Abstract
Purpose.
The accessory lacrimal glands are assumed to contribute to the production of tear fluid, but little is known about their function. The goal of this study was to conduct an analysis of gene expression by glands of Wolfring that would provide a more complete picture of the function of these glands.
Methods.
Glands of Wolfring were isolated from frozen sections of human eyelids by laser microdissection. RNA was extracted from the cells and hybridized to gene expression arrays. The expression of several of the major genes was confirmed by immunohistochemistry.
Results.
Of the 24 most highly expressed genes, 9 were of direct relevance to lacrimal function. These included lysozyme, lactoferrin, tear lipocalin, and lacritin. The glands of Wolfring are enriched in genes related to protein synthesis, targeting, and secretion, and a large number of genes for proteins with antimicrobial activity were detected. Ion channels and transporters, carbonic anhydrase, and aquaporins were abundantly expressed. Genes for control of lacrimal function, including cholinergic, adrenergic, vasoactive intestinal polypeptide, purinergic, androgen, and prolactin receptors were also expressed in gland of Wolfring.
Conclusions.
The data suggest that the function of glands of Wolfring is similar to that of main lacrimal glands and are consistent with secretion electrolytes, fluid, and protein under nervous and hormonal control. Since these glands secrete directly onto the ocular surface, their location may allow rapid response to exogenous stimuli and makes them readily accessible to topical drugs.
Human glands of Wolfring, isolated by laser microdissection, are enriched in genes for protein synthesis and secretion. They also express many genes for electrolyte and fluid secretion and control of lacrimal function, indicating that their function is similar to that of the main lacrimal gland.
Introduction
The accessory lacrimal glands, first described by Wolfring and Krause in the 19th century (see citations in Seifert et al.1), are located in the palpebral conjunctiva. Although much smaller, they have an acinar structure similar to that of the main lacrimal gland.1,2 These glands are assumed to contribute to the production of tear fluid, but due to their small size and inaccessibility, relatively little is known about their function. Reports using immunostaining of biopsy specimens and organ culture of human accessory gland fragments demonstrated that accessory glands express lactoferrin, lysozyme, epidermal growth factor (EGF), secretory IgA, and phospholipase A2.3–7 It has also been demonstrated by microscopy that the glands of Wolfring and Krause are innervated8–10 and that they express adrenergic receptors.11
Functionally, little is known about secretion of fluid by the accessory glands or the contribution of these glands to the tear film in animals or humans. Controversy exists in the older literature concerning the importance of accessory gland secretion and their relative contribution to basal and reflex tears.12–14 Two studies on animals, however, suggest that accessory gland secretion is functionally significant. Bergmanson et al. demonstrated that the rabbit conjunctiva contains glands similar to the glands of Wolfring.15 In a rabbit model of keratoconjunctivitis sicca in which the main lacrimal gland duct was occluded and the Harderian and nictitans glands were removed, Gilbard et al. reported that several topically applied secretogogues, including vasoactive intestinal polypeptide (VIP), forskolin, IBMX, and 8-bromo cAMP, stimulated tear secretion.16 This fluid may have been from the accessory glands described by Bergmanson, since a topically applied agent would not be expected to have a strong effect on the main lacrimal gland. Maitchouk et al.17 reported an 80% to 90% decrease in tear secretion after removal of the main, exorbital lacrimal gland from squirrel monkeys. This was followed by recovery of Schirmer scores to approximately 30% of normal by 20 weeks after surgery, with no ocular surface pathology. The investigators showed by MALDI-TOF mass spectrometry that the protein composition of tears from the operated and control eyes was similar. They concluded that the recovery of tear secretion was due to compensation by the accessory lacrimal glands, suggesting similar functions of main and accessory glands.
Previous investigations of the accessory glands, most of which used histologic methods and microscopy, focused on a limited number of proteins per study. The development of methods for genomic analysis since the time when many of the studies cited above were conducted makes possible a more comprehensive analysis of accessory lacrimal glands. The goal of the present study was to extend the understanding of accessory glands by isolating glands of Wolfring from human eyelid biopsy samples using laser microdissection, followed by cDNA microarray analysis of gene expression in the glands. The primary hypothesis was that the glands of Wolfring express genes for secretory proteins known to be present in tears and also proteins known to be present in the main lacrimal glands that are involved in processes such as ion and fluid secretion or control and stimulation of secretory processes.
Materials and Methods
Human Eyelid Tissue and Lacrimal Glands
Eyelid tissue removed during lid resection surgery was obtained from Mark Hatton, MD. The tissues were embedded in tissue freezing medium within approximately 30 minutes of removal and stored at −80°C. One human main lacrimal gland, in storage at the Schepens Eye Research Institute, was obtained as discarded tissue from orbital surgery procedures at Massachusetts Eye and Ear. A second main lacrimal gland was obtained from the National Disease Research Interchange (Philadelphia, PA). These were also stored at −80°C. The Institutional Review Board of the Schepens Eye Research Institute approved this use of human tissue that would have been discarded if not used for research. The project protocol adhered to the tenets of the Declaration of Helsinki. Information on the tissues from which the data in this report were obtained is given in Table 1.
Table 1. .
Patient Information for Tissues Used in This Study
|
Age |
Sex |
Surgery/Cause of Death |
| Gland of Wolfring | ||
| 65 | M | Lower lid laxity |
| 84 | F | Lower lid laxity |
| 83 | M | Lower lid laxity |
| 80 | M | Lower lid laxity |
| 61 | M | Lower lid laxity* |
| 28 | F | Floppy lid syndrome* |
| Main lacrimal gland | ||
| 58 | M | Glioblastoma† |
| 57 | M | Orbital surgery |
Used for RT-PCR and immunostaining only.
From National Disease Research Interchange.
Identification of Accessory Glands, Assessment of RNA Quality, and Laser Microdissection
To determine whether the RNA in eyelid samples and main lacrimal glands was of good quality, an 8 μm frozen section was cut and RNA was extracted with an Arcturus PicoPure RNA isolation kit (Life Technologies Corp., Carlsbad, CA) using the manufacturer's protocol for a “scrape assay.” The RNA was reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) and analyzed for GAPDH expression by real-time PCR. Samples from the PCR assay were also analyzed by electrophoresis on a 1% agarose gel.
Since each eyelid contains only three to five glands of Wolfring, it was necessary to cut multiple serial sections of each sample to determine if a gland was present. The sections were stained with 0.1% toluidine blue O in water. When an accessory gland was identified, an image was captured by microscopy and SPOT imaging software (Diagnostic Instruments, Inc., Sterling Heights, MI). The sample was then stored at −80°C until use for laser microdissection.
For laser microdissection (LMD), 7 μm frozen sections were collected on PEN slides (Leica Microsystems, Wetzlar, Germany). Generally six to eight sections were placed on a slide, and eight slides were prepared from the gland of Wolfring of each eyelid sample. The large number of sections was prepared to ensure that an adequate amount of RNA could be extracted. Acinar cells were collected from main lacrimal glands by the same method. The sections were fixed with 70% ethanol, stained with hematoxylin and eosin under RNase-free conditions, and air dried. The glands of Wolfring were immediately collected using a Leica AS LMD laser microdissection system. All of the samples from a single gland obtained by LMD were pooled. Because the size of the glands varied from eyelid to eyelid, no information is available on the amount of tissue or number of cells collected. RNA was extracted from the tissue, purified using the Arcturus PicoPure kit, and stored at −80°C.
Microarray Analysis
RNA samples were submitted to the Microarray Core facility at the Dana-Farber Cancer Institute, Harvard Medical School, for analysis. RNA quality was checked using a bioanalyser (Agilent Technologies, Santa Clara, CA). Four glands of Wolfring and two main lacrimal gland samples had RNA of sufficient quality to proceed with microarray analysis. The RNA was amplified and prepared for microarrays using the NuGEN Ovation V2 Amplification System (NuGEN Technologies, Inc., San Carlos, CA) as described on the DFCI Microarray Core website (http://chip.dfci.harvard.edu/index.php?option=com_content&task=view&id=14&Itemid=28, in the public domain). The cDNA was hybridized to Affymetrix Human Genome U133 Plus 2.0 3′ gene expression arrays (Affymetrix Microarray Solutions, Santa Clara, CA). Gene lists were generated from the expression scans using open source software (dChip, Harvard Medical School, https://sites.google.com/site/dchipsoft/, in the public domain) followed by further analysis using DAVID Functional Annotation Bioinformatics Microarray Analysis software (National Institute of Allergy in Infectious Diseases, NIH, Bethesda, MD, http://david.abcc.ncifcrf.gov/, in the public domain).18
RT-PCR for Validation of Microarray Data
Prevalidated quantitative PCR (qPCR) primer sets for mucin-like 1, β-defensin 124, frizzled-related protein 2, protein kinase Cε, and GAPDH were purchased from Qiagen (Valencia, CA). Gene expression analysis was performed by real-time RT-PCR using the ΔΔCt method according to manufacturer's recommendations, as described in the Qiagen RT2 qPCR Primer Assay Handbook. These methods are similar to those we have previously described.19,20
Immunofluorescence Microscopy
Primary antibodies against lysozyme, lactoferrin, muscarinic acetylcholine receptor 3, prolactin receptor, Maxi K channel, and aquaporin 5 (all polyclonal goat anti-human) and carbonic anhydrase VI (polyclonal rabbit anti-human) were purchased from Santa Cruz, Biotechnology, Inc. (Santa Cruz, CA). A polyclonal rabbit anti-rat antibody (known to react with human) against the intermediate conductance Ca2+-activated K+ channel 4 was purchased from Alomone Labs, Ltd. (Jerusalem, Israel). A polyclonal rabbit anti-human tear lipocalin antibody was a gift from Ben Glasgow (Department of Ophthalmology, University of California-Los Angeles).21 This antibody did not cross-react with lysozyme. Gordon Laurie and Robert McKown (Department of Cell Biology, University of Virginia, Charlottesville, VA, and Department of Biology, James Madison University, Harrisonburg, VA) provided a polyclonal rabbit anti-human antibody against lacritin.22
Eyelid tissue in tissue freezing medium, stored at −80°C, was warmed to −20°C. Frozen sections, 6 μm thick, were cut and stained with 0.1% toluidine blue to confirm the presence of a gland of Wolfring. Sections containing a gland of Wolfring were prepared and stained as previously described.23 Cover slips were mounted using Prolong Gold Antifade Reagent with DAPI (Life Technologies Corp.). Control slides were prepared by omitting the primary antibody and incubating with buffer alone during the primary antibody incubation.
Results
Tissue Acquisition and Assessment of RNA Quality
Eyelids from 16 patients were sectioned to identify glands of Wolfring. Of these, glands of adequate size were found in six samples, four of which yielded RNA of adequate quantity and quality for microarray analysis. Two of the samples were obtained after the microarray experiments were complete and were used for immunohistochemistry only. When present, the glands were found proximal to the meibomian glands, as expected, and had an acinar structure very similar to that of the main lacrimal gland (Fig. 1).
Figure 1. .
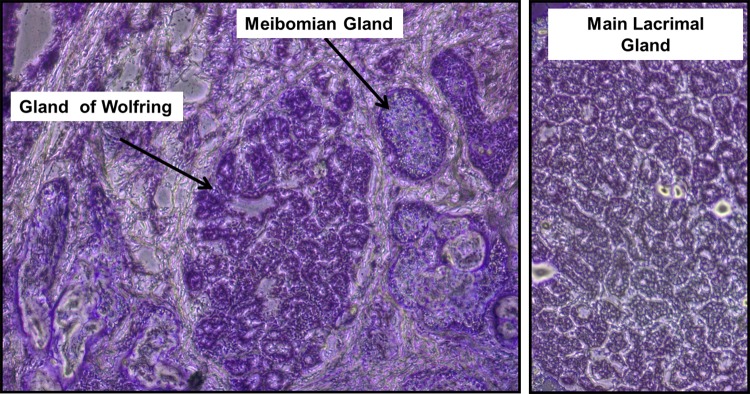
Frozen sections of human eyelid (left) and human main lacrimal gland (right) stained with toluidine blue O. Note the similarity of the structure of the gland of Wolfring to the acini of the main lacrimal gland (original magnification ×100).
Because the eyelids used in this study were surgical samples, when a gland of Wolfring was identified in an eyelid sample it was necessary to determine whether the RNA was of sufficient quality before proceeding with further analysis. As shown in Figure 2, GAPDH RT-PCR indicated that the RNA in all four samples was of good quality. The same was confirmed for two of the three main lacrimal glands used in this study (data not shown). RNA purified from gland of Wolfring and main lacrimal gland acini, obtained by LMD (Fig. 3), was also of adequate quality for microarray analysis. Two examples of bioanalyzer data are shown in Figure 4, with strong 18S and 28S rRNA peaks. Some baseline noise is evident; however, this was judged to be acceptable for surgical samples.
Figure 2. .
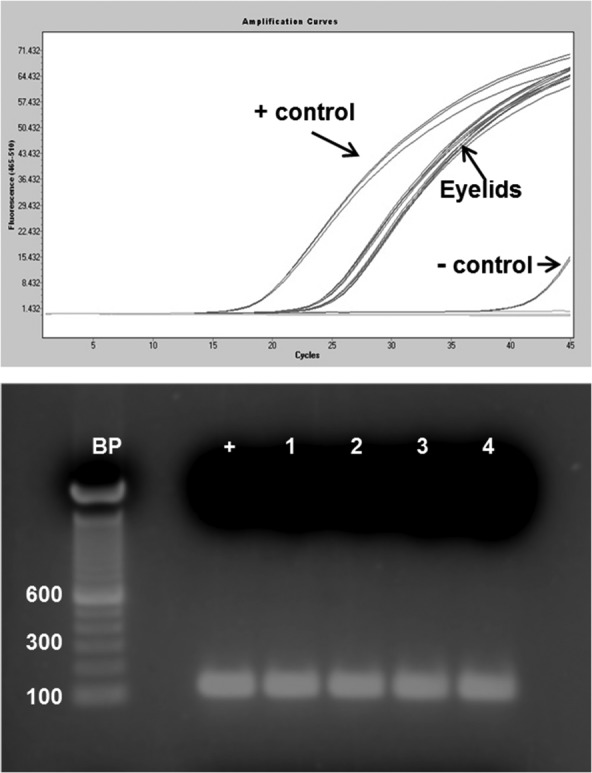
Integrity of RNA in eyelid samples. (A) GAPDH RT-PCR of RNA extracted from frozen sections of four eyelids (in triplicate). (B) 1% agarose gel of samples from (A), showing the GAPDH band (+ is the positive control). BP, 100 base pair ladder.
Figure 3. .
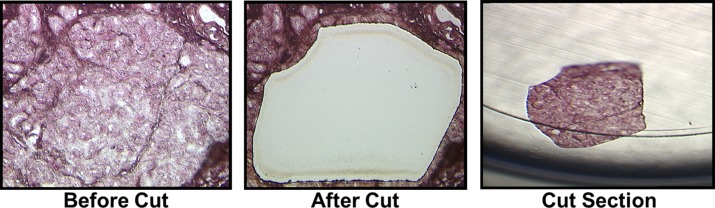
Laser microdissection of a gland of Wolfring from a frozen eyelid section, showing the gland in situ (left), the eyelid section after removal of the gland (center) (original magnification ×100), and the gland of Wolfring tissue isolated from the remainder of the eyelid (right) (original magnification ×50). Tissue was stained with hematoxylin and eosin under RNAase-free conditions.
Figure 4. .
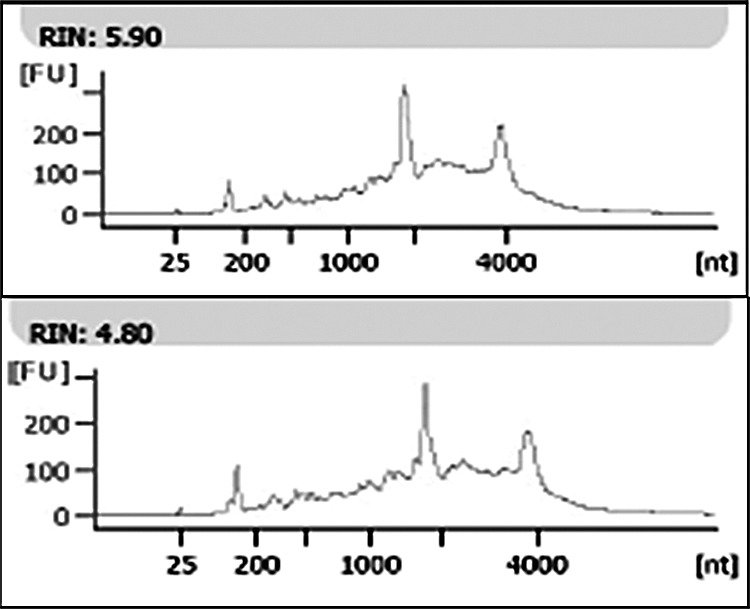
Representative bioanalyzer data demonstrating good-quality RNA extracted from two glands of Wolfring isolated by laser microdissection, as indicted by strong 18S and 28S rRNA peaks. The baseline noise indicates some degradation, as expected in human surgical tissue. Samples with RNA integrity values (RIN) of 4 or greater were submitted for microarray analysis.
Gene Expression in Glands of Wolfring
Ranking of the top 5000 genes expressed by the gland of Wolfring (see Supplementary Table S1, http://www.iovs.org/content/53/11/6738/suppl/DC1) showed that of the 24 most highly expressed genes, 9 have particularly high relevance to lacrimal gland function (Table 2). These include polymeric immunoglobulin receptor, tear lipocalin, lactoferrin, lysozyme, and lacritin, all of which are abundant proteins in the tear fluid. Secretory phospholipase A2, group IIA (Gene ID 5320, intensity 13,805), was also identified in the gland of Wolfring. Four other genes, known to be expressed in salivary glands and of possible relevance to lacrimal function (as discussed below), were expressed in the gland of Wolfring. These include submaxilary gland androgen-regulated protein (Gene ID 10879, intensity 20,385.7), small breast epithelial mucin (also known as mucin-like 1, Gene ID 118430, intensity 20,760.2), histatin 1 (Gene ID 3346, intensity 11,948.5), and mucin 7 (Gene ID 4589, intensity 7550.7).
Table 2. .
The Top 24 Most Highly Expressed Genes in the Gland of Wolfring Include 9 Genes of Particular Relevance to Lacrimal Function
|
Name |
Rank |
Gene ID |
Intensity* |
| Proline-rich lacrimal 1 | 1 | 58503 | 32,347.6 ± 626.0 |
| Polymeric immunoglobulin receptor | 2 | 5284 | 31,460.9 ± 898.3 |
| Lipocalin 1 (tear prealbumin) | 3 | 3933 | 29,758.8 ± 2,057.2 |
| Lactotransferrin | 4 | 4057 | 29,675.6 ± 1,764.5 |
| Proline-rich 4 (lacrimal) | 8 | 11272 | 27,294.0 ± 319.1 |
| Secretoglobin, family 2A, member 1 | 9 | 4246 | 26,779.4 ± 1,594.2 |
| Prolactin-induced protein | 10 | 5304 | 26,756.3 ± 1,334.2 |
| Lysozyme | 12 | 10859 | 25,275.6 ± 421.1 |
| Lacritin | 24 | 90070 | 22,780.6 ± 609.9 |
Intensities were calculated for normalized data, mean ± SD, n = 4 (see also Supplementary Table S1, http://www.iovs.org/content/53/11/6738/suppl/DC1).
Most of the highly expressed genes listed in Table 2 code for secretory proteins, suggesting that the glands of Wolfring are very active in protein synthesis and secretion. Functional Annotation Clustering analysis using DAVID software showed that the glands are enriched in genes involved in protein synthesis, targeting, and secretion (Table 3). Complete lists of the genes included in the functional clusters are provided in Supplementary Table S2 (see Supplementary Materials, http://www.iovs.org/content/53/11/6738/suppl/DC1).
Table 3. .
The Glands of Wolfring Appear to Be Enriched in Genes Related to Protein Synthesis, Targeting, and Secretion, as Determined by Functional Annotation Clustering Analysis Using DAVID
|
Functional Classification |
Enrichment Score |
| Ribosomal proteins | 43.14 |
| RNA processing | 17.35 |
| RNA splicing | 16.99 |
| Protein transport and localization | 10.65 |
| Endoplasmic reticulum | 6.18 |
| Signal recognition particle | 2.74 |
| Vesicle targeting | 2.12 |
| Golgi vesicles | 1.79 |
Enrichment scores ≥1.3 are significant. The 3000 most highly expressed genes listed previously (see Supplementary Table S1, http://www.iovs.org/content/53/11/6738/suppl/DC1) were submitted for classification at high stringency, and 2294 unique DAVID IDs were returned. The complete list of genes included in the functional clusters is provided in Supplementary Table S2 (see Supplementary Materials, http://www.iovs.org/content/53/11/6738/suppl/DC1).
The entire gene list was searched manually for genes related to ion and water transport, yielding numerous genes related to these functions (Table 4). As expected in a transporting tissue, Na+/K+ ATPase was strongly expressed; and all three subunits, α, β, and γ (FXYD domain), were expressed at similar intensities. Numerous sodium, potassium, and chloride channel genes were identified as well as genes for transporters of these ions. Of note, several genes thought to be involved in K+ and Cl− secretion, based on our previous study of main lacrimal gland ducts,23 were strongly expressed. These are highlighted in bold font in Table 4. Genes involved in bicarbonate secretion, specifically sodium/bicarbonate cotransporters and carbonic anhydrase, were also identified. Finally, three aquaporin genes were strongly expressed.
Table 4. .
Representative Genes Related to Ion and Water Transport Expressed in Gland of Wolfring
|
Name |
Gene ID |
Intensity* |
| Sodium channels | ||
| Sodium channel, nonvoltage-gated 1 alpha | 6337 | 2,816.2 ± 927.8 |
| Sodium channel, voltage gated, type II, alpha 2 | 6326 | 313.6 ± 54.5 |
| Sodium channel, voltage gated, type I, alpha | 6323 | 239.8 ± 59.6 |
| Potassium channels | ||
| Potassium intermediate/small conductance calcium-activated channel, subfamily N, member 4 | 3783 | 4,190.8 ± 1,211.2 |
| Potassium voltage-gated channel, shaker-related subfamily, beta member 1 | 7881 | 1,932.7 ± 469.8 |
| Hyperpolarization-activated cyclic nucleotide-gated potassium channel 3 | 57657 | 589.1 ± 75.9 |
| Potassium large conductance calcium-activated channel, subfamily M, alpha member 1 (Maxi K) | 3778 | 538.8 ± 149.6 |
| Chloride channels | ||
| Chloride channel 3 | 1182 | 7,406.0 ± 399.1 |
| Chloride channel, nucleotide sensitive, 1A | 1207 | 1,038.9 ± 301.9 |
| Chloride channel 5 | 1184 | 808.1 ± 227.9 |
| Calcium channels | ||
| Transient receptor potential cation channel, subfamily M, member 7 | 54822 | 1,240.1 ± 430.1 |
| Calcium channel, voltage dependent, L type, alpha 1D subunit | 776 | 1,165.4 ± 281.1 |
| Calcium channel, voltage dependent, P/Q type, alpha 1A subunit | 773 | 1,064.4 ± 169.7 |
| Transporters† | ||
| ATPase, Na+/K+ transporting, alpha 1 polypeptide | 476 | 11,115.4 ± 1,584.8 |
| ATPase, Na+/K+ transporting, beta 1 polypeptide | 481 | 13,625.7 ± 1,276.1 |
| FXYD domain containing ion transport regulator 3 | 5349 | 10,075.4 ± 1,125.1 |
| Solute carrier family 12 (sodium/potassium/chloride transporters), member 2 | 6558 | 10,020.7 ± 739.8 |
| Solute carrier family 4, sodium bicarbonate cotransporter, member 7 | 9497 | 4,205 ± 779.8 |
| Solute carrier family 9 (sodium/hydrogen exchanger), member 1 | 6548 | 2,849.6 ± 414.9 |
| Solute carrier family 12 (potassium/chloride transporters), member 8 | 84561 | 1,202.8 ± 235.6 |
| Solute carrier family 4, sodium bicarbonate cotransporter, member 5 | 57835 | 1,002.7 ± 340.5 |
| Solute carrier family 24 (sodium/potassium/calcium exchanger), member 3 | 57419 | 242.1 ± 71.1 |
| Solute carrier family 7 (cationic amino acid transporter), member 1 | 6541 | 7,977.3 ± 472.7 |
| Carbonic anhydrase | ||
| Carbonic anhydrase II | 760 | 7,008 ± 1,808.1 |
| Carbonic anhydrase XII | 771 | 845.3 ± 246.2 |
| Carbonic anhydrase VI | 765 | 526.4 ± 275.0 |
| Aquaporins | ||
| Aquaporin 3 | 360 | 15,058 ± 981.4 |
| Aquaporin 5 | 362 | 11,271.2 ± 1,546.3 |
| Aquaporin 1 | 358 | 7306.6 ± 2,406.8 |
Genes involved in K+ and Cl− secretion23 are bold.
Intensities were calculated for normalized data, mean ± SD, n = 4.
The gland of Wolfring expresses many solute carrier family genes (see Supplementary Table S3, http://www.iovs.org/content/53/11/6738/suppl/DC1, for a more extensive list).
Based on evidence that the glands of Wolfring are innervated,8–11 we searched the gene list for neurotransmitter receptor genes. Consistent with parasympathetic control, receptors for both acetylcholine and VIP were identified. The glands also express genes for adrenergic receptors. Evidence for control of the glands by hormones and cytokines is provided by the expression of genes for androgen, estrogen, prolactin, EGF, and histamine H1 receptors (Table 5).
Table 5. .
Genes Involved in Control of Lacrimal Function, Expressed in Gland of Wolfring
|
Name |
Gene ID |
Intensity* |
| Cholinergic receptor, muscarinic 3 | 1131 | 1735.3 ± 759 |
| Cholinergic receptor, muscarinic 1 | 1128 | 119 ± 105.6 |
| Butyrylcholinesterase | 590 | 72.2 ± 24.3 |
| Vasoactive intestinal peptide receptor 1 | 7433 | 1234.9 ± 244.9 |
| Vasoactive intestinal peptide receptor 2 | 7434 | 114.5 ± 18.2 |
| Adrenergic, alpha-2A-, receptor | 150 | 582.8 ± 185.1 |
| Adrenergic, beta-2-, receptor | 154 | 440.9 ± 200.8 |
| Adrenergic, alpha-1A-, receptor | 148 | 377.1 ± 68.5 |
| Purinergic receptor P2X, 1 | 5023 | 496.1 ± 68.2 |
| Purinergic receptor P2X, 4 | 5026 | 356.1 ± 150.6 |
| Purinergic receptor P2X, 5 | 5025 | 304.2 ± 34.4 |
| Purinergic receptor P2Y, G-protein coupled, 8 | 286530 | 1453.8 ± 494.1 |
| Androgen receptor (dihydrotestosterone receptor) | 367 | 260.0 ± 273.1 |
| Estrogen receptor 1 | 2099 | 543.1 ± 379.4 |
| Estrogen receptor 2 | 2100 | 125.2 ± 53.0 |
| Prolactin receptor | 5618 | 329.8 ± 161.0 |
| Epidermal growth factor receptor | 1956 | 1234.0 ± 100.7 |
| Epidermal growth factor (beta-urogastrone) | 1950 | 2874.7 ± 484.3 |
| Histamine receptor H1 | 3269 | 130.5 ± 31.1 |
Intensities were calculated for normalized data, mean ± SD, n = 4.
The vast majority of the genes expressed by the gland of Wolfring were expressed at equal intensity in the main lacrimal gland. Analysis with dChip, however, identified 67 genes that were expressed at levels at least 4-fold higher (P ≥ 0.05) in gland of Wolfring than in the main lacrimal gland (see Supplementary Table S4, http://www.iovs.org/content/53/11/6738/suppl/DC1). In the main lacrimal gland, 15 genes were expressed at levels 4-fold greater, or more, than in the gland of Wolfring. The data are presented with the caveat that the comparison is made with only two main lacrimal glands. Human lacrimal tissue with RNA of sufficient quality for microarray analysis is not readily available and, with exceptions,24 studies of gene expression in human lacrimal glands have generally been conducted with low sample numbers.25,26 Several of the genes with large differences in expression levels and/or relevance to lacrimal function are listed in Table 6. Of particular interest were mucin-like 1 (also known as small breast epithelial mucin27,28) and secreted frizzled-related protein, because of their high level of expression in gland of Wolfring, as well as β-defensin 124 and protein kinase Cε (PKCε) because of their relevance to lacrimal gland and tear film function. These genes were chosen for confirmation by RT-PCR. Results were qualitatively in agreement with microarray data for mucin-like 1 (relative expression = 117), frizzled-related protein 2 (relative expression = 7.3) and β-defensin (relative expression = 5.3). The relative expression of PKCε in main lacrimal gland, as compared to gland of Wolfring, was only 0.66, probably due to the very low expression intensity of this gene in the tissues (Table 6).
Table 6. .
Selected Genes Expressed at Significantly Higher Levels in the Gland of Wolfring (GW) Than in the Main Lacrimal Gland (MLG), Ranked in Order of Fold Difference (Δ) Where Positive Numbers Indicate Higher Levels in the Gland of Wolfring
|
Name |
Gene ID |
Intensity GW |
Intensity MLG |
Δ |
P Value |
| Mucin-like 1* | 118430 | 16,601.8 | 499.2 | +33.3 | 0.048 |
| Secreted frizzled-related protein 2* | 6423 | 2,441.6 | 91.1 | +26.8 | 0.011 |
| Carbonic anhydrase VI | 765 | 526.3 | 21.4 | +24.6 | 0.034 |
| Glutathione S-transferase A1 | 2938 | 560.8 | 27.2 | +20.6 | 0.027 |
| Adrenergic, alpha-2A-, receptor | 150 | 582.8 | 70.2 | +8.3 | 0.03 |
| Proenkephalin | 5179 | 184.5 | 23.2 | +8.0 | 0.049 |
| Defensin, beta 124* | 245937 | 202.1 | 33.3 | +6.1 | 0.037 |
| G protein-coupled receptor 30 (GPRC estrogen receptor 1) | 266977 | 26.1 | 5.6 | +4.9 | 0.045 |
| Amylase, alpha 1A; salivary | 276 | 9,236.8 | 1978.8 | +4.7 | 0.014 |
| Histamine receptor H1 | 3269 | 130.5 | 31.5 | +4.1 | 0.008 |
| Major histocompatibility complex, class II, DR beta 4 | 3126 | 46.7 | 222.0 | −4.75 | 0.032 |
| Protein kinase C, epsilon | 5581 | 10.6 | 66.2 | −6.2 | 0.016 |
| Zinc finger and BTB domain containing 16 | 7704 | 104.3 | 1634.5 | −15.7 | 0.022 |
GW: n = 4; MLG: n = 2 (see also supplementary Table S4, http://www.iovs.org/content/53/11/6738/suppl/DC1).
Confirmed by RT-PCR; see text for description.
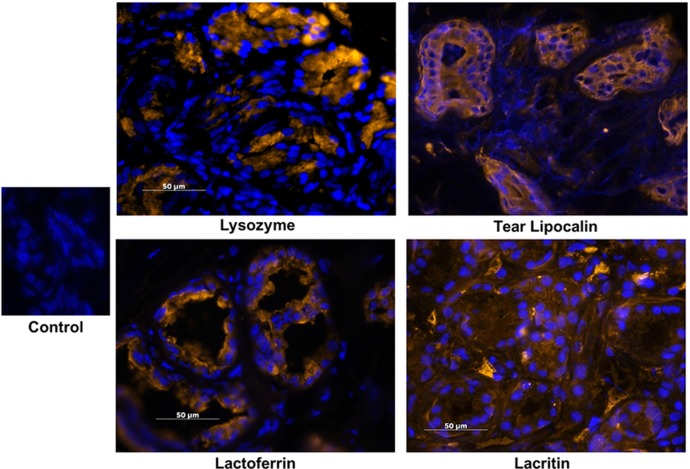
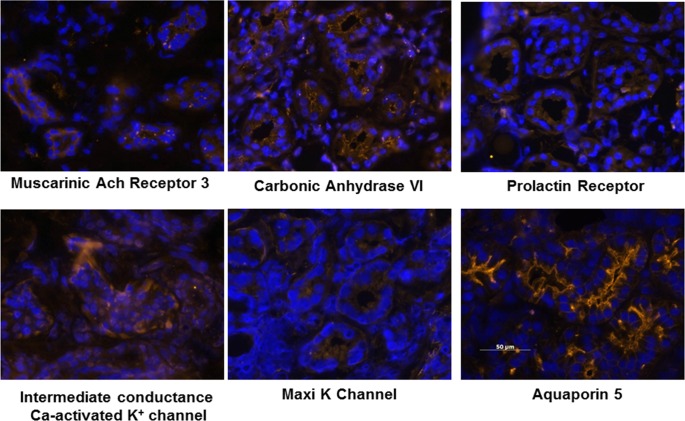
The expression of several genes was also confirmed by immunohistochemistry on frozen sections of eyelid samples containing gland of Wolfring for the presence of their gene products. Intense immunolocalization of the secreted proteins lysozyme, lactoferrin, tear lipocalin, and lacritin, all of which had high levels of gene expression (Table 2), was detected in the glands of Wolfring (Fig. 5). The glands also showed binding of antibodies to proteins involved in secretion of ions and water for which genes were expressed at relatively high levels (Tables 4 and 5). These included the muscarinic acetylcholine receptor 3, intermediate conductance Ca-activated K+ channel, the Maxi K channel, carbonic anhydrase VI, and aquaporin 5 (Fig. 6). The gland of Wolfring also bound antibodies for the prolactin receptor, which may be involved in several lacrimal functions including fluid secretion.
Figure 5. .
Immunohistochemistry on frozen sections of gland of Wolfring using antibodies to several proteins known to be present in tear fluid. Nuclei were stained with DAPI. The control section was exposed to rhodamine-conjugated secondary antibody alone.
Figure 6. .
Immunohistochemistry on frozen sections of gland of Wolfring for several proteins known to be involved in electrolyte and fluid secretion. Nuclei were stained with DAPI.
Discussion
Genes for Secretory Proteins
The profile of gene expression in the glands of Wolfring and the confirmation of the translation of several prominent genes into functionally important proteins, as shown by immunostaining, indicate that these glands are very similar in function to the main lacrimal gland. Only a limited number of genes were differentially expressed between the two gland types, and none were exclusively expressed in the accessory glands. As discussed below, comparison of the various genes listed in Table 2 and Tables 4 through 6 to reports in the literature shows that many of these genes and proteins have previously been identified in the main lacrimal glands of humans and other mammals and that the secretory proteins have been identified in tears.
As indicated by Functional Annotation Clustering (Table 3) and the genes identified in Table 2, the glands of Wolfring are apparently highly active in synthesis and secretion of proteins. As discussed below, the most highly expressed genes coding for these proteins are of importance to tear function. The expression of three of these proteins that are well known for their importance in the function of the lacrimal system, lactoferrrin, lysozyme, and polymeric immunoglobulin receptor (secretory component), was reported in the gland of Wolfring many years ago.4,7 Our data confirm these reports and demonstrate, at the level of gene expression, the predominance of these tear proteins in accessory glands.
Two other well-known tear proteins that we report for the first time as being highly expressed by the glands of Wolfring are tear lipocalin and lacritin. Tear lipocalin is one of the two most abundant tear proteins.29,30 It is a lipid-binding protein and has been identified in the main lacrimal gland.21 We now show that it is one of the four most highly expressed genes in the gland of Wolfring. The lipocalin protein stained intensely in the acinar cells of the gland, suggesting that the accessory glands are important in tear lipocalin secretion.
Lacritin is a more recently discovered protein, named for its original identification in the main lacrimal gland.22 This protein is secreted in the tears and has multiple functions, including antimicrobial activity, promotion of mitosis, and stimulation of tear secretion.31,32 Lacritin is also expressed by cornea, conjunctiva, and meibomian glands.33 We have now demonstrated that lacritin is among the most abundant transcripts in the glands of Wolfring, suggesting that these glands secrete lacritin directly onto the ocular surface.
Secretoglobin, SCGB2A1, is also strongly expressed in the gland of Wolfring (Table 2). Secretoglobins have been identified in human and rabbit tears, as well as the secretions of the prostate, salivary gland, and uterus.34–36 While the precise functions of these proteins have not been confirmed, they are thought to be involved in inflammatory modulation and wound repair, two important processes at the ocular surface. Remington et al.37 detected the mRNA for secretoglobin in rabbit lacrimal gland. The expression of the gene, and the protein has also been reported in human main lacrimal gland. The high level of expression of SCGB2A1 in the glands of Wolfring suggests that the accessory glands also contribute to the secretoglobin that is present in tears.
The genes for three proline-rich proteins, related to genes for similar proteins originally identified in salivary gland, were strongly expressed in the glands of Wolfring. These include proline-rich lacrimal 1, proline-rich lacrimal 4, and submaxillary gland androgen-regulated protein 3B (Table 2), which have previously been identified in the main lacrimal gland.38–40 The proline-rich lacrimal 1 protein translation product, also known as basic proline-rich lacrimal protein,39 has been identified in human tears.41 The gene for this protein was the most highly expressed gene in the accessory glands in the present study. These proteins are of interest in tears because of evidence that they have antimicrobial function in saliva.42 This suggests similar functions in tears.
Among the proteins known to be present salivary glands, histatin-1 has not previously been reported in lacrimal glands. Histatins are abundant in saliva. Their antifungal activity, especially against Candida albicans, is well documented.43 It has recently been reported that histatin can promote wound healing in an in vitro epithelial cell model, and the authors of the report suggested that the presence of histatin in saliva may explain the rapid healing of wounds of the oral mucosa.44 The expression of a gene for histatin in the gland of Wolfring is intriguing since this protein might have similar functions on the ocular surface.
All of the proteins discussed above, as well as phospholipase A2 (listed in Supplementary Table S1, http://www.iovs.org/content/53/11/6738/suppl/DC1, and previously identified in gland of Wolfring45) and β-defensin (Table 6), have known or putative antimicrobial or immunomodulatory functions. Since the accessory lacrimal glands are located in the conjunctiva, they are in close contact with the tear film and other components of the ocular surface. These glands may provide a rapid-response, protective mechanism since signaling molecules from bacteria and fungi or immunogenic molecules might possibly have a direct effect on the accessory glands. This could result in secretion of the proteins discussed above directly into the tear film without the need for activation of the reflex pathway required for stimulation of the main lacrimal gland.
Finally, two of the mucin-related genes expressed by the glands of Wolfring, mucin 7 and mucin-like 1, merit comment. Mucin 7 was the only secreted mucin gene detected in this study. This small, soluble mucin is secreted by salivary glands. The gene is also expressed in the main lacrimal gland, but the protein cannot be detected in tears.46 The identification of the mucin 7 gene in the gland of Wolfring contributes support to the hypothesis that these glands function similarly to the main lacrimal gland.
The gene for mucin-like 1, also known as small breast epithelial mucin (SBEM),27,28 is highly expressed in the gland of Wolfring, and is apparently expressed at a level 33-fold higher in the accessory glands than in the main lacrimal gland (Table 6). It has previously been reported that this small glycoprotein is expressed in mammary epithelium and is overexpressed in breast tumors. It is not expressed in other common sites of primary tumors such as colon, lung, pancreas, and prostate. Until recently it was thought that the only other site of SBEM expression was the salivary gland, and therefore it has been suggested that this gene may be a good marker for metastatic breast cancer.47,48 Our findings, and the report that SBEM is expressed by meibomian glands,33 expand the known tissue expression of this small mucin of unknown function.
Genes for Ion and Water Transport
The channels, transporters, and mechanisms involved in ion and water secretion by the main lacrimal gland have been studied extensively49–53 and have been recently reviewed by Dartt.54 The genes for ion channels and transporters listed in Table 4 that are expressed in the glands of Wolfring, and the prominent expression of aquaporin 5 on the luminal membranes of the acini (Fig. 6), suggest that these glands function similarly to the main lacrimal gland in contributing to the electrolytes and fluid of the tear film. Although fluid secretion by accessory glands cannot easily be measured directly, these observations help to answer questions raised in the past14 and support evidence from Gilbard et al. and Maitchouk et al. that accessory glands contribute significantly to the tear fluid.16,17
In this context, we call attention to two ions in the tears that are of particular importance to the ocular surface epithelium, bicarbonate and potassium. Bicarbonate is the most abundant buffer in tears. We have previously reported that it is required for recovery of the corneal epithelial barrier after damage.55,56 Williams and Watsky showed that bicarbonate is required for maintaining coupling between corneal epithelial cells via gap junctions.57 The current study shows that the glands of Wolfring express sodium/bicarbonate cotransporters and a sodium/hydrogen exchanger. Carbonic anhydrase is strongly expressed on the luminal membranes, as shown in Figure 6. These observations support a role for the accessory glands in secreting bicarbonate.
It is well known that lacrimal gland fluid and tears have a high K+ concentration, 20 to 25 mM. We have previously reported that the duct cells of rat exorbital glands express K+ and Cl− channels and transporters in an orientation on the basal and apical membranes that is consistent with secretion of K+ into the ductal lumen.23 Ding et al. have confirmed these observations in the ducts of rabbit main lacrimal gland.58 We have also recently shown that high extracellular K+ can protect corneal epithelial cells from the apoptotic effects of UVB radiation, suggesting a role for the high levels of this ion in tears in protecting the cornea from the adverse effects of ambient UV exposure.59,60 As highlighted in Table 4 and Figure 6, the glands of Wolfring express most of the same channels and transporters related to K+ secretion that have been identified in the main lacrimal gland, although CFTR was not present. This indicates a possible contribution of these glands to the elevated K+ concentration in tears.
Genes Involved in Control of Lacrimal Function
The control of electrolyte and protein secretion by the main lacrimal gland via parasympathetic, sympathetic, purinergic, and EGF signaling has been well documented.54,61 The innervation of the glands of Wolfring by parasympathetic and sympathetic nerves indicates that these glands are under similar control.8–11 We have now demonstrated for the first time the expression of genes for cholinergic, VIP, and purinergic receptors, and have confirmed the expression of the gene for adrenergic receptors. The presence of EGF in accessory lacrimal glands has previously been reported,5 and we have now detected the expression of the genes for EGF and its receptor. The expression of purinergic receptors is of interest since it has been reported by Murikami et al. that topical P2Y agonists stimulate tear secretion.62 The authors concluded that this response was due to stimulation of P2Y receptors in the conjunctiva. Our observations suggest that the increased tear secretion was due to stimulation of the accessory glands.
The expression of genes for androgen, estrogen, and prolactin receptors (Tables 5 and 6) indicates that, as in the main lacrimal gland,63–65 gene expression and gland function are under hormonal control in the glands of Wolfring. This is evident since several of the most highly expressed genes in the glands of Wolfring (Table 2), polymeric immunoglobin receptor, secretoglobin,35,66 prolactin-induced protein (known to be expressed in main lacrimal gland)67, and submaxilary gland androgen-regulated protein40, are influenced by androgens and prolactin. The changes in levels of androgen, estrogen, and prolactin that occur after menopause, with aging, and in pregnancy are known to have adverse effects on the main lacrimal gland, especially affecting immune function and causing inflammation and decreased fluid secretion.63,68–70 These effects have not been studied directly in the human accessory glands, although it is well known that ocular surface inflammation occurs in dry eye disease.71 It has, however, been reported that the accessory lacrimal glands are inflamed in dry eye dogs.72 If also the case in humans, this may explain the efficacy of topical cyclosporine-A in stimulating tear secretion and increasing tear breakup time in some patients.73
Conclusions
This study has produced a large-scale description of gene expression in human gland of Wolfring. The data indicate that the function of these glands is very similar to that of the main lacrimal glands and support the proposal by Tripathi and Tripathi that these glands are of the same embryonic origin.74 An understanding of accessory gland function is important for the understanding of ocular surface biology and dry eye disease because (1) accessory glands may contribute a functionally significant portion of the tear fluid and its various components; (2) while accessory glands might be especially vulnerable to damage due to inflammation or infection of the ocular surface, due to their location in the conjunctiva, they are also in a position to respond quickly to insults by inflammatory mediators and microbes; and (3) owing to their location, accessory glands can more easily be treated with topically applied drugs than the main lacrimal gland.
Supplementary Material
Acknowledgments
The authors thank Edward Fox, PhD, and Changzhong Chen, MS, Dana Farber Cancer Institute, Harvard Medical School, Boston, Massachusetts, for assistance with microarray analysis; and Suzanne K. Freitag, MD, Massachusetts Eye and Ear, Harvard Medical School, for providing one of the eyelid samples.
Footnotes
Supported by a sabbatical leave and research fellowship granted by Calvin College (JLU), NIH Grant EY03306 (IKG), and the Den Ouden Undergraduate Research Fellowship and West Michigan Optometric Scholarship (REVD).
Disclosure: J.L. Ubels, None; I.K. Gipson, None; S.J. Spurr-Michaud, None; A.S. Tisdale, None; R.E. Van Dyken, None; M.P. Hatton, None
References
- 1.Seifert P, Spitznas M, Koch F, Cusumano A. The architecture of human accessory lacrimal glands. Ger J Ophthalmol. 1993;2:444–454 [PubMed] [Google Scholar]
- 2.Jordan DR, Anderson RB, Mamalis N. Accessory lacrimal glands. Ophthalmic Surg. 1990;21:146–147 [PubMed] [Google Scholar]
- 3.Allansmith MR, Gillette TE. Secretory component in human ocular tissues. Am J Ophthalmol. 1980;89:353–361 [DOI] [PubMed] [Google Scholar]
- 4.Gillette TE, Allansmith MR, Greiner JV, Janusz M. Histologic and immunohistologic comparison of main and accessory lacrimal tissue. Am J Ophthalmol. 1980;89:724–730 [DOI] [PubMed] [Google Scholar]
- 5.Obata H, Horiuchi H, Dobashi Y, Oka T, Sawa M, Machinami R. Immunohistochemical localization of epidermal growth factor in human main and accessory lacrimal glands. Jpn J Ophthalmol. 1993;37:113–121 [PubMed] [Google Scholar]
- 6.Aho HJ, Saari KM, Kallajoki M, Nevalainen TJ. Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Invest Ophthalmol Vis Sci. 1996;37:1826–1832 [PubMed] [Google Scholar]
- 7.Hunt S, Spitznas M, Seifert P, Rauwolf M. Organ culture of human main and accessory lacrimal glands and their secretory behaviour. Exp Eye Res. 1996;62:541–554 [DOI] [PubMed] [Google Scholar]
- 8.Seifert P, Spitznas M. Demonstration of nerve fibers in human accessory lacrimal glands. Graefes Arch Clin Exp Ophthalmol. 1994;232:107–114 [DOI] [PubMed] [Google Scholar]
- 9.Seifert P, Stuppi S, Spitznas M. Distribution pattern of nervous tissue and peptidergic nerve fibers in accessory lacrimal glands. Curr Eye Res. 1997;16:298–302 [DOI] [PubMed] [Google Scholar]
- 10.Seifert P, Spitznas M. Vasoactive intestinal polypeptide (VIP) innervation of the human eyelid glands. Exp Eye Res. 1999;68:685–692 [DOI] [PubMed] [Google Scholar]
- 11.Esmaeli-Gutstein B, Hewlett BR, Harvey JT. Characterization of adrenergic receptors in the accessory lacrimal glands of the upper eyelid. Ophthal Plast Reconstr Surg. 1999;15:245–251 [DOI] [PubMed] [Google Scholar]
- 12.Jones LT. The lacrimal secretory system and its treatment. Am J Ophthalmol. 1966;62:47–60 [DOI] [PubMed] [Google Scholar]
- 13.Scherz W, Dohlman CH. Is the lacrimal gland dispensable? Keratoconjunctivitis sicca after lacrimal gland removal. Arch Ophthalmol. 1975;93:281–283 [DOI] [PubMed] [Google Scholar]
- 14.Jordan A, Baum J. Basic tear flow. Does it exist? Ophthalmology. 1980;87:920–930 [DOI] [PubMed] [Google Scholar]
- 15.Bergmanson JP, Doughty MJ, Blocker Y. The acinar and ductal organisation of the tarsal accessory lacrimal gland of Wolfring in rabbit eyelid. Exp Eye Res. 1999;68:411–421 [DOI] [PubMed] [Google Scholar]
- 16.Gilbard JP, Rossi SR, Heyda KG, Dartt DA. Stimulation of tear secretion by topical agents that increase cyclic nucleotide levels. Invest Ophthalmol Vis Sci. 1990;31:1381–1388 [PubMed] [Google Scholar]
- 17.Maitchouk DY, Beuerman RW, Ohta T, Stern M, Varnell RJ. Tear production after unilateral removal of the main lacrimal gland in squirrel monkeys. Arch Ophthalmol. 2000;118:246–252 [DOI] [PubMed] [Google Scholar]
- 18.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57 [DOI] [PubMed] [Google Scholar]
- 19.Kunert KS, Keane-Myers AM, Spurr-Michaud S, Tisdale AS, Gipson IK. Alteration in goblet cell numbers and mucin gene expression in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2001;42:2483–2489 [PubMed] [Google Scholar]
- 20.Gipson IK, Spurr-Michaud S, Argüeso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506 [DOI] [PubMed] [Google Scholar]
- 21.Glasgow BJ. Tissue expression of lipocalins in human lacrimal and von Ebner's glands: colocalization with lysozyme. Graefes Arch Clin Exp Ophthalmol. 1995;233:513–522 [DOI] [PubMed] [Google Scholar]
- 22.Sanghi S, Kumar R, Lumsden A, et al. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J Mol Biol. 2001;310:127–139 [DOI] [PubMed] [Google Scholar]
- 23.Ubels JL, Hoffman HM, Srikanth S, Resau JH, Webb CP. Gene expression in rat lacrimal gland duct cells collected using laser capture microdissection: evidence for K+ secretion by duct cells. Invest Ophthalmol Vis Sci. 2006;47:1876–1885 [DOI] [PubMed] [Google Scholar]
- 24.Jäger K, Bönisch U, Risch M, Worlitzsch D, Paulsen F. Detection and regulation of cationic amino acid transporters in healthy and diseased ocular surface. Invest Ophthalmol Vis Sci. 2009;50:1112–1121 [DOI] [PubMed] [Google Scholar]
- 25.Ubels JL, Dennis MH, Rigatti BW, Vergnes JP, Beatty R, Kinchington PR. Nuclear retinoic acid receptors in the lacrimal gland. Curr Eye Res. 1995;14:1055–1062 [DOI] [PubMed] [Google Scholar]
- 26.Ozyildirim AM, Wistow GJ, Gao J, et al. The lacrimal gland transcriptome is an unusually rich source of rare and poorly characterized gene transcripts. Invest Ophthalmol Vis Sci. 2005;46:1572–1580 [DOI] [PubMed] [Google Scholar]
- 27.Colpitts TL, Billing P, Granados E, et al. Identification and immunohistochemical characterization of a mucin-like glycoprotein expressed in early stage breast carcinoma. Tumour Biol. 2002;23:263–278 [DOI] [PubMed] [Google Scholar]
- 28.Skliris GP, Hubé F, Gheorghiu I, et al. Expression of small breast epithelial mucin (SBEM) protein in tissue microarrays (TMAs) of primary invasive breast cancers. Histopathology. 2008;52:355–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fullard RJ, Kissner DM. Purification of the isoforms of tear specific prealbumin. Curr Eye Res. 1991;10:613–628 [DOI] [PubMed] [Google Scholar]
- 30.Glasgow BJ, Gasymov OK. Focus on molecules: tear lipocalin. Exp Eye Res. 2011;92:242–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKown RL, Wang N, Raab RW, et al. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res. 2009;88:848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samudre S, Lattanzio FA Jr, Lossen V, et al. Lacritin, a novel human tear glycoprotein, promotes sustained basal tearing and is well tolerated. Invest Ophthalmol Vis Sci. 2011;52:6265–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Richards SM, Lo K, Hatton M, Fay A, Sullivan DA. Changes in gene expression in human meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:2727–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glasgow BJ, Abduragimov AR, Gassymov OK, Faull KF, Yusifov TN, Lehrer RI. Characterization of a lipophilin in rabbit tears. Adv Exp Med Biol. 2002;506:573–580 [DOI] [PubMed] [Google Scholar]
- 35.Stoeckelhuber M, Messmer EM, Schmidt C, Xiao F, Schubert C, Klug J. Immunohistochemical analysis of secretoglobin SCGB 2A1 expression in human ocular glands and tissues. Histochem Cell Biol. 2006;126:103–109 [DOI] [PubMed] [Google Scholar]
- 36.Jackson BC, Thompson DC, Wright MW, et al. Update of the human secretoglobin (SCGB) gene superfamily and an example of “evolutionary bloom” of androgen-binding protein genes within the mouse Scgb gene superfamily. Hum Genomics. 2011;5:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remington SG, Crow JM, Nelson JD. Secretoglobins: lacrimal gland-specific rabbit lipophilin mRNAs. Invest Ophthalmol Vis Sci. 2008;49:2856–2862 [DOI] [PubMed] [Google Scholar]
- 38.Dickinson DP, Thiesse M. A major human lacrimal gland mRNA encodes a new proline-rich protein family member. Invest Ophthalmol Vis Sci. 1995;36:2020–2031 [PubMed] [Google Scholar]
- 39.Dickinson DP, Thiesse M. cDNA cloning of an abundant human lacrimal gland mRNA encoding a novel tear protein. Curr Eye Res. 1996;15:377–386 [DOI] [PubMed] [Google Scholar]
- 40.Isemura S. Nucleotide sequence of gene PBII encoding salivary proline-rich protein P-B. J Biochem. 2000;127:393–398 [DOI] [PubMed] [Google Scholar]
- 41.Fung KY, Morris C, Sathe S, Sack R, Duncan MW. Characterization of the in vivo forms of lacrimal-specific proline-rich proteins in human tear fluid. Proteomics. 2004;4:3953–3959 [DOI] [PubMed] [Google Scholar]
- 42.Ligtenberg AJ, Walgreen-Weterings E, Veerman EC, de Soet JJ, de Graaff J, Amerongen AV. Influence of saliva on aggregation and adherence of Streptococcus gordonii HG 222. Infect Immun. 1992;60:3878–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruissen AL, Groenink J, Helmerhorst EJ, et al. Effects of histatin 5 and derived peptides on Candida albicans. Biochem J. 2001;356:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oudhoff MJ, Bolscher JG, Nazmi K, et al. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J. 2008;22:3805–3812 [DOI] [PubMed] [Google Scholar]
- 45.Aho HJ, Saari KM, Kallajoki M, Nevalainen TJ. Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Invest Ophthalmol Vis Sci. 1996;37:1826–1832 [PubMed] [Google Scholar]
- 46.Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007;84:939–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miksicek RJ, Myal Y, Watson PH, Walker C, Murphy LC, Leygue E. Identification of a novel breast- and salivary gland-specific, mucin-like gene strongly expressed in normal and tumor human mammary epithelium. Cancer Res. 2002;62:2736–2740 [PubMed] [Google Scholar]
- 48.Liu ZZ, Xie XD, Qu SX, Zheng ZD, Wang YK. Small breast epithelial mucin (SBEM) has the potential to be a marker for predicting hematogenous micrometastasis and response to neoadjuvant chemotherapy in breast cancer. Clin Exp Metastasis. 2010;27:251–259 [DOI] [PubMed] [Google Scholar]
- 49.Mircheff AK. Lacrimal fluid and electrolyte secretion: a review. Curr Eye Res. 1989;8:607–617 [DOI] [PubMed] [Google Scholar]
- 50.Tan YP, Marty A, Trautmann A. High density of Ca2+-dependent K+ and Cl−channels on the luminal membrane of lacrimal acinar cells. Proc Natl Acad Sci U S A. 1992;89:11229–11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishida N, Hirai SI, Mita S. Immunolocalization of aquaporin homologs in mouse lacrimal glands. Biochem Biophys Res Commun. 1997;238:891–895 [DOI] [PubMed] [Google Scholar]
- 52.Walcott B, Birzgalis A, Moore LC, Brink PR. Fluid secretion and the Na+, K+, Cl− cotransporter in mouse exorbital lacrimal gland. Am J Physiol Cell Physiol. 2005;289:C860–867 [DOI] [PubMed] [Google Scholar]
- 53.Selvam S, Thomas PB, Gukasyan HJ, et al. Transepithelial bioelectrical properties of rabbit acinar cell monolayers on polyester membrane scaffolds. Am J Physiol Cell Physiol. 2007;293:C1412–1419 [DOI] [PubMed] [Google Scholar]
- 54.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez Bernal D, Ubels JL. Artificial tear composition and promotion of recovery of the damaged corneal epithelium. Cornea. 1993;12:115–120 [DOI] [PubMed] [Google Scholar]
- 56.Ubels JL, McCartney MD, Lantz WK, Beaird J, Dayalan A, Edelhauser HF. Effects of preservative-free artificial tear solutions on corneal epithelial structure and function. Arch Ophthalmol. 1995;113:371–378 [DOI] [PubMed] [Google Scholar]
- 57.Williams KK, Watsky MA. Bicarbonate promotes dye coupling in the epithelium and endothelium of the rabbit cornea. Curr Eye Res. 2004;28:109–120 [DOI] [PubMed] [Google Scholar]
- 58.Ding C, Parsa L, Nandoskar P, Zhao P, Wu K, Wang Y. Duct system of the rabbit lacrimal gland: structural characteristics and role in lacrimal secretion. Invest Ophthalmol Vis Sci. 2010;51:2960–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singleton KR, Will DS, Schotanus MP, et al. Elevated extracellular K+ inhibits apoptosis of corneal epithelial cells exposed to UV-B radiation. Exp Eye Res. 2009;89:140–151 [DOI] [PubMed] [Google Scholar]
- 60.Ubels JL, Van Dyken RE, Louters JR, Schotanus MP, Haarsma LD. Potassium ion fluxes in corneal epithelial cells exposed to UVB. Exp Eye Res. 2011;92:425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hodges RR, Vrouvlianis J, Scott R, Dartt DA. Identification of P2X3 and P2X7 purinergic receptors activated by ATP in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2011;52:3254–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murakami T, Fujihara T, Nakamura M, Nakata K. P2Y2 receptor stimulation increases tear fluid secretion in rabbits. Curr Eye Res. 2000;21:782–787 [DOI] [PubMed] [Google Scholar]
- 63.Sullivan DA. Tearful relationships? Sex, hormones, the lacrimal gland, and aqueous-deficient dry eye. Ocul Surf. 2004;2:92–123 [DOI] [PubMed] [Google Scholar]
- 64.Wood RL, Zhang J, Huang ZM, et al. Prolactin and prolactin receptors in the lacrimal gland. Exp Eye Res. 1999;69:213–226 [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Chiu CT, Nakamura T, et al. Elevated prolactin redirects secretory vesicle traffic in rabbit lacrimal acinar cells. Am J Physiol Endocrinol Metab. 2007;292:E1122–1134 [DOI] [PubMed] [Google Scholar]
- 66.Xiao F, Mirwald A, Papaioannou M, Baniahmad A, Klug J. Secretoglobin 2A1 is under selective androgen control mediated by a peculiar binding site for Sp family transcription factors. Mol Endocrinol. 2005;19:2964–2978 [DOI] [PubMed] [Google Scholar]
- 67.Myal Y, Iwasiow B, Cosby H, et al. Analysis of tissue- and hormone-specific regulation of the human prolactin-inducible protein/gross cystic disease fluid protein-15 gene in transgenic mice. J Mol Endocrinol. 1998;21:217–223 [DOI] [PubMed] [Google Scholar]
- 68.Schechter J, Carey J, Wallace M, Wood R. Distribution of growth factors and immune cells are altered in the lacrimal gland during pregnancy and lactation. Exp Eye Res. 2000;71:129–142 [DOI] [PubMed] [Google Scholar]
- 69.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326 [DOI] [PubMed] [Google Scholar]
- 70.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009;127:763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lemp MA, Baudoine C, Baum J. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5:75–92 [DOI] [PubMed] [Google Scholar]
- 72.Stern MA. Pathophysiology and allergy of the lacrimal functional unit: what goes wrong with our tear-secreting apparatus? Asian J Ophthalmol. 2005;7(S1):5–8 [Google Scholar]
- 73.Perry HD, Solomon R, Donnenfeld ED, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. 2008;126:1046–1050 [DOI] [PubMed] [Google Scholar]
- 74.Tripathi BJ, Tripathi RC. Evidence for the neuroectodermal origin of the human lacrimal gland. Invest Ophthalmol Vis Sci. 1990;31:393–395 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.