Abstract
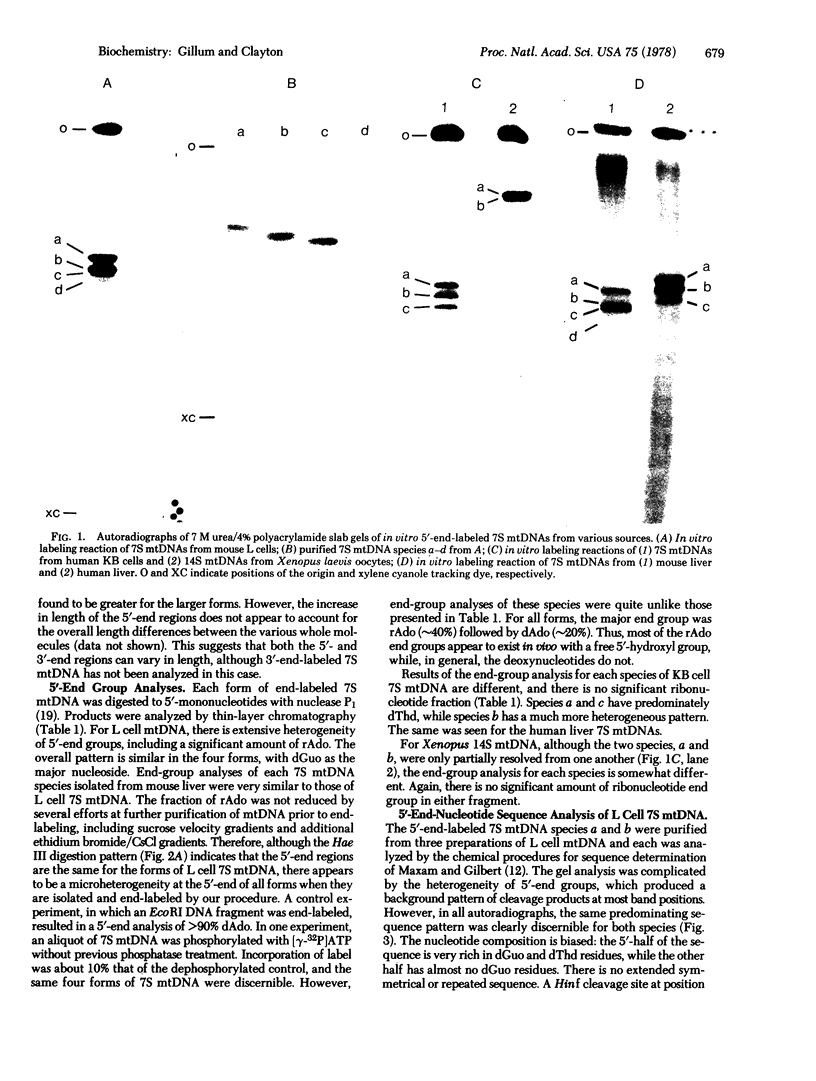
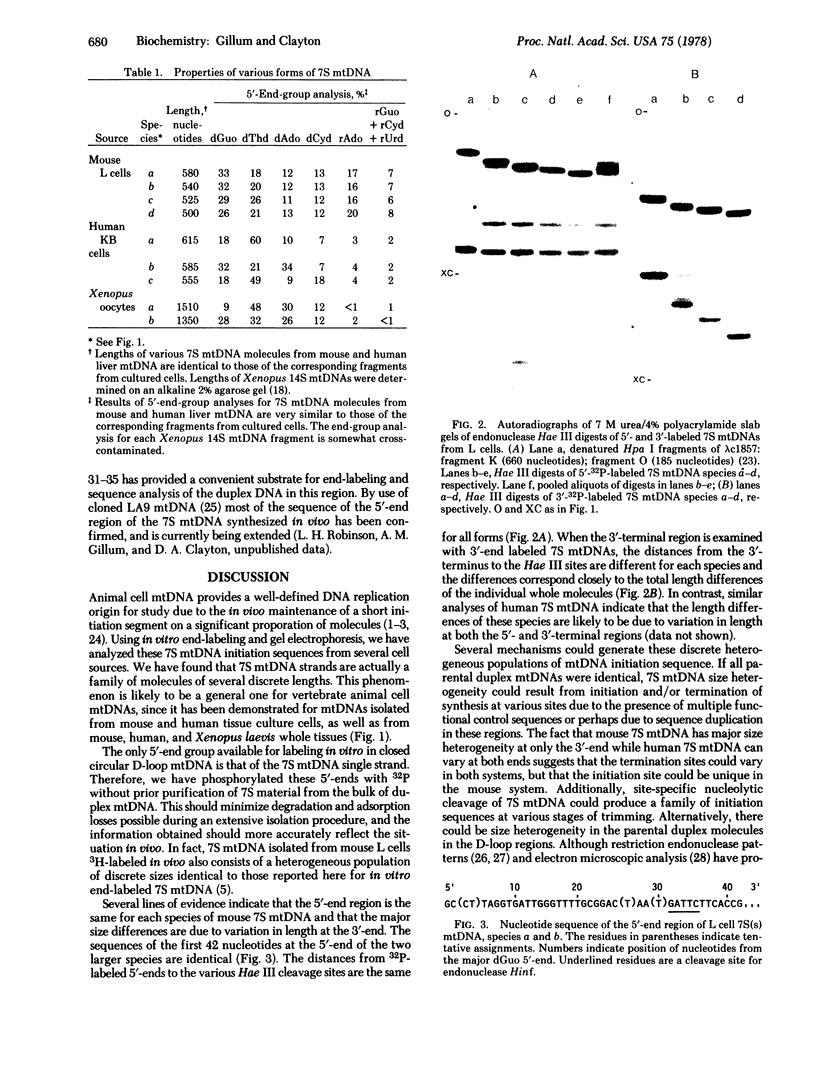
The single-stranded mitochondrial DNA (mtDNA) displacement-loop initiation sequence (7S mtDNA) is hydrogen-bonded at the origin of replication in animal cell mtDNA. Analysis of 7S mtDNA from several cell sources indicates that this initiation sequence exists as a family of fragments of relatively discrete lengths. mtDNA from both mouse L cells and mouse liver has four major sizes of 7S mtDNA fragments, ranging from 500 to 580 nucleotides in length. The 5′-end region of each of these species is the same; thus, the size heterogeneity is due primarily to differences in length at the 3′-end of these molecules. By contrast, 7S mtDNA from both human KB cells and human liver exists in three major forms, ranging from 555 to 615 nucleotides in length, due to differences at both terminal regions. The mtDNA initiation sequence from Xenopus laevis oocytes also exists in at least two forms, 1350 and 1510 nucleotides in length. Thus, the maintenance of multiple forms of mtDNA initiation sequence appears to be a general phenomenon of animal cells, although the precise mechanism of synthesis or processing of these forms is variable.
The sequence of 42 nucleotides at the 5′-end of 7S mtDNA from mouse L cells has been determined and found to be rich in dGuo and dThd residues, with no apparent palindromes or potential secondary structures. We thus present sequence information on the replication origin of mtDNA, as defined by the naturally occurring 7S mtDNA.
Keywords: DNA replication, 5′-terminal labeling, nucleotide sequence, RNA primer, Hae III endonuclease
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastia D. The nucleotide sequence surrounding the origin of DNA replication of Col E1. Nucleic Acids Res. 1977 Sep;4(9):3123–3142. doi: 10.1093/nar/4.9.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: asynchronous replication of strands, segregation of circular daughter molecules, aspects of topology and turnover of an initiation sequence. J Mol Biol. 1974 Jul 15;86(4):801–824. doi: 10.1016/0022-2836(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: topology of circular daughter molecules and dynamics of catenated oligomer formation. J Mol Biol. 1976 Jan 5;100(1):85–92. doi: 10.1016/s0022-2836(76)80036-8. [DOI] [PubMed] [Google Scholar]
- Blakesley R. W., Wells R. D. 'Single-stranded' DNA from phiX174 and M13 is cleaved by certain restriction endonucleases. Nature. 1975 Oct 2;257(5525):421–422. doi: 10.1038/257421a0. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Chang A. C., Lansman R. A., Clayton D. A., Cohen S. N. Studies of mouse mitochondrial DNA in Escherichia coli: structure and function of the eucaryotic-procaryotic chimeric plasmids. Cell. 1975 Oct;6(2):231–244. doi: 10.1016/0092-8674(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Fisher P. A., Korn D. DNA polymerase-alpha. Purification and structural characterization of the near homogeneous enzyme from human KB cells. J Biol Chem. 1977 Sep 25;252(18):6528–6535. [PubMed] [Google Scholar]
- Francisco J. F., Simpson M. V. The occurrence of two types of mitochondrial DNA in rat populations as detected by EcoRI endonuclease analysis. FEBS Lett. 1977 Jul 15;79(2):290–294. doi: 10.1016/0014-5793(77)80805-3. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg R. L. Mitochondrial DNA in Xenopus laevis oocytes. I. Displacement loop occurrence. Dev Biol. 1974 Jun;38(2):346–355. doi: 10.1016/0012-1606(74)90012-8. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Site-specific cleavage of single-stranded DNA by a Hemophilus restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2555–2558. doi: 10.1073/pnas.72.7.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Roychoudhury R., Wu R. Nucleotide sequence with elements of an unusual two-fold rotational symmetry in the region of origin of replication of SV40 DNA+. Biochem Biophys Res Commun. 1976 Apr 5;69(3):678–686. doi: 10.1016/0006-291x(76)90929-3. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Grossman L. I., Robberson D. L., Watson R., Vinograd J. The replication and structure of mitochondrial DNA in animal cells. Cold Spring Harb Symp Quant Biol. 1974;38:281–288. doi: 10.1101/sqb.1974.038.01.031. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehaug J. R., Kleppe K. Effect of salts and polyamines on T4 polynucleotide kinase. Biochemistry. 1975 Mar 25;14(6):1225–1229. doi: 10.1021/bi00677a021. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Philipps G. R. Analysis of purified tRNA species by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Dec;44(2):345–357. doi: 10.1016/0003-2697(71)90220-x. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Newbold J. E., Hutchison C. A., 3rd, Edgell M. H. Specific cleavage analysis of mammalian mitochondrial DNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4496–4500. doi: 10.1073/pnas.72.11.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A., Morrow J. F. Cleavage of replicating forms of mitochondrial DNA by EcoRI endonuclease. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4447–4451. doi: 10.1073/pnas.71.11.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Replication of mitochondrial DNA in mouse L cells and their thymidine kinase - derivatives: displacement replication on a covalently-closed circular template. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3810–3814. doi: 10.1073/pnas.69.12.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. H., Landy A. HindII, HindIII, and HpaI restriction fragment maps of bacteriophage lambda DNA. Gene. 1977 Sep;2(1):1–31. doi: 10.1016/0378-1119(77)90019-1. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Brutlag D., Clayton D. A. The mitochondrial DNA of Drosophila melanogaster exists in two distinct and stable superhelical forms. Cell. 1977 Oct;12(2):471–482. doi: 10.1016/0092-8674(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. N., Dhar R., Weissman S. M. Nucleotide sequence of a fragment of SV40 DNA that contains the origin of DNA replication and specifies the 5' ends of "early" and "late" viral RNA. III. Construction of the total sequence of EcoRII-G fragment of SV40 DNA. J Biol Chem. 1977 Jan 10;252(1):355–367. [PubMed] [Google Scholar]
- Tomizawa J. I., Ohmori H., Bird R. E. Origin of replication of colicin E1 plasmid DNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1865–1869. doi: 10.1073/pnas.74.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchinsky M. F., Shershneva L. P. A simple method of quantitative recovery of nucleotides from thin layers. Anal Biochem. 1973 Aug;54(2):315–318. doi: 10.1016/0003-2697(73)90358-8. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Dawid I. B. Mapping of mitochondrial DNA of individual sheep and goats: rapid evolution in the D loop region. Cell. 1977 Jul;11(3):571–583. doi: 10.1016/0092-8674(77)90075-7. [DOI] [PubMed] [Google Scholar]