Abstract
BACKGROUND
Contact lens induced papillary conjunctivitis (CLPC) continues to be a major cause of dropout during contact lens extended wear. This retrospective study explores risk factors for the development of CLPC during silicone hydrogel lens extended wear.
METHODS
Data from 205 subjects enrolled in the Longitudinal Analysis of Silicone Hydrogel Contact Lens (LASH) study wearing lotrafilcon A silicone hydrogel lenses for up to 30 days of continuous wear were used to determine risk factors for CLPC in this secondary analysis of the main cohort. The main covariates of interest included substantial lens-associated bacterial bioburden, and topographically determined lens base curve-to-cornea fitting relationships. Additional covariates of interest included history of prior adverse events, time of year, race, education level, gender and other subject demographics. Statistical analyses included univariate logistic regression to assess the impact of potential risk factors on the binary CLPC outcome, and Cox proportional hazards regression to describe the impact of those factors on time-to-CLPC diagnosis.
RESULTS
Across 12 months of follow-up, 52 subjects (25%) experienced CLPC. No associations were found between CLPC development and the presence of bacterial bioburden, lens-to-cornea fitting relationships, history of prior adverse events, gender or race. CLPC development followed the same seasonal trends as the local peaks in environmental allergans.
CONCLUSIONS
Lens fit and biodeposits, in the form of lens associated bacterial bioburden, were not associated with the development of CLPC during extended wear with lotrafilcon A silicone hydrogel lenses.
Introduction
Contact lens induced papillary conjunctivitis (CLPC) was first reported by Spring in 19741. The incidence of CLPC varies greatly by lens type and wearing modality and has been reported anywhere from 1.5 to 47.5%2. The incidence dropped from 36% to 4.5% in a retrospective study performed by Porazinski and Donshik3 by refitting patients that were replacing lenses greater than every 4 weeks with lenses replaced every 1 day to 3 weeks. With the increased use of silicone hydrogel lenses for extended wear4 there has been an increase in inflammatory reactions in the eye, including CLPC2,5. The pro-inflammatory changes that occur on the ocular surface when wearing extended wear lenses are secondary to tear stagnation, localized pressure, and a closed eye environment producing a subclinical inflammatory state coupled with frictional rubbing of the lens on the upper palpebral conjunctiva2.
Contact lens dropout related to discomfort has received attention in the recent literature6,7. During daily and extended wear, one of the leading causes of discomfort and discontinuation is CLPC4,6. CLPC does not cause permanent damage but leads to increased lens movement and awareness, itching and mucous discharge and may require the patient to discontinue contact lens wear until the condition clears.
CLPC can present as either a local reaction or a generalized state where papillae are enlarged and spread across the entire palpebral conjunctiva2,8. Local CLPC is defined when the papillae are confined to at most 2 sections of the upper tarsal plate. Although it is unclear what causes CLPC, it has been hypothesized that local CLPC is caused by mechanical trauma, while general CLPC is caused by an immunological response to bio-deposits that accumulate on patients’ contact lenses 4,8. Local papillae have been found to develop in an area of protruding sutures or corneal ulcers8,9,10 or in response to the lens’ edge2 rubbing against upper palpebral conjunctiva indicating that constant contact with a stimulus can cause such localized response. Silicone hydrogel lenses which have a higher modulus of elasticity compared to their low Dk counterparts are thought to contribute to such mechanical trauma associated with local CLPC2,11,12. Alternatively, Skotnitsky and colleagues found that patients wearing aspheric lenses appear to suffer from local CLPC less than those wearing spherical lenses because the aspheric lens better mimics the shape of the cornea2. In another study, Skotnitsky et al also noted that contact lens wearers suffering from allergies are more prone to develop general CLPC during allergy season9 because of the involvement of Type 1 hypersensitivity, and Zhao et al. discovered a higher level of IgE present in the tears of patients with diagnosed CLPC13. Bio-deposits with exposure of the upper lid to allergens that are found on the contact lens surface could be the initiating factor and as a result a CLPC immunologic or hypersensitivity reaction occurs4. In fact, in the days of thermal disinfection, general CLPC was thought to be related to denatured proteins on the lens surface secondary to the heat disinfection process.
The Longitudinal Analysis of Silicone Hydrogel (LASH) Contact Lens study was a prospective cohort study that included 205 subjects wearing lotrafilcon A lenses for up to 30 days of continuous wear and followed for 12 months. The most common reason for dropout from the study was the development of CLPC. Bacterial lens contamination was found to be associated with a 4-8 fold increase in risk for the development of corneal inflammatory events14. Contact lens bacterial bioburden may be considered one form of a bio-deposit that leads to a clinically evident or subclinical inflammatory response on the ocular surface. Currently, there is no published literature exploring the potential connection between bioburden and CLPC yet the link is biologically plausible. Additionally, in previous studies, the lens to cornea fitting relationship as a risk factor for CLPC has not been explored in detail. The LASH Study assessed corneal topography and lens microbial contamination in detail and thus this cohort affords an opportunity to study these risk factors for CLPC development during SH lens continuous wear. Therefore, in this analysis, topographically determined lens fitting relationships and bacterial lens contamination are the primary covariates assessed for an association with contact lens induced papillary conjunctivitis.
Methods
This study is a secondary analysis of data from the LASH Study. The LASH Study was a prospective cohort study of subjects fit to the lotrafilcon A silicone hydrogel lens (Ciba Vision, Duluth, GA) for up to 29 nights or 30 consecutive days of continuous wear, with monthly disposal, and followed for 1 year. The primary outcome was the time to development of a corneal infiltrative event and the study was powered for the primary analysis. The study was approved by the University Hospitals Case Medical Center Institutional Review Board and followed all the Tenets of the Declaration of Helsinki. The LASH Study cohort and design have been detailed elsewhere11,12.In brief, healthy subjects were at least 15 years of age, with refractive errors between +6.00 Diopters (D) to −10.00 D, minimal or no astigmatism, flat and steep keratometry readings between 39.00 D and 48.00 D, and no contraindications to continuous wear lens use. Subjects returned for visits after 1 week of extended wear, and then after 1, 4, 8 and 12 months of continuous wear. At every visit, each eye was clinically assessed via detailed slit lamp examinations and corneal topography using the Orbscan II system (Bausch & Lomb, Rochester, NY). Each visit also included an assessment of lens fit, posterior lens debris, and front surface lens wetting and deposits. The Institute for Eye Research (IER) grading scales15 were used for grading upper tarsal plate redness and roughness, limbal redness, bulbar redness, conjunctival staining in 4 peripheral corneal zones, and corneal staining in 5 corneal zones. At selected visits, the lids, conjunctivae and lenses were cultured and the bioburden assessed as described previously14. Substantial bioburden was identified if a lens harbored any pathogenic species or higher than normal levels of commensal species as described previously14.
Key measures at baseline for the 205 enrolled LASH subjects included demographic (see Table 1) and clinical variables, in addition to lens neophyte status (has not worn contact lenses within the last 12 months), and an indicator of prior adverse events. Other than traditional assessments of lens movement, lag and sag, there is no practical clinically-derived method for assessing posterior tear lens thickness or edge standoff. Therefore, lens-to-ocular surface fitting relationships were assessed by determining the topographically derived corneal Best Fit Sphere (BFS) compared to the lens base curve. That is, the difference between the base curve of the lens and corneal BFS was the fitting variable of interest. This was thought to be the best available surrogate for overall lens draping which could influence lid mechanics and interactions.
Table 1.
Demographic and other baseline summaries, overall and by eventual CLPC status
| Variables | Full dataset, N=205 | CLPC, n=52 | No CLPC, n=153 | |||
|---|---|---|---|---|---|---|
| n | percent | n | percent of CLPC group |
n | percent of non- CLPC group |
|
| Sex | ||||||
| Female | 157 | 76.6% | 39 | 75.0% | 118 | 77.1% |
|
Age (mean 32.8 y, range 15-62) |
||||||
| Under 21 | 26 | 12.7% | 4 | 7.7% | 22 | 14.4% |
| 21-50 | 163 | 79.5% | 45 | 86.5% | 118 | 77.1% |
| 50+ | 16 | 7.8% | 3 | 5.8% | 13 | 8.5% |
|
Previous soft lens
wear experience |
||||||
| Current or recent | 152 | 74.1% | 37 | 71.2% | 115 | 75.2% |
| Neophytes | ||||||
| Never wore | 21 | 10.2% | 7 | 13.5% | 14 | 9.2% |
| Discontinued more than 12 months ago |
32 | 15.6% | 8 | 15.4% | 24 | 15.7% |
| Race | ||||||
| Caucasian | 115 | 56.1% | 27 | 51.9% | 88 | 57.5% |
| African-American | 49 | 23.9% | 14 | 26.9% | 35 | 22.9% |
| Asian | 31 | 15.1% | 7 | 13.5% | 24 | 15.7% |
| Other | 10 | 4.9% | 4 | 7.7% | 6 | 3.9% |
|
Education (highest level achieved, 203 reported) |
||||||
| High School | 17 | 8.4% | 1 | 1.9% | 16 | 10.6% |
| Some College | 53 | 26.1% | 14 | 26.9% | 39 | 25.8% |
| College Degree (4- year) |
61 | 30.0% | 17 | 32.7% | 44 | 29.1% |
| Graduate Work | 72 | 35.5% | 20 | 38.5% | 52 | 34.4% |
|
Smoking Status (201 reported) |
||||||
| Current | 21 | 10.4% | 6 | 11.5% | 15 | 10.1% |
| Never | 170 | 84.6% | 43 | 82.7% | 127 | 85.2% |
| Former | 10 | 5.0% | 3 | 5.8% | 7 | 4.7% |
|
History of previous adverse events (204 reported) |
||||||
| Yes | 90 | 44.1% | 24 | 46.2% | 66 | 43.4% |
| No | 93 | 45.6% | 28 | 53.8% | 65 | 42.8% |
| N/A | 21 | 10.3% | 0 | 0.0% | 21 | 13.8% |
| Contact lens power | ||||||
| Greater than +/− 5.00 D |
63 | 30.7% | 1 | 1.9% | 62 | 40.5% |
The primary outcome, CLPC, was defined as an increase from baseline in the level of redness and/or roughness of two or more grades. Data was drawn from the eye experiencing the event of interest (CLPC) or the right eye for event free subjects. If the subject experienced bilateral CLPC at a single visit, data from the more severely affected eye was used.
Thirty microbial contaminants were cultured. Levels of bioburden were split into 4 groups: no bacteria cultured (0), normal flora in expected quantities present (1), normal flora but cultured in abnormal quantities (2), pathologic bacteria present (3). For the purpose of this analysis, these groups were collapsed into a dichotomous variable; groups 0 and 1 were classified as “negative bioburden” whereas groups 2 and 3 were classified as “positive bioburden”. In brief, normal flora or organisms of low pathogenicity including coagulase negative staphylococci and Bacillus were classified as abnormal if more than 10 colony forming units were cultured from the lens, and Corynebacterium was classified as abnormal if more than 100 colony forming units were cultured. All other pathogenic bacteria were classified as abnormal at any degree. Bioburden data, for those affected with CLPC, was included only if the culture was done prior to the CLPC diagnosis in the affected eye.
The seasonality of the diagnosis of CLPC was assessed to see if it followed the same pattern as the environmental allergy season in Cleveland, Ohio. This was done because it has been reported that a reaction to allergens is something common to those suffering from general CLPC 8,9.
Eye-level (as opposed to person-level) statistical analyses included logistic regression models to describe the impact of potential risk factors on the binary CLPC outcome, and Cox proportional hazards models to describe the impact of those factors on time-to-CLPC diagnosis. Initially, univariate logistic regression was used to test each of the exploratory variables independently on the outcome of CLPC status. As none of the univariate analyses showed significance, there was no formal multivariate model building. However, selected biologically plausible covariates were added to multivariate models to assess the combined effects of the resulting set of variables on the outcome.
Results
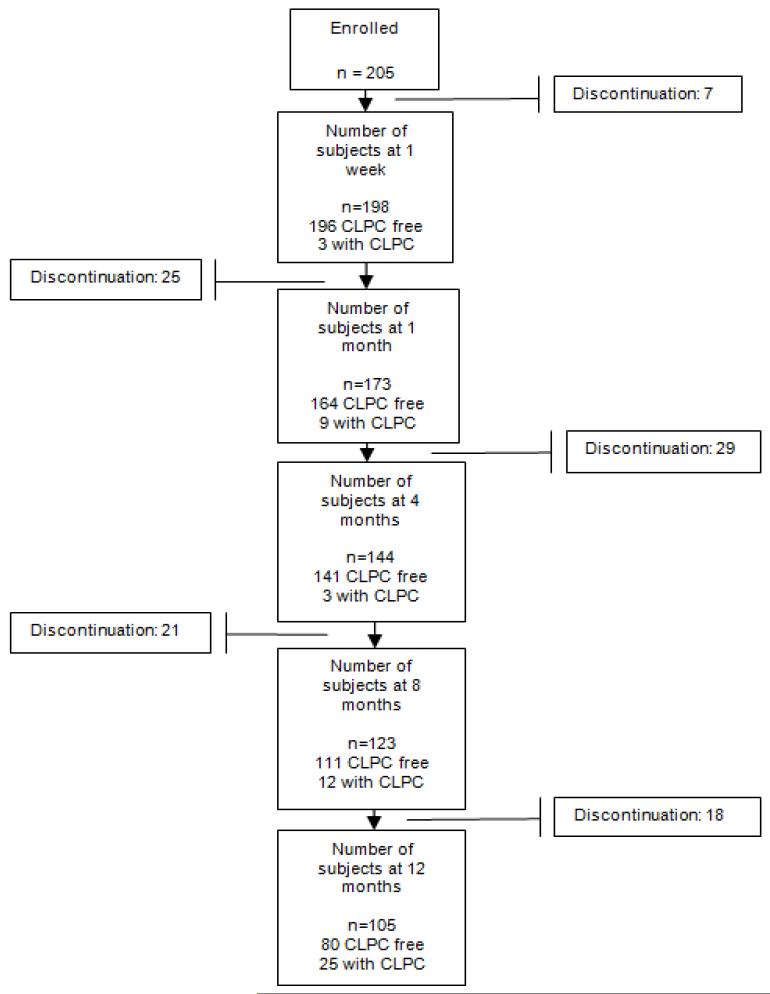
Figure 1 depicts the pattern of retention in the study. It describes the time-course of the 52 subjects with CLPC and CLPC-related dropout over the 12 month follow-up period; other reasons for dropout have been outlined and previously published in the original LASH Study main analysis.14 The 52 (of 205) subjects with CLPC contributed to a gross incidence of 25%, but a cumulative unadjusted probability of experiencing CLPC (using Kaplan-Meier survival estimates) of 60.5% (95% confidence interval 38.3% - 82.7%). Six of the subjects experienced CLPC very late in the study follow-up (more than 365 days since baseline) and thus skew the cumulative incidence proportion; if those 6 patients are removed, the Kaplan-Meier unadjusted cumulative probability of experiencing CLPC is 31.3%.
Figure 1.
Flow chart of subject retention and discontinuation
Table 2 shows the breakdown of bioburden (0 for negative bioburden/1 for positive bioburden) by location of culture and the outcome of CLPC by affected eye. Table 3 displays the univariate odds ratios for the covariates we assessed. None of the variables were significant at p=0.05. Since the main hypothesis of the study was to see if bioburden or the lens-to-cornea fitting relationship had a significant role in the outcome of CLPC, a few multivariate logistic regression models were created exploring these effects. None of the models returned any significant associations and are therefore not presented.
Table 2.
Number and Percentage of patients with substantial bioburden at each culture location stratified by presence or absence of CLPC.
| Lid | Conjunctiva | Lens | |
|---|---|---|---|
| Right Eye | |||
| no CLPC | 120/153 (78%) |
15/153 (10%) |
33/133 (25%) |
| CLPC | 28/35 (80%) |
5/35 (14%) |
8/32 (25%) |
| Left Eye | |||
| no CLPC | NA | NA | NA |
| CLPC | 14/17 (82%) |
2/17 (12%) |
3/17 (18%) |
One eye of each patient was assessed over time; eyes of patients with eventual CLPC, and if no CLPC was present then the right eye was assessed
NA: not applicable; Numerator in each cell= Number of eyes with substantial bioburden Denominator= Number of eyes where CLPC and bioburden data were available for assessment in that category.
Table 3.
Univariate Odds Ratios
| Odds Ratio | 95% Confidence intervals | ||
|---|---|---|---|
| Age | 1.00 | 0.98 | 1.03 |
| Gender (referent group - male) | |||
| Female | 0.89 | 0.44 | 1.90 |
|
Ethnicity (referent group - Caucasian) |
|||
| Asian | 0.95 | 0.34 | 2.36 |
| African American | 1.30 | 0.60 | 2.75 |
| Other | 2.17 | 0.52 | 8.18 |
|
Smoking Status (referent group non-smokers) |
|||
| Smokers | 1.17 | 0.40 | 3.05 |
|
Education (referent group - high school) |
|||
| College degree | 1.42 | 0.64 | 3.18 |
| Graduate | 1.41 | 0.66 | 3.08 |
| Difference in curvature | 0.79 | 0.20 | 3.06 |
| Lid bioburden | 1.03 | 0.49 | 2.29 |
| Conjunctival bioburden | 1.43 | 0.52 | 3.62 |
| Contact lens bioburden | 0.88 | 0.39 | 1.87 |
| Neophyte | 1.32 | 0.64 | 2.64 |
| Previous adverse events | 1.16 | 0.61 | 2.18 |
Univariate Cox Proportional Hazards Regression results are displayed in Table 4. There was a trend toward significance in the hazard of developing CLPC for African Americans and neophytes. As in the logistic regression models, different multivariate Cox Proportional Hazard Model with biologically plausible forced covariates were run, however, none of the models returned any significant associations and are therefore not presented.
Table 4.
Univariate Hazard Ratios for the Risk of Developing CLPC
| Hazard Ratio | 95% Confidence interval | ||
|---|---|---|---|
| Age | 1.01 | 0.98 | 1.03 |
| Female (referent group male) | 1.08 | 0.57 | 2.03 |
| Asian (referent group Caucasian) | 1.31 | 0.56 | 3.06 |
| African American | 1.71 | 0.90 | 3.37 |
| Other (ethnicity) | 1.50 | 0.52 | 4.30 |
|
Smoker (referent group non- smoker) |
1.27 | 0.54 | 2.98 |
|
College degree (referent group high school) |
1.45 | 0.72 | 2.90 |
| Graduate degree | 1.31 | 0.67 | 2.56 |
| Difference in curvature | 0.97 | 0.31 | 3.07 |
| Presence of lid bioburden | 0.71 | 0.35 | 1.42 |
| Presence of conjunctival bioburden | 1.22 | 0.55 | 2.71 |
|
Presence of contact lens
bioburden |
0.79 | 0.40 | 1.54 |
| Neophyte | 1.54 | 0.84 | 2.82 |
| Previous adverse events | 1.26 | 0.73 | 2.18 |
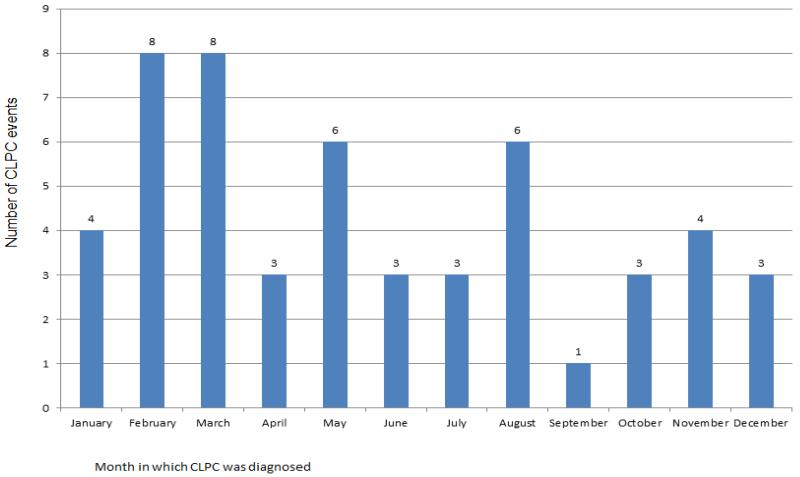
Assessment of seasonality of the initial CLPC diagnoses is displayed in Figure 2. The data does show a pattern of seasonality. The three common allergens in Ohio start in March (tree pollen), May (grass pollen), and August (ragweed)16 and the Figure 2 shows an increase of CLPC diagnosis in those same months with 38.5% of those with CLPC diagnosed in those months (20 of 52).
Figure 2.
Number of CLPC events by month
Discussion
This secondary analysis of the LASH Study assessed whether lens fit and/or levels of bioburden are factors in the development of the inflammatory response associated with CLPC. Because this study was not designed to assess CLPC as an outcome and was a pilot analysis of the potential association, it was not powered to significantly answer this question. However, the sample size is rather large and if a trend was noticed it would warrant further research in this area. Since no trend was detected, we do not feel there is a further need to consider lens or ocular bioburden as a factor in CLPC development.
To our knowledge, we are the first to explore microbial bioburden as a potential risk factor for CLPC development and there was sufficient reason for doing so. The impact of microorganisms on other chronic inflammatory conditions such as atopic keratoconjunctivits (AKC), atopic dermatitis/eczema, allergic airway inflammation and asthma has been explored in many other studies. For example, microorganisms have been implicated in eczematous exacerbations in atopic eczema/dermatitis syndrome (AEDS) which has been substantiated by the observed clinical benefit of antibacterial and antimycotic treatement 17, 18. Colonization may evoke immune responses which may trigger disease manifestations. Alternatively, toxins secreted by some organisms, such as Staphylococcus aureus, intensify inflammation as has been observed in AEDS19.
At the ocular surface, in patients with AKC, there is a markedly high occurrence of S. aureus on the lids and conjunctiva 20 and in AKC patients with ulcerative blepharitis there is a strikingly high percentage of colonization with Candida species 21. Furthermore, S. aureus secreted enterotoxins are involved in the pathophysiology of AKC 22. However, it has also been postulated that some of these associations may not be causative; that is, the abnormal colonization patterns may be secondary to deficiencies of naturally occurring antibacterial agents in the affected area 23, 24.
There is a known association between allergic asthma and high household levels of total and Gram-negative bacteria 25. This response is thought to be mediated by toll-like receptor activation of the inflammatory cascade by the bacterial protein, flagellin 26. Toll-like receptors (TLR) are a family of evolutionarily conserved glycoprotein cell receptors that recognize microbial components known as pathogen-associated molecular patterns. They trigger the innate immune response and link innate and adaptive immunity. They recognize a wide range of exogenous and endogenous molecules including those on protozoa, viruses and bacteria. For example, TLR-4 recognizes cell wall lipopolysaccarides on Gram-negative bacteria and TLR-2 recognizes peptidoglycans of Gram-positive bacteria. TLRs are expressed ubiquitously throughout tissues in the body including the cornea and conjunctiva. Of relevance to this study, TLRs have been identified in the epithelium of healthy conjunctiva 27, which act as defense mechanisms towards microbial agents in contact with the ocular surface. If the contact lens is abnormally coated with microbes, then held in contact with the ocular surface and upper tarsal conjunctiva (as is it during 30-day continuous wear), the TLR response may be upregulated resulting in an inflammatory papillary reaction visualized as CLPC. In this regard, Bonini et al have shown an overexpression of TLR-4 mRNA and protein in the conjunctiva of patients with vernal keratoconjuctivitis. Furthermore, TLR activation has been shown to contribute to allergic airway inflammation via microbial stimulation 28, and at the ocular surface, S. aureus accelerates experimental allergic conjunctivitis in a TLR-2 dependant manner 29. Therefore, lens-associated bioburden, in the presence of TLRs in the conjunctival epithelium, may be responsible in part for the development of CLPC.
Despite the strong evidence that microbial stimulation of TLRs, found in conjunctival epithelium, activate other allergic conditions, we were not able to demonstrate an association, or even a trend, between lens associated bioburden and CLPC during contact lens continuous wear. This data agrees in part with others who have explored microbial colonization of lid margins and chronic allergic conjunctivitis and found no association 30. Other factors likely play a larger role than bacterial stimulation of the immune response, and may lend more credibility to the denatured protein or mechanical stimulation hypotheses in atopic individuals. Atopy has been suspected as a causative factor in the development of CLPC4,9. Using graphical models, seasonality was assessed for the month that CLPC was diagnosed to see if there was a pattern of CLPC presenting itself during allergy season. Atopic related CLPC is expected to be more of the general type. Only 3 subjects experienced “general” CLPC in this study and therefore looking only at this subgroup is not productive. Nonetheless, looking at the sample of all 52 CLPC subjects, a possible upsurge in CLPC was noted in the spring and summer months. In Ohio, environmental allergens can be experienced year-round. The most common to the area are tree pollen (March-June), grass pollen (May-July), and Ragweed (August-October)16.
Our attempt to isolate a mechanical effect on CLPC was by surveying the difference between the best-fit sphere of the corneal surface and the lens base curve as a variable predicting CLPC. Because the lotrafilcon A lens studied was non-aspheric and had the highest modulus of elasticity of any silicone hydrogel lens, and these variables have been associated with mechanically induced CLPC,2, 11, 12 there was rationale to assess some sort of soft lens fitting parameter in this study. This may be an overly simplistic way to determine a variable mechanical effect amongst subjects, and may not have provided a relevant measure of an “edge-effect” that is thought to influence CLPC. Future studies in this area should use other imaging technology such as anterior segment optical coherence tomography (OCT) to quantify conjunctival lens edge standoff as a variable that may be associated with CLPC development.
Acknowledgments
Grant Support: This work was supported by NEI: K23 EY015270 with indirect support for laboratories and coordination from the Ohio Lions Eye Research Foundation and Research to Prevent Blindness. The clinicalTrials.gov identifier is NCT00727402.
References
- 1.Spring TF. Reaction to hydrophilic lenses. Med J Aust. 1974;1:449–450. doi: 10.5694/j.1326-5377.1974.tb50817.x. [DOI] [PubMed] [Google Scholar]
- 2.Skotnitsky C. [accessed 11/11/11];Contact lens induced papillary conjunctivitis (CLPC) & high Dk SH lenses. 2007 Feb; http://www.siliconehydrogels.org/editorials/feb_07.asp.
- 3.Porazinski AD, Donshik PC. Giant papillary conjunctivitis in frequent replacement contact lens wearers a retrospective study. CLAO J. 1999;25:142–147. [PubMed] [Google Scholar]
- 4.Skotnitsky CC, Naduvilath TJ, Sweeney DF, Sankaridurg PR. Two presentations of contact lens-induced papillary conjunctivitis (CLPC) in hydrogel lens wear: local and general. Optom Vis Sci. 2006 Jan;83(1):27–36. doi: 10.1097/01.opx.0000195565.44486.79. [DOI] [PubMed] [Google Scholar]
- 5.Skotnitsky C, Sankaridurg P, Sweeney DF, Holden B. General and local contact lens induced papillary conjunctivitis (CLPC) Clin Exp Optom. 2002 May;85(3):193–197. doi: 10.1111/j.1444-0938.2002.tb03034.x. [DOI] [PubMed] [Google Scholar]
- 6.Sankaridurg PR, Sweeney DS, Sharma S, Gora R, Naduvilath T, Ramachandran L, et al. Adverse events with extended wear of disposable hydrogels: results for the first 13 months of wear. Ophthalmology. 1999;106:1671–1680. doi: 10.1016/S0161-6420(99)90346-9. [DOI] [PubMed] [Google Scholar]
- 7.Ozkan J, Mandathara P, Krishna P, Sankaridurg P, Naduvilath T, Willcox MD, Holden B. Risk factors for corneal inflammatory and mechanical events with extended wear silicone hydrogel contact lenses. Optom Vis Sci. 2010 Nov;87(11):847–53. doi: 10.1097/OPX.0b013e3181f6f97d. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton F, Stretton S, Papas E, Skotnitsky C, Sweeney DF. Silicone hydrogel contact lenses and the ocular surface. Ocul Surf. 2006 Jan;4(1):24–43. doi: 10.1016/s1542-0124(12)70262-8. [DOI] [PubMed] [Google Scholar]
- 9.Skotnitsky C. [accessed 11/11/11];Contact lens papillary conjunctivitis – an update. 2005 Jul; http://www.siliconehydrogels.org/featured_review/featured_review_jul_05.asp.
- 10.Donshik PC, Ballow M, Luistro A, Samartino L. Treatment of contact lens-induced giant papillary conjunctivitis. The CLAO Journal. 1984 Oct;10(4):346–350. [PubMed] [Google Scholar]
- 11.Friederich D. Contact lens-induced papillary conjunctivitis and silicone hydrogel lenses. The AOA’s CLCS Newsletter. 2011 May; [Google Scholar]
- 12.Weissman BA, Yeung KK. [accessed 11/13/2011];Conjunctivitis, Giant Papillary. 2011 May; http://emedicine.medscape.com/article/1191641-overview.
- 13.Zhao Z, Fu H, Skotnitsky CC, Sankaridurg PR, Wilcox MD. IgE antibody on worn highly oxygen-permeable silicone hydrogel contact lenses from patients with contact lens-induced papillary conjunctivitis (CLPC) Eye Contact Lens. 2008 Mar;34(2):117–21. doi: 10.1097/ICL.0b013e318145a4e5. [DOI] [PubMed] [Google Scholar]
- 14.Szczotka-Flynn L, Lass JH, Sethi A, Debanne S, Benetz BA, Albright M, Gillespie B, Kuo J, Jacobs MR, Rimm A. Risk factors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5421–30. doi: 10.1167/iovs.10-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute for Eye Research . Institute for Eye Research Grading Scales; [Accessed Sept 10, 2013]. Available http://contactlensupdate.com/2012/11/20/brien-holden-vision-institute-grading-scales/ [Google Scholar]
- 16. [accessed 03/23/2012]; http://www.ohioallergy.com/faq.php.
- 17.Breuer K, Haussler S, Kapp A, Werfel T. Staphylococcus aureus:colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol. 2002 Jul;147(1):55–61. doi: 10.1046/j.1365-2133.2002.04872.x. [DOI] [PubMed] [Google Scholar]
- 18.Lintu P, Savolainen J, Kortekangas-Sovolainen O, Kalimo K. Systemic ketokonazole is an effective treatment of atopic dermatitis with IgE-mediated hypersensitivity to yeasts. Allergy. 2001 Jun;56(6):512–7. doi: 10.1034/j.1398-9995.2001.056006512.x. [DOI] [PubMed] [Google Scholar]
- 19.Campbell DE, Kemp AS. Proliferation and production of interferon-gamma (IFN-gamma) and IL-4 in response to Staphylococcus aureus and staphylococcal superantigen in childhood atopic dermatitis. Clin Exp Immunol. 1997 Feb;107(2):392–7. doi: 10.1111/j.1365-2249.1997.278-ce1172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nivenius E, Montan PG, Chryssanthouw E, Jung K, van Hage-Hamsten M, van der Ploeg I. No apparent association between periocular and ocular microcolonization and the degree of inflammation in patients with atopic keratoconjunctivitis. Clin Exp Allergy. 2004 May;34(5):725–30. doi: 10.1111/j.1365-2222.2004.1950.x. [DOI] [PubMed] [Google Scholar]
- 21.Huber-Spitzy V, Bohler-Sommeregger K, Arocker-Mettinger E, Grabner G. Ulcerative blepharitis in atopic patients – is Candida species the causative agent? Br J Ophthalmol. 1992 May;76(5):272–4. doi: 10.1136/bjo.76.5.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujishima H, Okada N, Dogru M, Baba F, Tomita M, Abe J, Matsumoto K, Saito H. The role of Staphylococcol enterotoxin in atopic keratoconjunctivitis and corneal ulceration. Allergy. 2012 Jun;67(6):799–803. doi: 10.1111/j.1398-9995.2012.02818.x. [DOI] [PubMed] [Google Scholar]
- 23.Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002 Aug;119(2):433–9. doi: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 24.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002 Oct 10;347(15):1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 25.Ross MA, Curtis L, Scheff PA, Hryhorczuk DO, Ramakrishnan V, Wadden RA, Persky VW. Association of asthma symptoms and severity with indoor bioaerosols. Allergy. 2000 Aug;55(8):705–11. doi: 10.1034/j.1398-9995.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RH, Maruoka S, Whitehead GS, Foley JF, Flake GP, Sever ML, Zeldin DC, Kraft M, Garantziotis S, Nakano H, Cook DN. The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens. Nat Med. 2012 Nov;18(11):1705–10. doi: 10.1038/nm.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonini S, Micera A, Iovieno A, Lambiase A, Bonini S. Expression of Toll-like receptors in healthy and allergic conjunctiva. Ophthalmology. 2005 Sep;112(9):1528. doi: 10.1016/j.ophtha.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Xiang Y, Yao X, Liu Y, Gong W, Yoshimura T, Wang JM. The active contribution of Toll-like receptors to allergic airway inflammation. Int Immunopharmacol. 2011 Oct;11(10):1391–8. doi: 10.1016/j.intimp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung SH, Nam KH, Kweon MN. Staphylococcus aureus accelerates an experimental allergic conjunctivitis by Toll-like receptor 2-dependant manner. Clin Immunol. 2009 Apr;131(1):170–7. doi: 10.1016/j.clim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Tuft SJ, Ramakrishnan M, Seal DV, Kemeny DM, Buckley RJ. Role of Staplylococcus aureus in chronic allergic conjunctivitis. Ophthalmology. 1992 Feb;99(2):180–4. doi: 10.1016/s0161-6420(92)31995-5. [DOI] [PubMed] [Google Scholar]