutide 0.75 mg (−0.47% [−5.1 mmol/mol]; −0.63 to −0.31% [−6.9 to −3.4 mmol/mol]). Fig. 2B shows HbA1c values over time up to 52 weeks.
Figure 2.
Efficacy and safety measures through the treatment period. A: Change in HbA1c from baseline at 26 and 52 weeks, ANCOVA LOCF. B: HbA1c over time, MMRM. C: Percentage of patients achieving HbA1c targets at 26 and 52 weeks. D: Change in FPG over time, MMRM. E: Change in weight over time, MMRM. Data presented are LS mean ± SE. ††P < 0.001, superiority vs. sitagliptin; ‡‡P < 0.001, superiority vs. placebo; #, *P < 0.05 vs. sitagliptin and placebo, respectively; ##, **P < 0.001 vs. sitagliptin and placebo, respectively.
The LS mean HbA1c changes from baseline to 26 weeks were −1.22 ± 0.05 (−13.3 ± 0.6), −1.01 ± 0.06 (−11.0 ± 0.7), −0.61 ± 0.05 (−6.7 ± 0.6), and 0.03 ± 0.07% (0.3 ± 0.8 mmol/mol) for dulaglutide 1.5 mg, dulaglutide 0.75 mg, sitagliptin, and placebo, respectively (Fig. 2A). Compared with placebo, LS mean changes from baseline were significantly greater (P < 0.001, all comparisons) with dulaglutide 1.5 mg (LS mean difference −1.26% [−13.8 mmol/mol]), dulaglutide 0.75 mg (−1.05% [−11.5 mmol/mol]), and sitagliptin (−0.64% [−7.0 mmol/mol]). Compared with sitagliptin, LS mean changes from baseline at 26 weeks were significantly greater with both dulaglutide doses (P < 0.001, both comparisons).
After 52 weeks of treatment, the percentage of patients attaining the target HbA1c goal of <7.0% (53 mmol/mol) was significantly higher in dulaglutide 1.5-mg and dulaglutide 0.75-mg arms (58 and 49%, respectively) compared with sitagliptin (33%) (P < 0.001, both comparisons) (Fig. 2C). At the same end point, 42 and 29% of patients in the dulaglutide 1.5- and 0.75-mg arms, respectively, achieved HbA1c targets of ≤6.5% (48 mmol/mol) compared with 19% in the sitagliptin arm (P < 0.001, both comparisons). At 26 weeks, the percentage of patients achieving HbA1c values <7.0% (53 mmol/mol) was significantly higher with dulaglutide 1.5 mg (61%) and dulaglutide 0.75 mg (55%) compared with sitagliptin (38%) (P < 0.001, both comparisons) and placebo (21%) (P < 0.001, both comparisons) (Fig. 2C). At the same time point, 47 and 31% of dulaglutide 1.5 mg– and dulaglutide 0.75 mg–treated patients, respectively, achieved HbA1c values ≤6.5% (48 mmol/mol) compared with 22% with sitagliptin (P < 0.001, both comparisons) and 13% with placebo (P < 0.001, both dulaglutide comparisons; P = 0.005, sitagliptin comparison).
Values of FPG significantly decreased within 2 weeks with both dulaglutide doses and with sitagliptin and remained steady thereafter, while the placebo arm exhibited smaller and slower decreases over time (Fig. 2D). Compared with sitagliptin, LS mean changes from baseline at 52 weeks in FPG were significantly greater (P < 0.001, both comparisons) with dulaglutide 1.5 mg (LS mean difference −26 mg/dL) and dulaglutide 0.75 mg (−13 mg/dL). All three active arms had significantly greater changes in FPG from baseline to 26 weeks compared with placebo (Fig. 2D).
The mean changes in body weight from baseline to 52 weeks were (LS mean ± SE [ANCOVA with LOCF]) significantly greater (P < 0.001, both comparisons) for dulaglutide 1.5 mg (−3.03 ± 0.22 kg) and dulaglutide 0.75 mg (−2.60 ± 0.23 kg) compared with sitagliptin (−1.53 ± 0.22 kg) (LS mean difference −1.50 and −1.07 kg, respectively). Both dulaglutide doses were associated with significantly greater (P < 0.001) reductions in body weight compared with placebo and sitagliptin at 26 weeks. The effect on body weight was maintained in all arms over time (Fig. 2E).
β-Cell function, estimated by HOMA2-%B at 52 weeks, increased numerically in all arms versus baseline (Supplementary Table 2). The changes observed at 52 weeks were significantly greater with dulaglutide doses compared with sitagliptin (P < 0.001). No differences were observed among arms with respect to insulin sensitivity of the peripheral tissues, estimated by HOMA2-%S (Supplementary Table 2). Both dulaglutide arms were associated with a greater effect on HOMA2-%B versus placebo at 26 weeks (P < 0.001, both comparisons). A significantly lower (P = 0.026) effect on HOMA2-%S was associated with dulaglutide 0.75 mg compared with placebo; no other differences were noted for change in HOMA2-%S at this time point.
At 52 weeks, a decrease in LDL cholesterol with dulaglutide 1.5 mg and an increase with sitagliptin was observed, resulting in significant between-treatment difference (P = 0.03) (Supplementary Table 2). At 26 weeks, significantly greater decreases in total and LDL cholesterol were observed with dulaglutide 1.5 mg compared with placebo (P < 0.001 and 0.007, respectively). No other differences were observed for total, LDL, and HDL cholesterol or triglycerides between dulaglutide and sitagliptin doses at 52 weeks and placebo at 26 weeks.
Safety
Table 2 summarizes incidence of deaths, overall and serious adverse events, and individual adverse events occurring in at least 5% of patients at 26 and 52 weeks. Four patients (dulaglutide 1.5 mg: one; sitagliptin: two; placebo [during the sitagliptin period]: one) died during this trial. The patient treated with dulaglutide 1.5 mg died of a nonhemorrhagic stroke 6 months after randomization. The incidence of adverse events was similar in dulaglutide arms versus sitagliptin at 52 weeks and versus placebo at 26 weeks. The incidence of gastrointestinal (GI) adverse events (nausea, diarrhea, and vomiting), and the adverse event of decreased appetite was significantly higher (P < 0.05) with dulaglutide- compared with sitagliptin-treated patients. Similar results were observed for comparisons between dulaglutide- and placebo-treated patients. The incidence of nausea, diarrhea, and vomiting with dulaglutide 1.5 mg and dulaglutide 0.75 mg peaked within the first 2 weeks of treatment and gradually declined to stable levels (1–6% incidence) between 8 and 52 weeks of treatment.
Table 2.
Safety assessments, change from baseline in vital signs, and TE dulaglutide ADAs through 26 and 52 weeks
| Variable | 26 weeks |
52 weeks |
|||||
|---|---|---|---|---|---|---|---|
| DU 1.5 mg (N = 304) | DU 0.75 mg (N = 302) | SITA (N = 315) | PL (N = 177) | DU 1.5 mg (N = 304) | DU 0.75 mg (N = 302) | SITA (N = 315) | |
| Death | 1 (<1) | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 0 (0) | 2 (<1) |
| Serious adverse events | 17 (6) | 10 (3) | 11 (4) | 6 (3) | 26 (9) | 16 (5) | 17 (5) |
| Adverse events, (patients with ≥1 event) | 208 (68)# | 204 (68)# | 185 (59) | 111 (63) | 233 (77) | 231 (77) | 219 (70) |
| TE adverse events, (≥5% patients) | |||||||
| SOC: GI events | 116 (38)##,** | 97 (32)##,* | 55 (18) | 41 (23) | 126 (41)## | 111 (37)## | 73 (23) |
| Nausea | 51 (17)##,** | 38 (13)##,* | 14 (4) | 7 (4) | 53 (17)## | 42 (14)## | 16 (5) |
| Vomiting | 36 (12)##,** | 22 (7)#,** | 6 (2) | 1 (1) | 39 (13)## | 23 (8)# | 7 (2) |
| Diarrhea | 39 (13)##,* | 27 (9)## | 8 (3)* | 11 (6) | 44 (15)## | 30 (10)## | 9 (3) |
| Abdominal pain | 13 (4) | 11 (4) | 6 (2) | 3 (2) | 18 (6) | 12 (4) | 10 (3) |
| Dyspepsia | 13 (4) | 14 (5) | 8 (3) | 2 (1) | 14 (5) | 18 (6) | 9 (3) |
| Abdominal distension | 12 (4) | 12 (4) | 3 (1) | 1 (1) | 12 (4) | 14 (5) | 3 (1) |
| SOC: infections and infestations | 89 (29) | 71 (24) | 74 (24) | 36 (20) | 111 (37) | 97 (32) | 101 (32) |
| Nasopharyngitis | 25 (8) | 24 (8) | 26 (8) | 13 (7) | 35 (12) | 35 (12) | 36 (11) |
| URI | 12 (4) | 12 (4) | 4 (1) | 2 (1) | 16 (5) | 16 (5) | 12 (4) |
| UTI | 11 (4) | 16 (5) | 11 (4) | 9 (5) | 15 (5) | 18 (6) | 15 (5) |
| Other adverse eventsa | |||||||
| Hyperglycemia | 4 (1)#,** | 5 (2)#,** | 14 (4)* | 19 (11) | 16 (5) | 23 (8) | 29 (9) |
| Decreased appetite | 29 (10)##,** | 16 (5)# | 5 (2) | 3 (2) | 29 (10)## | 17 (6)# | 7 (2) |
| Back pain | 12 (4) | 13 (4) | 10 (3) | 7 (4) | 15 (5) | 18 (6) | 15 (5) |
| Headache | 20 (7) | 20 (7) | 19 (6) | 9 (5) | 26 (9) | 23 (8) | 23 (7) |
| Arthralgia | 7 (2) | 10 (3) | 4 (1) | 4 (2) | 11 (4) | 14 (5) | 8 (3) |
| Dizziness | 3 (1) | 13 (4) | 8 (3) | 4 (2) | 5 (2) | 14 (5) | 10 (3) |
| Discontinuation due to adverse events | 21 (7) | 12 (4) | 14 (4) | 24 (14) | 33 (11) | 23 (8) | 30 (10) |
| Vital signs | |||||||
| SBP (mmHg) | −1.7 ± 0.7* | −1.4 ± 0.7* | −1.9 ± 0.7* | 1.1 ± 0.9 | −0.8 ± 0.7 | −0.5 ± 0.7 | −0.5 ± 0.7 |
| DBP (mmHg) | −0.4 ± 0.4 | −0.2 ± 0.4 | −1.1 ± 0.4* | 0.7 ± 0.6 | 0.3 ± 0.5 | 0.2 ± 0.5 | −0.2 ± 0.5 |
| Pulse rate (bpm) | 2.6 ± 0.5##,** | 1.9 ± 0.5#,* | −0.1 ± 0.5 | −0.2 ± 0.7 | 2.4 ± 0.5## | 2.1 ± 0.5## | −0.3 ± 0.5 |
| TE dulaglutide ADAs | |||||||
| Dulaglutide ADAs | 2 (<1) | 6 (2) | 1 (<1) | 0 (0) | 2 (<1) | 7 (2) | 2 (<1) |
| Neutralizing dulaglutide ADAs | 2 (<1) | 0 (0) | 0 (0) | 0 (0) | 2 (<1) | 0 (0) | 0 (0) |
Data are n (%) or LS mean (± SE) unless otherwise indicated.
DU, dulaglutide; PL, placebo; SITA, sitagliptin; SOC, system organ class; URI, upper respiratory infection; UTI, urinary tract infection.
#, *P < 0.05 vs. sitagliptin and placebo, respectively.
##, **P < 0.001 vs. sitagliptin and placebo, respectively.
aMultiple SOCs.
The incidence of study discontinuation due to adverse events at 52 weeks was similar across arms (dulaglutide 1.5 mg: 33 [10.9%]; dulaglutide 0.75 mg: 23 [7.6%]; sitagliptin: 30 [9.5%]); the most common adverse events causing study discontinuation were hyperglycemia and nausea. Discontinuation because of nausea was only reported with dulaglutide (dulaglutide 1.5 mg: 8 [2.6%]; dulaglutide 0.75 mg: 3 [1.0%]), and the majority of these patients (6) discontinued within the initial 2 weeks of study drug administration. During the placebo-controlled period, hyperglycemia and nausea were also the most common adverse events causing discontinuation. All cases of discontinuation due to nausea during this period (eight patients) occurred in dulaglutide arms. There were more patients from the placebo arm (17 patients [9.6%]) who discontinued the study due to hyperglycemia compared with active treatments (dulaglutide 1.5 mg: 4 [1.3%]; dulaglutide 0.75 mg: 1 [0.3%]; and sitagliptin: 6 [1.9%]).
The incidence of total hypoglycemia at 52 weeks was 10.2% for dulaglutide 1.5 mg, 5.3% for dulaglutide 0.75 mg, and 4.8% for sitagliptin; mean (SD) 1-year adjusted rates (events/patient/year) were 0.4 (1.6), 0.3 (2.6), and 0.1 (1.1), respectively. The incidence and rates at 26 weeks for dulaglutide- and sitagliptin-treated patients were similar to those observed at 52 weeks; incidence and rate in the placebo arm at 26 weeks were 1.1 and 0.1% (0.9), respectively. There were no severe hypoglycemic events reported during this trial.
There were three events of acute pancreatitis confirmed by adjudication (sitagliptin: two; placebo [during the sitagliptin period]: one). Increases in median values of serum lipase, total amylase, and pancreatic amylase (p-amylase) (within the normal range) were observed with both dulaglutide doses and sitagliptin (Supplementary Table 2). Increase in total amylase was significantly greater for both dulaglutide doses than for sitagliptin during the 52-week treatment period (P = 0.016, for both comparisons); increase in p-amylase for dulaglutide 1.5 mg was also significantly greater compared with sitagliptin (P = 0.008). There were no differences between the dulaglutide and sitagliptin arms in the incidence of treatment-emergent (TE) high values during this period. All active treatments demonstrated significant (P < 0.001) increase in median values of pancreatic enzymes (except sitagliptin for total amylase) and higher incidence of TE high lipase values in comparison with placebo during the placebo-controlled period (P < 0.001, dulaglutide 1.5 mg; P = 0.015, dulaglutide 0.75 mg; and P = 0.023, sitagliptin). TE high p-amylase values were also more common in both dulaglutide arms than placebo (P = 0.032, dulaglutide 1.5 mg; and P = 0.02, dulaglutide 0.75 mg).
Systolic blood pressure (SBP) decreased in all active treatment arms, with the greatest reductions observed during the first 3–6 months (Table 2). Decrease in SBP from baseline up to 26 weeks was significantly greater with both dulaglutide doses and sitagliptin compared with placebo (P < 0.05). There were no differences between dulaglutide arms and sitagliptin at this time point or at 52 weeks. Changes in diastolic blood pressure (DBP) were small (Table 2), and there were no significant differences between active treatments at 26 or 52 weeks. An increase of ∼2 to 3 bpm in LS mean pulse rate was observed with both dulaglutide doses at 26 and 52 weeks in comparison with no relevant changes in the sitagliptin and placebo arms at these time points (Table 2).
There were no cases of thyroid cancer during the 52-week observational period. There were no differences among arms in calcitonin levels at 26 or 52 weeks.
Nine patients treated with dulaglutide had TE dulaglutide ADAs during the treatment period (1.3%) (Table 2). Dulaglutide neutralizing antibodies were present in two of these nine patients, and no patient developed native-sequence GLP-1 neutralizing antibodies. None of these patients had hypersensitivity events. The incidence of hypersensitivity events across the groups was very low and similar (data not shown).
Conclusions
The results of the AWARD-5 study showed that in patients unable to attain glycemic targets with metformin monotherapy, addition of dulaglutide 1.5-mg or dulaglutide 0.75-mg doses provided significantly greater improvement in HbA1c compared with sitagliptin after 52 weeks and placebo after 26 weeks. Accordingly, a higher percentage of dulaglutide-treated patients attained HbA1c targets in comparison with sitagliptin. The differences in HbA1c relative to sitagliptin for dulaglutide 1.5 mg (−0.71% [−7.8 mmol/mol]) and dulaglutide 0.75 mg (−0.47% [−5.1 mmol/mol]) are considered clinically relevant (22). Dulaglutide also exhibited greater weight reduction than sitagliptin. GI system–related adverse events were the most commonly reported events in patients treated with dulaglutide and more frequent than with placebo or sitagliptin. Discontinuation rates across the groups at 26 and 52 weeks were comparable to the rates observed in similar diabetes trials previously reported in the medical literature (23–25).
The observed effects of dulaglutide and sitagliptin on HbA1c in AWARD-5 are similar to those reported in other patient populations treated with these agents. Grunberger et al. (11) and Umpierrez et al. (12) showed a similar effect of dulaglutide on HbA1c as add-on therapy to diet or in combination with OAMs. In the current study, the glucose-lowering effect of sitagliptin was −0.61 ± 0.05% (−6.7 ± 0.6 mmol/mol) and −0.39 ± 0.06% (4.3 ± 0.7 mmol/mol) at 26 and 52 weeks, respectively. This decrease in HbA1c is consistent with results from other studies with similar patient populations and study duration (6,26,27). Greater improvements in glycemic control with dulaglutide were also evident in the significantly higher percentage of patients who achieved HbA1c targets of <7.0% (53 mmol/mol) and ≤6.5% (48 mmol/mol) than with sitagliptin. In addition, the results of this trial showed that the difference in glucose-lowering effects between dulaglutide and sitagliptin was sustained over a longer period of time (i.e., 52 weeks) compared with previously published trials that compared sitagliptin to a GLP-1 receptor agonist for up to 26 weeks (5,6).
One possible explanation of these differences in glucose-lowering effects relates to the greater degree of GLP-1 receptor stimulation with receptor agonists than with DPP-4 inhibitors (28). It has been suggested that longer-acting receptor agonists are associated with persistently elevated GLP-1 receptor agonist concentrations, while DPP-4 inhibitors increase concentration of GLP-1 mostly after meals (29–31). Assessment of pancreatic β-cell function using the HOMA2 model may support these explanations. Dulaglutide demonstrated greater effects on β-cell function compared with sitagliptin, suggesting that a drug that provides high pharmacological levels of exogenous GLP-1 receptor agonist may be more effective than a drug that modestly increases concentration of endogenous GLP-1. One caveat to this comparison; HOMA2 assesses β-cell function in the fasting state when dulaglutide levels were continuously elevated, while during this same time phase for patients treated with sitagliptin, endogenous GLP-1 was, most likely, in its basal range (non–nutrient-stimulated).
Dulaglutide-treated patients achieved an early improvement (after 2 weeks) in fasting glucose consistent with the reported pharmacokinetic characteristics of the drug (7). This early effect is important from a clinical perspective, in that it is a readily available measure of glycemic response, allowing patients and health care providers to gauge an indication of therapeutic effect soon after the initiation of therapy.
The main determinants of weight gain in patients with diabetes are changes in HbA1c and type of glucose-lowering intervention (32). Dulaglutide treatment resulted in significantly greater decreases in body weight compared with sitagliptin throughout the duration of the treatment period, despite its larger effect on HbA1c. The mechanisms of weight loss with GLP-1 receptor agonists are probably related to their actions in the GI and/or central nervous system (33,34).
Safety assessments of dulaglutide in this trial are consistent with the known effects of the GLP-1 receptor agonist class. The incidence of hypoglycemic events remained very low in both dulaglutide arms, despite the robust effect on glycemic control. This is consistent with the known mechanism of action of dulaglutide, which enhances insulin secretion in a glucose-dependent fashion without causing β-cell overstimulation and hyperinsulinemia (35).
Both dulaglutide doses were associated with a higher incidence of GI adverse events, most commonly nausea, vomiting, and diarrhea. There were no cases of pancreatitis reported in patients treated with dulaglutide. The observed increases within the normal range in median values of pancreatic enzymes with dulaglutide and sitagliptin were similar in magnitude to the changes reported with liraglutide (36). The mechanism that causes these increases is unknown, and there were no observed clinical consequences of these findings.
Cardiovascular system observations included a decrease in SBP and an increase in pulse rate. The magnitude of the observed changes in pulse rate was similar to that reported with other GLP-1 receptor agonists (6,27,37). The incidence of dulaglutide ADAs was very low (1.3%) compared with other GLP-1 receptor agonists (38,39). There were no associated systemic or injection site hypersensitivity reactions in ADA-positive patients. Additionally, hypersensitivity events overall were rare and balanced in incidence across the arms. These data suggest a low risk of hypersensitivity reactions with dulaglutide.
The AWARD-5 trial confirmed that dulaglutide is an effective treatment option in metformin-treated patients who require further treatment intensification. Both doses were superior in glucose lowering to sitagliptin and placebo. Despite a greater decrease in HbA1c, patients who received dulaglutide exhibited a decrease in weight and low risk of hypoglycemia. Dulaglutide has a similar safety profile to that of other agents from the GLP-1 receptor agonist class. These results suggest a favorable benefit/risk profile for dulaglutide as an add-on intervention in metformin-treated type 2 diabetic patients.
Article Information
Acknowledgments. The authors thank the AWARD-5 team and staff for conducting this study and Oralee Johnson Varnado, PhD, Eli Lilly and Company, for writing assistance.
Funding and Duality of Interest. This work is sponsored by Eli Lilly and Company. M.N. received research grants from Berlin-Chemie AG/Menarini, Eli Lilly and Company, Merck Sharp & Dohme, Novartis Pharma AG, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, MetaCure Inc., Roche Pharma AG, Novo Nordisk, and Tolerx, Inc. for participation in multicenter clinical trials and received consulting fees or/and honoraria for membership in advisory boards or/and honoraria for speaking from Amylin Pharmaceuticals, Inc., AstraZeneca, Mjölndal, Berlin-Chemie AG/Menarini, Boehringer Ingelheim, Bristol-Myers Squibb, Diartis Pharmaceuticals, Inc., Eli Lilly and Company, F. Hoffmann-La Roche Ltd., GlaxoSmithKline, Intarcia Therapeutics, Inc., MannKind Corporation, Merck Sharp & Dohme GmbH, Merck Sharp & Dohme, Novartis Pharma AG, Novo Nordisk A/S, Novo Nordisk Pharma GmbH, Sanofi, Takeda, Versartis, and Wyeth Research, including reimbursement for travel expenses in connection with the above-mentioned activities. R.S.W. participated in multicenter clinical trials sponsored by Eli Lilly and Company, Medtronic, GlaxoSmithKline, Sanofi, Biodel, and AstraZeneca. G.E.U. is supported in part by research grants from the American Diabetes Association (7-03-CR-35), Public Health Service grant UL1-RR-025008 from the Clinical and Translational Science Awards Program (M01-RR-00039), National Institutes of Health, and National Center for Research Resources and by unrestricted research grants from pharmaceutical companies (Sanofi and Merck Sharp & Dohme) to Emory University. B.G. has received fees for consultancy, advisory boards, speaking, travel, or accommodation from GlaxoSmithKline, Eli Lilly and Company, Merck Sharp & Dohme, AstraZeneca, Bristol-Myers Squibb, Pfizer, Novo Nordisk, Sanofi, Novartis, Abbott, LifeScan, Roche Diagnostics, Medtronic, and Menarini. Z.S. and Z.M. are employees of Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.N., R.S.W., G.E.U., and B.G. researched data, contributed to the discussion, and reviewed and edited the manuscript. Z.S. and Z.M. researched data and wrote the manuscript. Z.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Portions of this study were presented at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013 and the 49th Annual European Association for the Study of Diabetes Meeting, Barcelona, Spain, 23–27 September 2013.
Footnotes
Clinical trial reg. no. NCT00734474, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-2761/-/DC1.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ, Orskov C, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett 1987;211:169–174 [DOI] [PubMed] [Google Scholar]
- 4.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987;2:1300–1304 [DOI] [PubMed] [Google Scholar]
- 5.Bergenstal RM, Wysham C, Macconell L, et al. DURATION-2 Study Group . Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431–439 [DOI] [PubMed] [Google Scholar]
- 6.Pratley RE, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group . Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–1456 [DOI] [PubMed] [Google Scholar]
- 7.Barrington P, Chien JY, Tibaldi F, Showalter HD, Schneck K, Ellis B. LY2189265, a long-acting glucagon-like peptide-1 analogue, showed a dose-dependent effect on insulin secretion in healthy subjects. Diabetes Obes Metab 2011;13:434–438 [DOI] [PubMed] [Google Scholar]
- 8.Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev 2010;26:287–296 [DOI] [PubMed] [Google Scholar]
- 9.Data on file, Eli Lilly and Company and/or one of its subsidiaries
- 10.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 11.Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with Type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabet Med 2012;29:1260–1267 [DOI] [PubMed] [Google Scholar]
- 12.Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ, 3rd, EGO Study Group . The effects of LY2189265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab 2011;13:418–425 [DOI] [PubMed] [Google Scholar]
- 13.Geiger MJ, Skrivanek Z, Gaydos B, Chien J, Berry S, Berry D. An adaptive, dose-finding, seamless phase 2/3 study of a long-acting glucagon-like peptide-1 analog (dulaglutide): trial design and baseline characteristics. J Diabetes Sci Tech 2012;6:1319–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skrivanek Z, Berry S, Berry D, et al. Application of adaptive design methodology in development of a long-acting glucagon-like peptide-1 analog (dulaglutide): statistical design and simulations. J Diabetes Sci Tech 2012;6:1305–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer K, Colvin K, Braunecker B, et al. Operational challenges and solutions with implementation of an adaptive seamless phase 2/3 study. J Diabetes Sci Tech 2012;6:1296–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association . World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–926 [PubMed] [Google Scholar]
- 17.Skrivanek ZCJ, Gaydos B, Heathman M, Geiger M, Milicevic Z, Eds. Dose-Finding Results in an Adaptive Trial of Dulaglutide Combined with Metformin in Type 2 Diabetes (AWARD-5). Chicago, IL, American Diabetes Association, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Skrivanek Z, Chien JY, Gaydos B, Heathman M, Geiger MJ, Milisevic Z. Dose finding results in an adaptive trial of dulaglutide combined with metformin in type 2 diabetes (AWARD-5). Diabetologia 2013;56(Suppl. 1):S402. [DOI] [PubMed] [Google Scholar]
- 19.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 20.Workgroup on Hypoglycemia, American Diabetes Association . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]
- 21.Dmitrienko A, Tamhane AC, Wiens BL. General multistage gatekeeping procedures. Biom J 2008;50:667–677 [DOI] [PubMed] [Google Scholar]
- 22.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zinman B, Gerich J, Buse JB, et al. LEAD-4 Study Investigators . Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009;32:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nauck M, Frid A, Hermansen K, et al. LEAD-2 Study Group . Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garber A, Henry R, Ratner R, et al. LEAD-3 (Mono) Study Group . Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373:473–481 [DOI] [PubMed] [Google Scholar]
- 26.Chapell R, Gould AL, Alexander CM. Baseline differences in A1C explain apparent differences in efficacy of sitagliptin, rosiglitazone and pioglitazone. Diabetes Obes Metab 2009;11:1009–1016 [DOI] [PubMed] [Google Scholar]
- 27.Pratley RE, Nauck MA, Bailey T, et al. 1860-LIRA-DPP-4 Study Group . Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: a randomized, open-label trial. Diabetes Care 2012;35:1986–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008;24:2943–2952 [DOI] [PubMed] [Google Scholar]
- 29.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, Sitagliptin Study 021 Group . Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006;29:2632–2637 [DOI] [PubMed] [Google Scholar]
- 30.Degn KB, Juhl CB, Sturis J, et al. One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 2004;53:1187–1194 [DOI] [PubMed] [Google Scholar]
- 31.Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005;78:675–688 [DOI] [PubMed] [Google Scholar]
- 32.Fonseca V, McDuffie R, Calles J, et al. ACCORD Study Group Determinants of weight gain in the Action to Control Cardiovascular Risk in Diabetes Trial. Diabetes Care 2013;36:2162–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998;101:515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev 1999;20:876–913 [DOI] [PubMed] [Google Scholar]
- 35.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 36.Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 2013;381:117–124 [DOI] [PubMed] [Google Scholar]
- 37.Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011;96:1301–1310 [DOI] [PubMed] [Google Scholar]
- 38.Russell-Jones D. The safety and tolerability of GLP-1 receptor agonists in the treatment of type-2 diabetes. Int J Clin Pract 2010;64:1402–1414 [DOI] [PubMed] [Google Scholar]
- 39.Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant 2005;20(Suppl. 6):vi3–vi9 [DOI] [PubMed] [Google Scholar]